Abstract
Background
There is a well-recognized need for a new generation of single photon emission computed tomography (SPECT) perfusion tracers with improved myocardial extraction over a wide flow range. Radiotracers that target complex I of the mitochondrial electron transport chain have been proposed as a new class of myocardial perfusion imaging agents. 7-(Z)-[125I]iodorotenone (125I-ZIROT) has demonstrated superior myocardial extraction and retention characteristics in rats and in isolated perfused rabbit hearts. We sought to fully characterize the biodistribution and myocardial extraction versus flow relationship of 123I-ZIROT in an intact large-animal model.
Methods and Results
The 123I-ZIROT was administered during adenosine A2A agonist-induced hyperemia in 5 anesthetized dogs with critical left anterior descending (LAD) stenoses. When left circumflex (LCx) flow was maximal, 123I-ZIROT and microspheres were coinjected and the dogs were euthanized 5 minutes later. 123I-ZIROT biodistribution was evaluated in 2 additional dogs by in vivo planar imaging. At 123I-ZIROT injection, transmural LAD flow was unchanged from baseline (mean±SEM, 0.90±0.22 versus 0.87±0.11 mL/[min · g]; P=0.92), whereas LCx zone flow increased significantly (mean±SEM, 3.25±0.51 versus 1.00±0.17 mL/[min · g]; P<0.05). Myocardial 123I-ZIROT extraction tracked regional myocardial flow better than either thallium-201 or 99mTc-sestamibi from previous studies using a similar model. Furthermore, the 123I-ZIROT LAD/LCx activity ratios by ex vivo imaging or well counting (mean±SEM, 0.42±0.08 and 0.45±0.1, respectively) only slightly underestimated the LAD/LCx microsphere flow ratio (0.32±0.09).
Conclusions
The ability of 123I-ZIROT to more linearly track blood flow over a wide range makes it a promising new SPECT myocardial perfusion imaging agent with potential for improved coronary artery disease detection and better quantitative estimation of the severity of flow impairment.
Keywords: coronary artery disease, diagnosis, myocardial perfusion imaging, radioisotope
There is a well-recognized need for a new generation of myocardial perfusion imaging (MPI) agents with improved myocardial extraction over a wide flow range.1–4 The “roll-off” phenomenon that occurs for most of the clinically used perfusion tracers results in underestimation of perfusion abnormalities, which can be a limitation in the detection of mild-to-moderate stenosis.5,6 In addition, more linear extraction of the tracer over a wide range of flow is a requirement for quantitative estimation of absolute blood flow.
It has long been recognized that cationic MPI agents, including the current clinical tracers 99mTc-sestamibi and 99mTc-tetrofosmin, accumulate in cardiac cells based on the mitochondrial membrane potential.7–10 Cationic tracers are nonspecifically trapped within the mitochondria because of their charge,11,12 and their accumulation correlates well with the 20% to 30% mitochondrial mass in the heart.13 More recently, radiotracers that specifically target complex I of the mitochondrial electron transport chain (mitochondrial complex I [MC-I] and nicotinamide adenine dinucleotide:ubiquinone oxidoreductase) have been proposed as a new class of MPI agents.14–18 These tracers are structural analogs of rotenone,14–16 fenazaquin,18 or pyridaben,17,19 3 well-known potent MC-I inhibitors.
Rotenone, a neutral, lipophilic, natural product, and its derivatives have been radiolabeled with either iodine14,15 or carbon and fluorine16 for single proton emission computed tomography (SPECT) and positron emission tomography (PET) imaging. In Sprague-Dawley rats, 7′-(Z)[125I]iodorotenone (125I-ZIROT) demonstrated myocardial uptake similar to 99mTc-sestamibi at 1 and 2 hours after injection, with significantly superior heart:lung ratios.14 Marshall et al15 demonstrated that 125I-ZIROT extraction and retention were greater than those of 99mTc-sestamibi in ex vivo–isolated, erythrocyte and albumin–perfused rabbit hearts. The objective of the present study was to evaluate the distribution of 123I-ZIROT and to fully characterize the myocardial flow/extraction relationship during vasodilator stress in an open-chest canine model of coronary stenosis.
Methods
The animal studies were conducted in compliance with the position of the American Heart Association on the use of research animals in accordance with the Principles of Laboratory Animal Care (National Institutes of Health publication 86 to 23, revised 1985). The animal protocols were approved by the Institutional Animal Care and Use Committees at both Lawrence Berkeley National Laboratory and the University of Virginia.
Preparation of 123I-ZIROT
Preparation of 123I-ZIROT was similar to the previously described synthesis of the iodine-125–labeled analog.14 Iodine-123 (0.1N in NaOH; MDS Nordion, Ottawa, Canada) was loaded onto an AG 4-X4 anion exchange resin column (Bio-Rad acetate form; 0.57×2 cm in a 1-mL syringe). The column was rinsed 5 times with 18 MΩ deionized water. The iodine-123 was eluted from the column with 4 mol/L NH4OH (0.4-mL fractions). The selected fraction was transferred into a 1-mL v-vial. The liquid was evaporated under a gentle stream of nitrogen at 115°C. The 7′-(Z)-tributylstannylrotenone14 (20 μg in 100 μL of dry acetonitrile) was added to the cooled vial equipped with a magnetic stir vane. NaOAc buffer (40 μL; 0.1 mol/L NaOAc and 1.3 mol/L HOAc, pH 3.5) and peracetic acid (10 μL) were added, and the reaction was stirred for 60 min at room temperature. The reaction was quenched with 10 μL of 0.1% N2S2O5 (aqueous), diluted with 350 μL of water, and injected onto a Whatman Partisil 10 ODS-3 high pressure liquid chromatography column (9.4 mm×50 cm; 60/40 MeOH/H2O, 6 mL/min). The product fractions were pooled, diluted 3 times with water, and passed through a Waters C18 Sep-Pak Light cartridge. The trapped product was eluted from the cartridge with 1 mL of United States Pharmacopeia ethanol through a Millex FG (22-μm) filter. The Sep-Pakfilter assembly was rinsed with 10 mL of sterile saline, providing a 9% ethanol/saline solution for injection. Radio thin-layer chromatographic evaluation of ZIROT was undertaken before injection of the tracer (1:1 ethyl acetate/hexane; Rf=0.44).
