Abstract
Background
Freezing of gait is a debilitating and common gait disturbance observed in individuals with Parkinson’s disease (PD). Although the underlying mechanisms of freezing remain unclear, bilateral coordination of steps, measured as a phase coordination index, has been suggested to be related to freezing. Phase coordination index has not, however, been measured during tasks associated with freezing such as turning and backward walking. Understanding how bilateral coordination changes during tasks associated with freezing may improve our understanding of the causes of freezing.
Methods
Twelve individuals with PD who freeze (freezers), 19 individuals with PD who do not freeze (non-freezers), and 10 healthy, age-matched older adults participated. General motor disease severity and freezing severity were assessed. Phase coordination index was calculated for all subjects during forward walking, backward walking, continuous turning in small radius circles, and turning in large radius circles.
Results
Freezers and non-freezers had similar disease duration and general motor severity. Stepping coordination (measured as phase coordination index) was significantly worse in freezers compared to non-freezers and controls. Turning and backward walking, tasks related to freezing, resulted in worse coordination with respect to forward walking. Coordination was associated with severity of freezing scores such that worse coordination was correlated with more severe freezing.
Conclusions
These results provide evidence that stepping coordination is related to freezing in people with PD. Identifying variables associated with freezing may provide insights into factors underlying this symptom, and may inform rehabilitative interventions to reduce its occurrence in PD.
Keywords: Parkinson Disease, coordination, freezing, gait, turning
INTRODUCTION
Freezing of gait (FOG) is a paroxysmal gait disturbance characterized as a sudden inability to produce effective stepping [1]. FOG affects 50% of individuals with Parkinson’s disease (PD), is directly related to falls [2], and is one of the most disabling and distressing symptoms of PD [3]. Despite the burden of FOG, its underlying causes are unclear.
FOG can occur during all types of gait, however it is most common during turning [4]. It is possible that the increase in FOG events during turning may be due in part to the asymmetric nature of the task. In particular, the temporal and spatial asymmetry of steps during turning (i.e. inner and outer legs cover different distances [5]) represents a more complex control problem than forward walking. The increased complexity of interlimb timing during turning may pose an additional challenge to the bilateral coordination of steps and could contribute to FOG [6, 7]. Recent studies have also suggested a potential relationship between backward walking and FOG. Though less evidence exists relating backward walking to FOG than turning to FOG, several gait characteristics, such as asymmetry, step length, and Functional Ambulation Profile (FAP [8]), are worsened in PD during backward walking with respect to forward. Further, individuals with PD who experience FOG (PD+FOG) exhibit more dysfunction during backward walking than those with PD not experiencing FOG (PD−FOG) [9]. Though evidence directly linking backward walking to FOG is lacking, backward walking represents a complex locomotor task which is more difficult for PD+FOG. Despite the dysfunction of backwards gait in PD+FOG, the link between backward gait and FOG is not well understood.
Several studies have investigated the coordination of bilateral stepping during gait, as quantified by the Phase Coordination Index (PCI). PCI has been shown to become worse with both age and PD [6, 7, 10–14]. Interestingly, among those with PD, PD+FOG subjects have worse coordination than PD−FOG [12]. These results, along with prior research suggesting FOG to be associated with abnormalities of spatiotemporal characteristics [15] and sequencing of gait [16], led to the hypothesis that freezing may be related to reduced bilateral coordination of stepping [7]. One limitation of these studies is that they only describe coordination of steps during forward walking. It remains unclear how coordination changes during walking tasks more commonly associated with freezing, e.g., turning and backward walking. If a relationship exists between FOG and coordination, tasks associated with freezing should result in more poorly coordinated gait, represented by higher PCI. If, however, PCI is not altered across these tasks, it would be unlikely that coordination is directly related to FOG. Said differently, if coordination and FOG are related, one would expect a co-variance of these measures across gait tasks.
Identifying factors associated with FOG may provide important insight into the underlying mechanisms of FOG, potentially informing rehabilitative interventions to reduce the incidence of FOG. Further, FOG is notoriously difficult to elicit in a laboratory setting. Identifying quantifiable variables that are closely related to FOG, such as PCI, is a first step in establishing surrogate measures for this symptom. These variables could be used to identify individuals at risk for FOG and track the progression of this symptom. Therefore, the goal of this study was to better understand the relationship between bilateral coordination of stepping and FOG. We determined how tasks associated with freezing (turning in small radius circles and backward walking) affected coordination with respect to tasks which elicit freezing less often (forward walking and turning in large radius circles). We hypothesized that tasks associated with freezing would result in higher PCI, i.e., worse coordination, and that this effect would be most pronounced in individuals who experience freezing (PD+FOG). We further hypothesized that there would be a direct relationship between FOG severity and PCI.