Surgical Preparation
Five fasted adult mongrel dogs (mean±SEM weight, 26.9±4.4 kg) were anesthetized with sodium pentobarbital (30 mg/kg IV), intubated, and ventilated with room air on a respirator with 5 cm of positive end-expiratory pressure. Anesthesia was maintained using an intravenous infusion of pentobarbital (2.5 mg/kg per hour). Saturation of peripheral oxygen and expiratory end-tidal CO2 were continuously monitored using a portable multiparameter vital signs monitor (Surgivet; Waukesha, WI). In addition, arterial blood gases were monitored throughout the experiment using a blood gas analyzer (ABL5; Radiometer America, Inc., Westlake, OH). SpO2,expCO2, pH, pO2, pCO2, and HCO3 were maintained at physiological levels. Lead II of the ECG was monitored continuously. Both femoral veins and the right jugular vein were isolated and cannulated using 8F polyethylene catheters for pentobarbital, fluid, 123I-ZIROT, and vasodilator administration. Both femoral arteries were isolated and cannulated with 8F polypropylene catheters for arterial blood sampling and radiolabeled microsphere reference blood withdrawal. A 7F catheter was inserted in the right carotid artery for continuous monitoring of the mean systemic arterial pressure.
A left thoracotomy was performed at the level of the fifth intercostal space, and the heart was suspended in a pericardial cradle. The heart was instrumented as previously described.5,6 A snare occluder and ultrasonic transducer were placed proximal to the first diagonal branch of the left anterior descending (LAD) coronary artery. A second flow transducer was placed on the left circumflex (LCx) coronary artery. The LAD and LCx flow, left atrial pressure, mean and end-diastolic left ventricular pressures, heart rate, and positive first derivative of left ventricular pressure with respect to time were continuously monitored and digitally recorded using LDS Ponemah software (DSI; St Paul, MN).
Experimental Protocol
The experimental protocol used in 5 dogs is depicted in Figure 1. After a brief stabilization period, 15-μM microspheres (Sr-85, Nb-95, or Sc-46; Perkin-Elmer Life Sciences, Downer’s Grove, Ill) were injected into the left atrial catheter to measure baseline resting blood flow. The microsphere technique is the “gold standard” for measuring regional myocardial blood flow, has been well validated,20 and is routinely used in our laboratory.5,6,21 Next, a critical LAD stenosis was produced by tightening the snare occluder, as previously described in detail.5,6,21 A critical stenosis was defined as that which produced no change in resting coronary flow but with the hyperemic response to a brief 10-s LAD occlusion completely abolished. Once the stenosis was established, a second set of microspheres was administered to document myocardial blood flow with the stenosis. Afterward, an intravenous infusion of the A2A adenosine receptor agonist ATL-146e (0.3 μg/kg per minute; PGx-Health, LLC) was initiated.22 When LCx flow was maximal, a mean±SEM of 109±49 MBq (3±1 mCi) of 123I-ZIROT and a third set of microspheres were coinjected. Five minutes later, the dogs were euthanized with an overdose of sodium pentobarbital.
Figure 1.
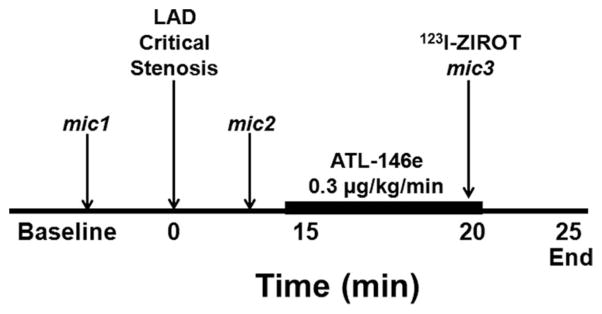
Experimental protocol. LAD, left anterior descending coronary artery; mic, radiolabeled microsphere injections.
Ex Vivo Heart Slice Imaging and Image Quantification
Immediately after euthanasia, the heart was removed and sliced into four 1-cm short-axis slices from apex to base. The heart slices were placed on a thin piece of cardboard, covered tightly with plastic wrap, and imaged directly on a low-energy all-purpose parallel hole collimator of a standard clinical γ camera (Siemens Orbiter). Images were collected using a 128×128 count matrix with a 20% window centered on the 159-keV iodine-123 photopeak for a total of 1×106 counts. The heart slice images were quantified by regions of interest (ROIs) drawn on the LAD defect area of the anteroseptal wall and on the normal LCx-perfused posterior wall, as previously described.5,6
Determination of Tracer Activities and Regional Myocardial Blood Flow
Each myocardial slice was then subdivided as previously described, resulting in 72 myocardial samples for each dog.5,6 γ Well counting was performed on these samples and on the blood reference samples to determine iodine-123 and microsphere activities using a γ well counter (MINAXI 5550; Perkin-Elmer Life Sciences). The tissue counts were corrected for background, decay, and isotope spillover; regional myocardial blood flow was calculated with specialized computer software (PCGERDA; Scientific Computing Solutions, LLC, Charlottesville, Va). Transmural activities and flow values were calculated as the weighted mean of the corresponding epicardial, midwall, and endocardial samples. The 5 transmural sections (15 segments) with the lowest flow values were classified as the stenotic LAD zone, and the 5 transmural sections with the highest flow values were classified as the normal LCx zone. For each of the 5 individual animals, raw data plots of ZIROT activities versus microsphere-derived flow at injection during vasodilator stress were created with all 72 myocardial tissue segments from each dog. A plot of data pooled from the 5 dogs was created by normalizing the activity data from each individual dog to the activity of a segment in the same dog, with a normal flow of ≈1.0 mL/(min · g), similar to previous studies5,6 from our laboratory.
Whole Body Planar Imaging
Two fasted adult mongrel dogs (weight, 20–25 kg) were sedated with sodium pentothal (30 mg/kg IV). The animals were not pretreated with potassium iodide to block thyroid uptake of free iodine-123. The dogs were intubated and maintained using inhaled isoflurane gas (2%). The 123I-ZIROT (148–222 MBq) was injected in a femoral vein. Serial planar images were acquired with a dual-head clinical γ camera (GE Millenium Hawkeye) using a low-energy, high-resolution, parallel-hole collimator and a 20% window centered on the 159-keV iodine-123 photopeak. The dynamic images were acquired from both heads in the left and right lateral views for 4 minutes each during the first hour and for 8 minutes each between 1 and 2 hours. In 1 dog, serial arterial blood samples (2 mL) were acquired at 5-s intervals for the first minute and then at 1.5, 2, 2.5, 3, 4, 5, 10, 15, 20, 30, and 60 min after 123I-ZIROT injection. The blood samples were counted in a γ well counter, and the blood clearance time constant was calculated by fitting the time-activity data with an exponential function.
Image Quantification
The dynamic image data sets acquired from the dual heads were summed at each point and were corrected for background and decay. The ROIs were drawn on the heart and liver, and the activities within each region were expressed as a percentage of the initial activity. Heart/liver ratios were calculated by dividing the mean counts in the heart ROI by the mean counts in the liver ROI.
Radiation Dosimetry
The ROIs were drawn in areas of minimal organ overlap. Residence times were calculated from the integral of the time activity curves for the following organs: brain, thyroid, heart, stomach, gallbladder, upper small and large intestines, kidney, liver, and urinary bladder. Radiation dose estimates were calculated using MIRDOSE3.1.23
Statistical Analysis
All statistical computations were performed with SYSTAT software (Systat Software, Inc, San Jose, Ca). The results were expressed as mean±SEM. Differences between means were assessed with either 1- or 2-way repeated-measures ANOVA, where appropriate, with Bonferroni correction for multiple comparisons, or a paired t test (ischemic/normal ratios), with values of P<0.05 considered significant. The myocardial flow-extraction relationship was analyzed by nonlinear regression using the following equation: y=a0*(1−exp[−a1/x]), according to the kinetic transport model of Gosselin and Stibitz.24
Results
Hemodynamic Parameters
The mean hemodynamic parameters are summarized in the Table. As expected, adenosine receptor agonist (ATL146e) infusion caused a slight transient decrease in mean arterial pressure and left ventricular pressure, with no significant changes in heart rate or left atrial pressure. Furthermore, the vasodilator resulted in no increase in mean ultrasonically measured LAD flow because of the presence of the critical stenosis on that vessel, but there was a significant increase in LCx flow from 37±8 to 121±29 (P<0.001 versus stenosis), resulting in a >3:1 flow disparity between the stenotic LAD and normal LCx coronary artery.
Absolute Myocardial Blood Flow
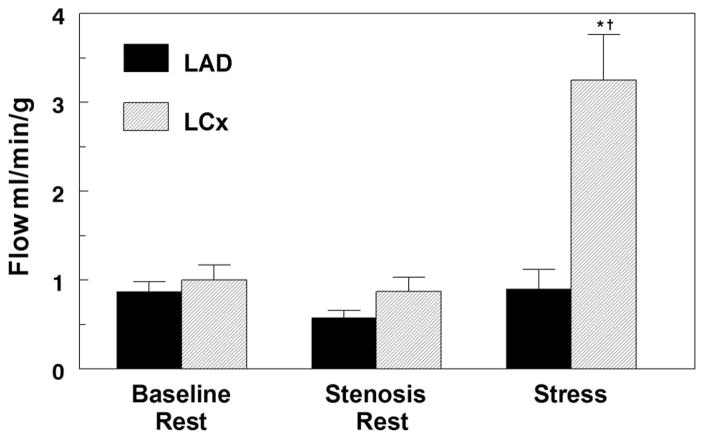
Figure 2 summarizes the microsphere-derived transmural regional myocardial blood flows. As can be seen, resting flow did not change in the LAD zone on setting of the critical stenosis. During peak vasodilator stress, LAD zone flow remained unchanged; however, there was a 3.3-fold increase in normal LCx zone flow, resulting in a nearly 4:1 mean flow disparity between the 2 zones when 123I-ZIROT was injected.
Figure 2.
Average transmural myocardial blood flow in the LAD and left circumflex (LCx) territories determined using microsphere injections at baseline, after setting the critical LAD stenosis, and during peak vasodilator stress at 123I-ZIROT injection. * P<0.001 vs baseline, and †P<0.001 vs LAD. n=5.
Myocardial ZIROT Uptake as a Function of Regional Blood Flow
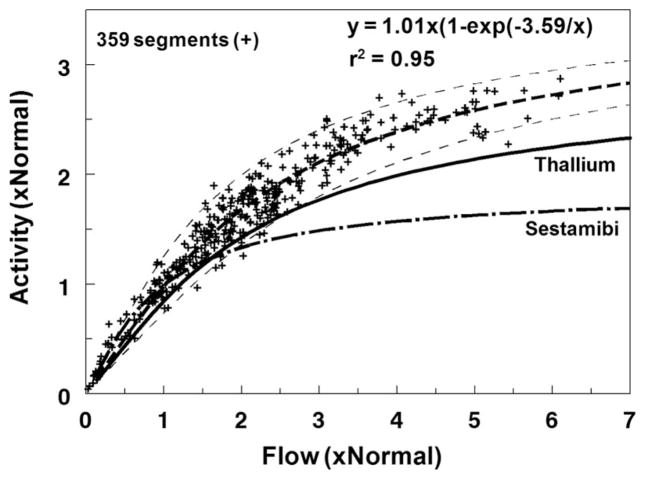
Figure 3 depicts the myocardial flow-extraction relationship obtained by γ well counting of the 359 myocardial segments obtained from all 5 animals. The thallium-201 and 99mTc-sestamibi flow-extraction curves from our previous studies using a similar canine model are shown on the same graphic.6 Myocardial 123I-ZIROT extraction tracked regional myocardial blood flow, as determined by the gold standard microspheres, over a wide range of flow, more linearly than either thallium-201 or 99mTc-sestamibi in our previous studies. As shown in Figure 3, 123I-ZIROT begins to underestimate true flow, as determined by the microsphere measurements, at >2 mL/(min · g). This is better than the current clinical flow tracers that deviate from unity between 1 and 1.5 mL/(min · g).
Figure 3.
Flow-extraction relationship. This graphic represents the pooled results from 359 myocardial segments obtained from the 5 experiments. 123I-ZIROT activity and flow in each myocardial segment were normalized to the area of normal flow (≈1 mL/[min · g]). The curve fit through the experimental data were of the form y=a0*(1−exp[−a1/x]) according to the kinetic transport model of Gosselin and Stibitz.24 The results previously obtained in our laboratory using the same animal model for thallium-201 and 99mTc-sestamibi are represented on the same graphic.6 r2 is the coefficient of determination.