METHODS
Participants
Ten healthy older adults, 19 “non-freezers” (PD−FOG), and 12 “freezers” (PD+FOG) participated (Table 1). Individuals with PD completed the Freezing of Gait Questionnaire (FOG-Q [17]) to assess freezing severity and to classify individuals as PD+FOG or PD−FOG. The FOG-Q is a self-assessment of FOG which consists of 6 questions, each scored from 0 to 4 (Maximum score- 24 points), with higher scores representing more severe freezing. Four questions address the frequency and duration of FOG and two questions assess general gait impairment. Patients were classified as PD+FOG if they answered ≥2 on question three of the FOG-Q [12], representing a frequency of FOG of at least once a week. Total FOG-Q (sum of scores on all questions) was determined for individuals with PD to assess the severity of FOG. The two questions not directly associated with FOG, as well as the criterion of a score of ≥2 on question 3 meant individuals classified as PD−FOG could have a non-zero FOG-Q total score. PD groups were matched for disease duration and severity, and all groups were matched for age. Disease severity was measured by part 3 (motor subscale) of the Movement Disorders Society Unified Parkinson Disease Rating Scale (MDS-UPDRS), as well as Hoehn and Yahr staging [18]. Individuals were excluded if they had any injury to the lower limbs within six months of testing, were unable to walk unassisted, or had neurological disorders other than PD. Individuals with PD were tested after a minimum 12-hour withdrawal from anti-parkinsonian medications. Experimental protocols were approved by the Human Research Protection Office of Washington University in St. Louis, and were in accordance with the Declaration of Helsinki. All subjects provided informed written consent prior to enrollment.
Table 1.
Subject characteristics; Mean (SD).
| Control n=10 | PD−FOG n=19 | PD+FOG n=12 | p-value | |
|---|---|---|---|---|
| Age | 69 (11) | 71 (9) | 72 (9) | 0.75 |
| Yrs with PD | - | 6.6 (5.1) | 8.0 (4.5) | 0.44 |
| MDS-UPDRS-3 | - | 41.6 (6.4) | 45.5 (15.2) | 0.34 |
| Hoehn & Yahr Stage | - | 2.37 (0.40) | 2.63 (0.83) | 0.26 |
| FOG-Q Total score | - | 4.2 (3.9) | 12.6 (4.1) | <0.001 |
Protocol
Six round footswitches (20mm diameter, 1mm thick; Motion Lab Systems; Baton Rouge, LA) were placed on the sole of each shoe (3 near the toes, and 3 near the heel) to determine the time of heel strike and toe off. Subjects then completed the following 6 gait tasks in random order: forward walking, backward walking, turning to the left and right in a small radius circle (radius = 0.6m), and turning to the left and right in a large radius circle (radius = 3m). Large radius turns have been suggested as a technique to reduce FOG during turning [19], and were included in this study to serve as a contrast for the small radius condition. Five to 8 trials of a 10 meter walk were completed for both forward and backward walking. One 60 second large radius circle trial was completed to the left and right, and three to five 20 second bouts of small radius turns were completed to the left and right. Subjects were instructed to perform all tasks at a comfortable, preferred pace. A telemetered system (Konigsberg Instruments, Pasadena, CA) transmitted footswitch data to the collection computer at 1000 Hz. Digital video was also acquired for each task. Data were collected using Cortex software (Motion Analysis Corp., Santa Rosa, CA USA).
Data analysis
PCI is a variable which integrates the accuracy and consistency of left- right stepping phases. The derivation of PCI has been described previously [11]. Briefly, PCI is the summation of two measures:
ϕCV – the coefficient of variation of the series of relative timing of the stepping of one leg (i.e. the timing of its heel strike) with respect to the gait cycle defined by 2 consecutive heel strikes (stride) of the other leg. The relative timing is represented by the value ϕ in degrees, which is the outcome of the time normalization with respect to the stride scaled to 360°. The ideal anti-phase stepping patter n yields ϕ=180°. ϕCV represents the consistency of phase generation.
-
ϕABS - the mean value of a series of absolute differences between the values of ϕi (i.e, the phase calculated for the ith stride) and 180°.
• ϕABS represents the overall accuracy in generating anti-phased stepping across all the steps of a walking trial.
Phase coordination index was calculated as the sum of ϕCV and PϕABS:
where PϕABS = 100·(ϕABS/180). Therefore, PCI consists of two relative values (ϕCV and PϕABS), both given as percentages. Periods of freezing and festination were identified by watching videos of gait which were time-synchronized with footswitch data. The period of festination and freezing, along with approximately one second before and after this period, was omitted from data analysis.
Statistical analysis
A one-way ANOVA determined statistical differences across age, and independent sample t-tests determined statistical differences for all other subject characteristics. As PCI was not different while turning to the left and right for small or large radius turns (see Results), PCI was collapsed across turning directions, leaving four gait tasks (forward walking, backward walking, large radius turns, and small radius turns). Therefore, a two-way mixed model ANOVA (group, 3 levels x walking condition, 4 levels) was used to determine the effects of both group and gait task on PCI as well as both components of PCI (PϕABS and ϕCV, see methods). Bonferroni correction for multiple comparisons was applied to all post hoc analyses. A recent review by Nutt and colleagues noted that while not all individuals with PD will experience FOG, those who do are likely on a spectrum of freezing severity [20]. With this in mind, we further tested the relationship between FOG and coordination by calculating Spearman’s ρ correlation statistic between severity of freezing (total FOG-Q score) and mean PCI across all tasks. Statistical analyses were run in SPSS (Chicago, IL).