Stenotic/Normal Count Ratios for Flow Versus Tracer Activities
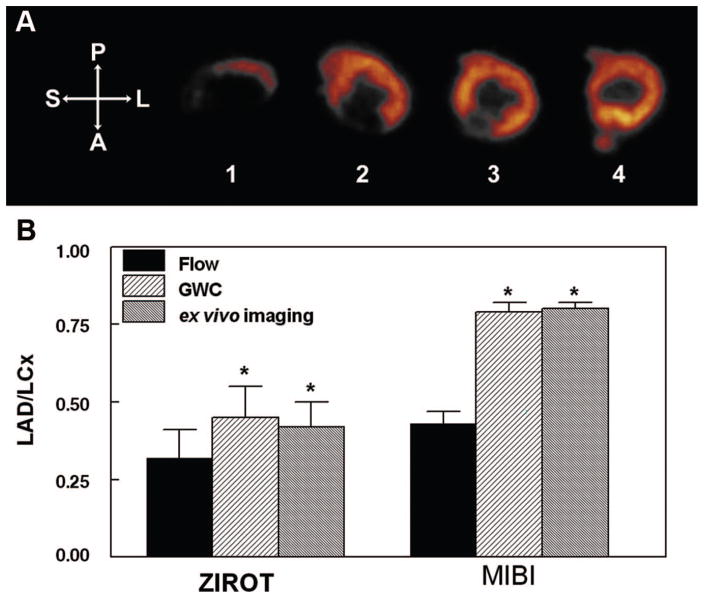
Representative ZIROT ex vivo heart slice images are displayed in Figure 4A. Large perfusion defects were easily observed in the anteroseptal wall (slices 1–3). The average defect:normal count ratio for the 3 slices with defects in this dog was 0.20. The bar graphs in Figure 4B compare the mean stenotic LAD/normal LCx ratios for flow versus 123I-ZIROT activities determined by either γ well counting of transmural myocardial segments or ex vivo image quantification. The lower the bar, the more severe the flow disparity between the stenotic and normal zones. As shown, using 2 separate and independent analyses of tracer deposition, 123I-ZIROT only slightly underestimated the true microsphere flow ratio. The 123I-ZIROT LAD/LCx flow ratio was 0.32±0.09, compared with LAD/LCX activity ratios of 0.45±0.10 by γ well counting (P<0.05 versus flow ratio) and 0.42±0.08 by ex vivo imaging (P<0.05 versus flow ratio). Also shown in Figure 4B are previous data evaluating 99mTc-sestamibi in the same model with comparable LAD-LCx flow disparity at injection (0.43±0.04).
Figure 4.
A, Representative example of 123I-ZIROT ex vivo imaging. Note the presence of a large defect on the anteroseptal segments of the most apical short-axis slices (slices 1–3). The defect:normal count ratio in this dog was 0.20. B, Comparison between the average transmural flow defect magnitude and the average 123I-ZIROT defect magnitude, as determined by GWC (γ well counting) or ex vivo imaging (n=5). For comparison, our previous results using the same animal model for 99mTc-sestamibi with comparable flow at injection are represented on the same graphic (n=9).6 *P<0.05 vs flow ratio. P indicates posterior; A, anterior; S, septal; L, lateral.
Determination of Biodistribution, Organ Kinetics, and Radiation Dosimetry Using Whole Body Dynamic Planar Imaging
Planar images of 123I-ZIROT at 15, 30, 60, and 120 min after injection are shown in Figure 5. The heart is easily identifiable by planar imaging as early as 15 min after injection. The low blood pool activity allows early visual delineation of both the left and right ventricle walls. This observation was supported by the serial blood sampling data in this dog that revealed that 123I-ZIROT rapidly cleared from the blood pool (t1/2=17 s), with <2% of the initial dose present in the blood pool at 15 min after injection. Limited uptake of free iodine-123 was seen in the thyroid during the 2-hour duration of the imaging study.
Figure 5.
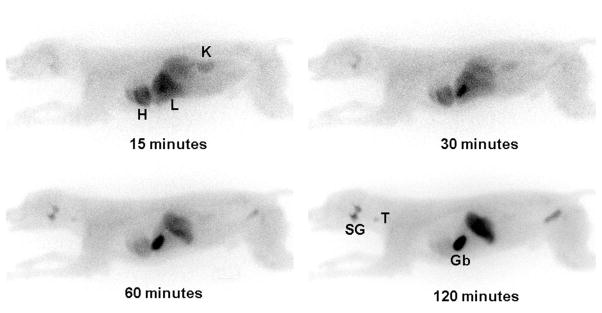
Representative whole body planar images of 123I-ZIROT at 15, 30, 60, and 120 min after injection. H indicates heart; Li, liver; K, kidney; GB, gall-bladder; T, thyroid; SG, salivary glands.
Both myocardial and liver uptake values were maximal at the 2-min point. Myocardial and liver clearance values were then moderate, with t1/2 values of ≈30 and 11 minutes, respectively (Figure 6A). At 15 min after 123I-ZIROT injection, the heart/liver activity ratio reached a plateau of 1.32±0.14 that was maintained for at least 2 hours (Figure 6B); there was progressive accumulation of the tracer in the gallbladder. The 123I-ZIROT uptake in the lungs was minimal, and the lungs were not visible by planar imaging at any point.
Figure 6.
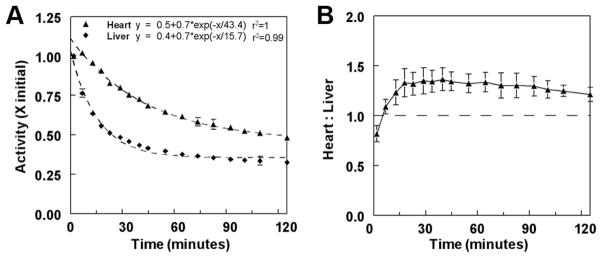
Results obtained from quantification of the in vivo planar images (n=2). The regions of interest (ROIs) were drawn around the heart and liver to determine the heart and liver kinetics (A) and the heart/liver ratio (B) over time.
Preliminary radiation dose estimates from the planar images were determined on selected organs, including the gallbladder wall, stomach, heart wall, kidneys, liver, thyroid, and salivary gland. The organ with the highest radiation dose was the gallbladder wall, with 1.14 rad/mCi injected. This was followed by the salivary gland, stomach, and thyroid, with doses of 0.66, 0.46, and 0.26 rad/mCi, respectively. The heart wall (0.03 rad/mCi) and the clearance organs, liver and kidney (0.04 rad/mCi), showed the least radiation dose. The whole body dose was 0.011 rad/mCi.
Discussion
Although SPECT imaging with thallium-201, 99mTc-sestamibi, and 99mTc-tetrofosmin has been the workhorse for clinical MPI, there are shortcomings with these agents. Despite the fact that they provide highly relevant diagnostic information about myocardial perfusion and viability, several known limitations have been identified.1 The roll-off phenomenon remains the principal limitation to the use of these MPI agents. Because of the underestimation of the highest flow values during a stress test, the activity ratio between a myocardial region with a mild impairment in flow reserve and a normal region might be close to unity, thus limiting the detection of an ischemic region.5,6 Therefore, there is a well-defined need for a new generation of perfusion tracers with improved myocardial extraction over a broader flow range. Moreover, quantitative flow measurement may be much more relevant than relative perfusion imaging for the evaluation of patients with homogeneous perfusion defects, such as in balanced coronary artery disease,25 cardiomyopathy,26 or endothelial dysfunction.27 Therefore, accurately tracking flow over a wide flow range is also 1 of the requirements for the determination of absolute myocardial blood flow.1,28 Because the prevalence of cardiovascular diseases associated with diffuse perfusion abnormalities, such as diabetes mellitus, is increasing, the need for quantitative flow measurement using either SPECT or PET is increasing.2 PET imaging, with better attenuation correction and higher spatial and temporal resolution than SPECT, is the best candidate for quantitative flow measurements in nuclear medicine. Nevertheless, even if the technique is not yet available, it is reasonable to think that the next generation of SPECT cameras, together with better attenuation correction and mathematical modeling, may enable the absolute measurement of myocardial blood flow using a single-photon perfusion tracer.
Rotenone derivatives radiolabeled with either iodine (123I-ZIROT) or fluorine-18 ([18F]fluorodihydrorotenone) have been proposed as new perfusion imaging agents.14–16 Rote-none is a natural product that has been extracted from the roots of the tropical plant Derris elliptica and has a nanomolar affinity for the MC-I of the electron transport chain.29 Because mitochondrial dysfunction has been reported in various pathological conditions, such as neurological diseases, rotenone derivatives have been previously radiolabeled with carbon-11 and tritium to evaluate the density of the MC-I in the brain and other tissues.30,31 Initial evaluations of labeled rotenone derivatives were focused on brain distribution relative to degenerative diseases. Once these compounds were labeled with PET and SPECT radioisotopes, their avidity for tissues rich in mitochondria, especially the heart, was noted.
Using mitochondria as a target for MPI is not new. Both sestamibi and tetrofosmin accumulate in the heart based on the mitochondrial membrane potential.8–10,12 Prior studies14 have shown that 125I-ZIROT demonstrates a high myocardial uptake in vivo in normal rats (3.71±0.20% ID/g at 1 h post-injection), with modest washout (2.96±0.24% ID/g at 2 h post-injection). Marshall et al15 evaluated 125I-ZIROT in an ex vivo model of isolated retrograde blood-perfused rabbit hearts and showed that both extraction and retention of 125I-ZIROT were significantly higher than that of sestamibi. Similarly, in the same model, [18F]fluorodihydrorotenone tracked flow better than thallium-201.16 The radiolabeled rotenone analogs have, therefore, demonstrated excellent characteristics as MPI agents. Accordingly, analogs of 2 other MC-I inhibitors, fenazaquin and pyridaben, have been subsequently radiolabeled with fluorine-18 and evaluated as new PET MPI agents.17–19,32–34 The results obtained in various animal models using the pyridaben analog 18F-BMS747158-02 have been encouraging.17,19,32–34 The MC-I inhibitor analogs can, therefore, be considered as a new class of MPI agents with excellent characteristics for both SPECT and PET imaging.
Myocardial Flow-Extraction Relationship
In the current study, infusion of the A2A adenosine receptor agonist ATL-146e22 increased flow by up to ≈6 times in normal zone myocardial segments, whereas flow in the critically stenotic LAD region was unchanged, producing a wide range of flow. Over this wide range, 123I-ZIROT tracked flow more linearly than was previously found using either thallium-201 or 99mTc-sestamibi.6 As shown in Figure 3, 123I-ZIROT begins to underestimate the true flow, as determined by the microsphere measurements, at >2 mL/(min · g). This is better than the current clinical flow tracers that deviate from unity between 1 and 1.5 mL/(min · g). Because of the more linear myocardial extraction, the 123I-ZIROT activity ratio between high- and low-flow regions more closely approximated the gold standard microsphere flow ratio in these same regions. Also, because of the more linear extraction, 123I-ZIROT produced large transmural perfusion defects on images.
Comparison With Other SPECT MPI Agents
Thallium-201 tracks regional myocardial blood flow better than the other clinically used SPECT MPI agents, 99mTc-sestamibi and 99mTc-tetrofosmin. Indeed, thallium-201 has higher first-pass extraction and the roll-off phenomenon occurs at higher flow rates than is observed with these 99mTc-labeled perfusion tracers.6,35 However, the physical properties of thallium-201 are not ideal because of its relatively long half-life, which limits the injected dose, and its suboptimal energy for SPECT camera detection. Therefore, despite the relatively high liver uptake, 99mTc-sestamibi and 99mTc-tetrofosmin are often preferred for SPECT MPI.36,37
We previously evaluated the thallium-201 and 99mTc-sestamibi flow-extraction relationship in a canine model with a similar degree of coronary stenosis as that used in the present study, as evidenced by comparable LAD/LCx flow ratios at tracer injection.6 The thallium-201 and 99mTc-sestamibi activity ratios obtained by γ well counting underestimated the degree of flow disparity to a greater extent than 123I-ZIROT (0.62±0.04 and 0.79±0.03 versus 0.45±0.10, respectively). This finding is in agreement with the results obtained by Marshall et al15,16 in the isolated perfused rabbit heart model.
The 99mTc-tetrofosmin flow-extraction relationship was first evaluated by Sinusas et al38 in a canine model of permanent LAD occlusion. When injected at peak adenosine or dipyridamole stress, there was good correlation between 99mTc-tetrofosmin extraction and absolute flow (r=0.84). However, the plateau in tetrofosmin extraction occurred at even lower flow rates than had been previously described for thallium-201 and 99mTc-sestamibi.6 These results were confirmed in our laboratory using a canine critical stenosis model similar to that used in the present study.35
99mTcN-NOET is a neutral lipophilic compound with a high myocardial first-pass extraction fraction.39,40 Calnon et al41 evaluated the 99mTcN-NOET myocardial flow-extraction relationship using a similar canine model as used in the present study. They reported a stenotic/normal 99mTcN-NOET activity ratio of 0.55±0.05 by well counting (versus 0.45±0.10 for 123I-ZIROT) and 0.58±0.64 by ex vivo imaging (versus 0.42±0.08 for 123I-ZIROT) in the presence of a stenotic/normal flow ratio similar to that obtained for 123I-ZIROT (0.33±0.04 versus 0.32±0.09, respectively). Despite its favorable characteristics and extensive preclinical and clinical testing, 99mTcN-NOET was ultimately not approved for MPI in humans.
ZIROT Biodistribution, Organ Clearance Kinetics, and Radiation Dosimetry
Remarkable in vivo stability of 123I-ZIROT was noted in this study. Pretreatment with potassium iodide is typical for studies involving radioiodinated tracers to prevent the accumulation of free radioiodine in the thyroid. In this study, potassium iodide was not administered, yet the accumulation of free radioiodine in the thyroid was surprisingly low, even 2 to 4 h after 123I-ZIROT injection (4-h data not shown). This is strong evidence that metabolic deiodination of 123I-ZIROT is minimal in dogs.
The myocardium was clearly identifiable on in vivo planar images at early points (<15 min), suggesting a favorable myocardial extraction associated with a rapid blood clearance. The 123I-ZIROT cardiac retention was moderate, with a t1/2 of ≈30 minutes. There are advantages and disadvantages for an MPI agent to be retained in the myocardium after injection.4 High myocardial retention allows gated imaging for simultaneous determination of relative perfusion and LV function. On the other hand, myocardial tracer clearance kinetics can be useful for absolute flow quantification using mathematical compartmental modeling.
A preliminary estimation of radiation dosimetry to several key organs and the whole body was determined for 123I-ZIROT. The estimated heart wall, clearance organs, and whole body doses are similar to those estimated for the current clinically approved MPI SPECT tracers.36,37 However, the gallbladder wall is the organ with the highest dose per mCi of tracer injected in the canine model and may be the organ that limits the injected dose of the tracer in human subjects. The protocols involved in the current studies used injected doses that range from 109 to 222 MBq (3–6 mCi). Suitable uptake and high-quality images were seen with 3 to 4 mCi injected into canine. This translates to 3.42 to 4.56 rad to the gallbladder wall, a dose that is generally recognized as safe and effective by the Food and Drug Administration (Code of Federal Regulations §361.1). The stability of 123I-ZIROT to deiodination, as previously noted, is also seen in the thyroid radiation dose estimate. Thyroid blocking with potassium iodide, a common practice for radioiodinated tracers, may be reduced or unnecessary with 123I-ZIROT. Given the preliminary radiation dose data in the canine, a sufficient 123I-ZIROT dose may be injected without undue dose burden to the patient and without compromising image quality.
Limitations of the Study
In this experimental study, we did not perform in vivo SPECT imaging of the myocardial uptake of 123I-ZIROT. The experimental protocol required euthanizing the animals at 5 min after tracer injection, which did not allow sufficient time to permit SPECT imaging. The reason for selecting the 5-min termination point was to permit comparison with our previous studies using other MPI agents, including Tl-201, under the same experimental conditions. In these earlier studies, the 5-min end point was chosen because it was sufficient to allow enough time for maximal extraction of the tracers being evaluated, based on blood pool availability, but did not allow time for Tl-201 to undergo appreciable redistribution. A second limitation of this study is that we did not perform measurements of myocardial first-pass extraction fraction. The first-pass extraction fraction is an important parameter that needs to be measured in a future study compared with other perfusion tracers. Nevertheless, given the extraction versus flow relationship presented in the current study, the first-pass myocardial extraction fraction for 123I-ZIROT can be inferred as higher than that of any of the currently approved myocardial perfusion radiotracers. Thus, although additional 123I-ZIROT studies appear warranted, other analogs of IROT are being sought with more favorable pharmacokinetics that might reduce the radiation dose burden to the gallbladder wall.
Summary and Conclusions
In a canine model of critical stenosis, 123I-ZIROT tracked regional myocardial blood flow over a wide range. In addition, the myocardium was clearly visible by in vivo planar imaging as early as 15 min after tracer injection. The ability to more linearly track changes in blood flow over a wide range, combined with favorable biodistribution properties, makes 123I-ZIROT a promising new SPECT MPI agent.
Table 1.
Table Hemodynamic Parameters at Baseline, After the Setting of the Stenosis, and During Vasodilator Stress at 123I-ZIROT Injection
| Parameters | Rest
|
Stress | |
|---|---|---|---|
| Baseline | Stenosis | ||
| HR, BPM | 123±15 | 121±13 | 135±19 |
| MAP, mm Hg | 102±7 | 99±6 | 80±8*† |
| LAP, mm Hg | 5±1 | 6±2 | 6±2 |
| LVP, mm Hg | 119±8 | 113±8* | 102±10*† |
| LVEDP, mm Hg | 6±2 | 7±2 | 6±2 |
| dP/dt, mm Hg/s | 2030±313 | 1779±148 | 2489±342† |
Data are given as mean±SEM. n=5. BPM indicates beats/minute; dP/dt, first derivative of LVP with respect to time; HR, heart rate; LAP, left atrial pressure; LVP, left ventricular pressure; LVEDP, left ventricular end-diastolic pressure; MAP, mean arterial pressure.
P<0.05 vs baseline.
P<0.05 vs stenosis.
CLINICAL PERSPECTIVE.
The clinical utility of radiotracer imaging of myocardial perfusion depends, in part, on the accuracy of the tracer reflecting myocardial blood flow and the relative biodistribution in cardiac versus noncardiac tissue. Current agents are limited by suboptimal energy characteristics, high subdiaphragmatic activity, or nonlinear uptake at high flow. The accuracy of a new myocardial perfusion radiotracer that targets complex I of the mitochondrial electron transport chain, 123I-ZIROT, was tested in an in vivo dog model of critical coronary artery stenosis with pharmacological stress-induced hyperemia. Myocardial 123I-ZIROT extraction tracked regional myocardial blood flow, as determined by the microspheres, over a wide range of flows, whereas the 123I-ZIROT LAD/LCx activity ratios obtained by ex vivo imaging only slightly underestimated the LAD/LCx microsphere flow ratio. 123I-ZIROT was identified in the myocardium 15 min after injection, with low lung and blood pool activity. These experiments indicate that 123I-ZIROT has advantages in extraction and biodistribution compared with either thallium-201 or 99mTc-sestamibi and, hence, could be a useful agent to image myocardial blood flow in humans.
Acknowledgments
Sources of Funding
This study was supported in part by contract DE-AC02–05CH11231 from the Director, Office of Science, Office of Biological and Environmental Research, Biological Systems Science Division of the US Department of Energy; and by grant EB000482 (HV) from the National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health.
Footnotes
Disclosures
None.
References
- 1.Beller GA, Bergmann SR. Myocardial perfusion imaging agents: SPECT and PET. J Nucl Cardiol. 2004;11:71–86. doi: 10.1016/j.nuclcard.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Cerqueira MD, Garcia EV, Gropler RJ, Udelson JE. Eighth Nuclear Cardiology Invitational Conference Park City, Utah, 2006. J Nucl Cardiol. 2007;14:e15–e25. doi: 10.1016/j.nuclcard.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 3.Beller GA, Watson DD. A welcomed new myocardial perfusion imaging agent for positron emission tomography. Circulation. 2009;119:2299–2301. doi: 10.1161/CIRCULATIONAHA.109.854919. [DOI] [PubMed] [Google Scholar]
- 4.Glover DK, Gropler RJ. Journey to find the ideal PET flow tracer for clinical use: are we there yet? J Nucl Cardiol. 2007;14:765–768. doi: 10.1016/j.nuclcard.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Glover DK, Ruiz M, Bergmann EE, Simanis JP, Smith WH, Watson DD, Beller GA. Myocardial technetium-99m-teboroxime uptake during adenosine-induced hyperemia in dogs with either a critical or mild coronary stenosis: comparison to thallium-201 and regional blood flow. J Nucl Med. 1995;36:476–483. [PubMed] [Google Scholar]
- 6.Glover DK, Ruiz M, Edwards NC, Cunningham M, Simanis JP, Smith WH, Watson DD, Beller GA. Comparison between 201Tl and 99mTc sestamibi uptake during adenosine-induced vasodilation as a function of coronary stenosis severity. Circulation. 1995;91:813–820. doi: 10.1161/01.cir.91.3.813. [DOI] [PubMed] [Google Scholar]
- 7.Carvalho PA, Chiu ML, Kronauge JF, Kawamura M, Jones AG, Holman BL, Piwnica-Worms D. Subcellular distribution and analysis of technetium-99m-MIBI in isolated perfused rat hearts. J Nucl Med. 1992;33:1516–1522. [PubMed] [Google Scholar]
- 8.Arbab AS, Koizumi K, Toyama K, Arai T, Araki T. Technetium-99m-tetrofosmin, technetium-99m-MIBI and thallium-201 uptake in rat myocardial cells. J Nucl Med. 1998;39:266–271. [PubMed] [Google Scholar]
- 9.Piwnica-Worms DP, Kronauge JF, LeFurgey A, Backus M, Hockett D, Ingram P, Lieberman M, Holman BL, Jones AG, Davison A. Mitochondrial localization and characterization of 99Tc-SESTAMIBI in heart cells by electron probe X-ray microanalysis and 99Tc-NMR spectroscopy. Magn Reson Imaging. 1994;12:641–652. doi: 10.1016/0730-725x(94)92459-7. [DOI] [PubMed] [Google Scholar]
- 10.Piwnica-Worms D, Kronauge JF, Delmon L, Holman BL, Marsh JD, Jones AG. Effect of metabolic inhibition on technetium-99m-MIBI kinetics in cultured chick myocardial cells. J Nucl Med. 1990;31:464–472. [PubMed] [Google Scholar]
- 11.Chiu ML, Kronauge JF, Piwnica-Worms D. Effect of mitochondrial and plasma membrane potentials on accumulation of hexakis (2-methoxyisobutylisonitrile) technetium(I) in cultured mouse fibroblasts. J Nucl Med. 1990;31:1646–1653. [PubMed] [Google Scholar]
- 12.Younes A, Songadele JA, Maublant J, Platts E, Pickett R, Veyre A. Mechanism of uptake of technetium-tetrofosmin, II: uptake into isolated adult rat heart mitochondria. J Nucl Cardiol. 1995;2:327–333. doi: 10.1016/s1071-3581(05)80077-7. [DOI] [PubMed] [Google Scholar]
- 13.Schaper J, Meiser E, Stammler G. Ultrastructural morphometric analysis of myocardium from dogs, rats, hamsters, mice, and from human hearts. Circ Res. 1985;56:377–391. doi: 10.1161/01.res.56.3.377. [DOI] [PubMed] [Google Scholar]
- 14.VanBrocklin HF, Hanrahan SM, Enas JD, Nandanan E, O’Neil JP. Mitochondrial avid radioprobes: preparation and evaluation of 7′(Z)-[125I]iodorotenone and 7′(Z)-[125I]iodorotenol. Nucl Med Biol. 2007;34:109–116. doi: 10.1016/j.nucmedbio.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marshall RC, Powers-Risius P, Reutter BW, Taylor SE, VanBrocklin HF, Huesman RH, Budinger TF. Kinetic analysis of 125I-iodorotenone as a deposited myocardial flow tracer: comparison with 99mTc-sestamibi. J Nucl Med. 2001;42:272–281. [PubMed] [Google Scholar]
- 16.Marshall RC, Powers-Risius P, Reutter BW, O’Neil JP, La Belle M, Huesman RH, VanBrocklin HF. Kinetic analysis of 18F-fluorodihydrorotenone as a deposited myocardial flow tracer: comparison to 201Tl. J Nucl Med. 2004;45:1950–1959. [PubMed] [Google Scholar]
- 17.Yalamanchili P, Wexler E, Hayes M, Yu M, Bozek J, Kagan M, Radeke HS, Azure M, Purohit A, Casebier DS, Robinson SP. Mechanism of uptake and retention of F-18 BMS-747158-02 in cardiomyocytes: a novel PET myocardial imaging agent. J Nucl Cardiol. 2007;14:782–788. doi: 10.1016/j.nuclcard.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Purohit A, Benetti R, Hayes M, Guaraldi M, Kagan M, Yalamanchilli P, Su F, Azure M, Mistry M, Yu M, Robinson S, Dischino DD, Casebier D. Quinazoline derivatives as MC-I inhibitors: evaluation of myocardial uptake using positron emission tomography in rat and non-human primate. Bioorg Med Chem Lett. 2007;17:4882–4885. doi: 10.1016/j.bmcl.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 19.Yu M, Guaraldi MT, Mistry M, Kagan M, McDonald JL, Drew K, Radeke H, Azure M, Purohit A, Casebier DS, Robinson SP. BMS-747158-02: a novel PET myocardial perfusion imaging agent. J Nucl Cardiol. 2007;14:789–798. doi: 10.1016/j.nuclcard.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Heymann MA, Payne BD, Hoffman JI, Rudolph AM. Blood flow measurements with radionuclide-labeled particles. Prog Cardiovasc Dis. 1977;20:55–79. doi: 10.1016/s0033-0620(77)80005-4. [DOI] [PubMed] [Google Scholar]
- 21.Hatada K, Ruiz M, Riou LM, Lima RL, Goode AR, Watson DD, Beller GA, Glover DK. Organ biodistribution and myocardial uptake, washout, and redistribution kinetics of Tc-99m N-DBODC5 when injected during vasodilator stress in canine models of coronary stenoses. J Nucl Cardiol. 2006;13:779–790. doi: 10.1016/j.nuclcard.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Glover DK, Ruiz M, Takehana K, Petruzella FD, Riou LM, Rieger JM, Macdonald TL, Watson DD, Linden J, Beller GA. Pharmacological stress myocardial perfusion imaging with the potent and selective A(2A) adenosine receptor agonists ATL193 and ATL146e administered by either intravenous infusion or bolus injection. Circulation. 2001;104:1181–1187. doi: 10.1161/hc3601.093983. [DOI] [PubMed] [Google Scholar]
- 23.Stabin MG. MIRDOSE: personal computer software for internal dose assessement in nuclear medicine. J Nucl Med. 1996;37:538–546. [PubMed] [Google Scholar]
- 24.Gosselin RE, Stibitz GR. Rates of solute absorption from tissue depots: theoretical considerations. Pflugers Archiv. 1970;318:85–98. doi: 10.1007/BF00586488. [DOI] [PubMed] [Google Scholar]
- 25.Lima RS, De Lorenzo A, Pantoja MR, Siqueira A. Incremental prognostic value of myocardial perfusion 99m-technetium-sestamibi SPECT in the elderly. Int J Cardiol. 2004;93:137–143. doi: 10.1016/S0167-5273(03)00149-9. [DOI] [PubMed] [Google Scholar]
- 26.van den Heuvel AF, van Veldhuisen DJ, van der Wall EE, Blanksma PK, Siebelink HM, Vaalburg WM, van Gilst WH, Crijns HJ. Regional myocardial blood flow reserve impairment and metabolic changes suggesting myocardial ischemia in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2000;35:19–28. doi: 10.1016/s0735-1097(99)00499-4. [DOI] [PubMed] [Google Scholar]
- 27.Soman P, Dave DM, Udelson JE, Han H, Ouda HZ, Patel AR, Karas RH, Kuvin JT. Vascular endothelial dysfunction is associated with reversible myocardial perfusion defects in the absence of obstructive coronary artery disease. J Nucl Cardiol. 2006;13:756–760. doi: 10.1016/j.nuclcard.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 28.Lodge MA, Bengel FM. Methodology for quantifying absolute myocardial perfusion with PET and SPECT. Curr Cardiol Rep. 2007;9:121–128. doi: 10.1007/BF02938338. [DOI] [PubMed] [Google Scholar]
- 29.Haley TJ. A review of the literature of rotenone, 1,2,12,12a-tetrahydro-8,9-dimethoxy-2-(1-methylethenyl)-1-benzopyrano[3,5-b]furo[2,3-h][1]benzopyran-6(6h)-one. J Environ Pathol Toxicol. 1978;1:315–337. [PubMed] [Google Scholar]
- 30.Charalambous A, Tluczek L, Frey KA, Higgins DS, Jr, Greenamyre TJ, Kilbourn MR. Synthesis and biological evaluation in mice of (2-[11C]methoxy)-6′,7′-dihydrorotenol, a second generation rotenoid for marking mitochondrial complex I activity. Nucl Med Biol. 1995;22:491–496. doi: 10.1016/0969-8051(94)00129-8. [DOI] [PubMed] [Google Scholar]
- 31.Talpade DJ, Greene JG, Higgins DS, Jr, Greenamyre JT. In vivo labeling of mitochondrial complex I (NADH:ubiquinone oxidoreductase) in rat brain using [(3)H]dihydrorotenone. J Neurochem. 2000;75:2611–2621. doi: 10.1046/j.1471-4159.2000.0752611.x. [DOI] [PubMed] [Google Scholar]
- 32.Higuchi T, Nekolla SG, Huisman MM, Reder S, Poethko T, Yu M, Wester HJ, Casebier DS, Robinson SP, Botnar RM, Schwaiger M. A new 18F-labeled myocardial PET tracer: myocardial uptake after permanent and transient coronary occlusion in rats. J Nucl Med. 2008;49:1715–1722. doi: 10.2967/jnumed.108.053967. [DOI] [PubMed] [Google Scholar]
- 33.Sherif HM, Saraste A, Weidl E, Weber AW, Higuchi T, Reder S, Poethko T, Henriksen G, Casebier D, Robinson S, Wester HJ, Nekolla SG, Schwaiger M. Evaluation of a novel (18)F-labeled positron-emission tomography perfusion tracer for the assessment of myocardial infarct size in rats. Circ Cardiovasc Imaging. 2009;2:77–84. doi: 10.1161/CIRCIMAGING.108.815423. [DOI] [PubMed] [Google Scholar]
- 34.Nekolla SG, Reder S, Saraste A, Higuchi T, Dzewas G, Preissel A, Huisman M, Poethko T, Schuster T, Yu M, Robinson S, Casebier D, Henke J, Wester HJ, Schwaiger M. Evaluation of the novel myocardial perfusion positron-emission tomography tracer 18F-BMS-747158-02: comparison to 13N-ammonia and validation with microspheres in a pig model. Circulation. 2009;119:2333–2342. doi: 10.1161/CIRCULATIONAHA.108.797761. [DOI] [PubMed] [Google Scholar]
- 35.Glover DK, Ruiz M, Yang JY, Smith WH, Watson DD, Beller GA. Myocardial 99mTc-tetrofosmin uptake during adenosine-induced vasodilatation with either a critical or mild coronary stenosis: comparison with 201Tl and regional myocardial blood flow. Circulation. 1997;96:2332–2338. doi: 10.1161/01.cir.96.7.2332. [DOI] [PubMed] [Google Scholar]
- 36.Wackers FJ, Berman DS, Maddahi J, Watson DD, Beller GA, Strauss HW, Boucher CA, Picard M, Holman BL, Fridrich R, Inglese E, Delaloye B, Bischof-Delaloye A, Camin L, McKusick K. Technetium-99m hexakis 2-methoxyisobutyl isonitrile: human biodistribution, dosimetry, safety, and preliminary comparison to thallium-201 for myocardial perfusion imaging. J Nucl Med. 1989;30:301–311. [PubMed] [Google Scholar]
- 37.Higley B, Smith FW, Smith T, Gemmell HG, Das Gupta P, Gvozdanovic DV, Graham D, Hinge D, Davidson J, Lahiri A. Technetium-99m-1,2-bis[bis(2-ethoxyethyl) phosphino]ethane: human biodistribution, dosimetry and safety of a new myocardial perfusion imaging agent. J Nucl Med. 1993;34:30–38. [PubMed] [Google Scholar]
- 38.Sinusas AJ, Shi Q, Saltzberg MT, Vitols P, Jain D, Wackers FJ, Zaret BL. Technetium-99m-tetrofosmin to assess myocardial blood flow: experimental validation in an intact canine model of ischemia. J Nucl Med. 1994;35:664–671. [PubMed] [Google Scholar]
- 39.Ghezzi C, Fagret D, Arvieux CC, Mathieu JP, Bontron R, Pasqualini R, de Leiris J, Comet M. Myocardial kinetics of TcN-NOET: a neutral lipophilic complex tracer of regional myocardial blood flow. J Nucl Med. 1995;36:1069–1077. [PubMed] [Google Scholar]
- 40.Riou LM, Unger S, Toufektsian MC, Ruiz M, Watson DD, Beller GA, Glover DK. Effects of increased lipid concentration and hyperemic blood flow on the intrinsic myocardial washout kinetics of (99m)TcN-NOET. J Nucl Med. 2003;44:1092–1098. [PubMed] [Google Scholar]
- 41.Calnon DA, Ruiz M, Vanzetto G, Watson DD, Beller GA, Glover DK. Myocardial uptake of (99m)Tc-N-NOET and (201)Tl during dobutamine infusion: comparison with adenosine stress. Circulation. 1999;100:1653–1659. doi: 10.1161/01.cir.100.15.1653. [DOI] [PubMed] [Google Scholar]