RESULTS
There was no age difference between groups (Table 1; p=0.75), and individuals with PD+FOG and PD-FOG were of similar disease severity (MDS-UPDRS-3, p=0.43; Hoehn & Yahr stage, p=0.26) and duration (p=0.44). The PD+FOG group exhibited significantly higher (p<0.001) total FOG-Q scores than the PD−FOG group (Table 1). Seven of twelve individuals in the PD+FOG group experienced FOG during the gait protocol. Within this group, backward walking and turning in small radius circles elicited freezing most frequently. Twenty five, 29, and 27 freezing events were observed during turning in small radius circles to the left, small radius circles to the right, and backward walking, respectively. In contrast, only one, two, and 7 freezing events were observed during forward walking, turning in large radius circles to the left and turning in large radius circles to the right, respectively.
As PCI data were not different when turning to the left or right (large turns: p=0.37; small turns: p=0.63; paired sample t-test), data from both turn directions were combined for large and small turns. Exemplar data from one control, one PD−FOG, and one PD+FOG subject are shown in Figure 1. PCI values were smallest during forward walking, increased slightly (i.e. coordination worsened) during large radius turning, further increased during backward walking, and were highest during small radius turning, where the highest shifts from anti-phase coordination (i.e, 180°) were observed. In all walking conditions, the control subject had the lowest and the PD+FOG subject had the highest PCI values.
Figure 1.
Stepping phase (ϕ) data of one subject from each group (control, PD−FOG, PD+FOG) for different walking tasks.
These differences were also observed at the group level (Figure 2), as a significant group effect was present (F2,38=16.5; p<0.001). Post hoc tests revealed PD+FOG had significantly higher PCI than PD−FOG (p=0.01), and PD−FOG had significantly higher PCI than controls (p=0.005; Bonferroni corrected). There was also a significant task effect (F2,83=78, p<0.001). Post hoc analyses showed PCI was statistically higher (i.e., worse bilateral coordination) when turning in small radius circles than when walking forward (p<0.001), turning in large radius circles (p<0.001), or walking backward (p=0.002; Figure 2). Backward walking also resulted in higher PCI than turning in large radius circles (p<0.001) and forward walking (p<0.001). Large turns had a significant effect on PCI with respect to forward (p=0.002), but this effect was substantially less pronounced than the effect of small turns or backward walking on PCI. In addition, there was a task by group interaction effect (F4,83=3.0, p=0.02) such that the difference in PCI across groups was largest during backward walking and small turns. The two subcomponents of PCI, PϕABS and ϕCV, represent the temporal accuracy and consistency of steps, respectively (see Methods). Both subcomponents exhibited significant group and task effects similarly to PCI (PϕABS: Group effect: F2,38=14.9, p<0.001, Task effect F2,71=44, p<0.001; ϕCV: Group effect F2,38=13.6, p<0.001, Task effect F2,87=95, p<0.001).
Figure 2.
Mean and SD of Phase coordination index (PCI) for PD+FOG, PD−FOG, and controls across gait tasks. Individual subject data plotted around mean of each group. Significant task (p<0.001) and group (p<0.001) effects were observed.
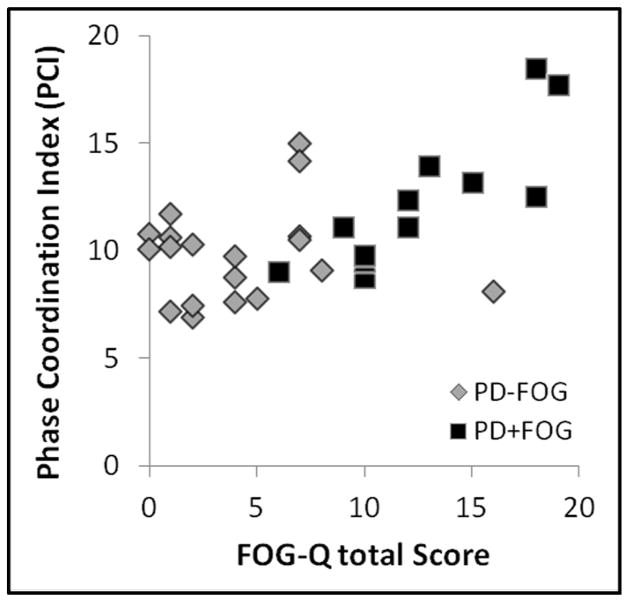
A significant relationship was observed between freezing severity (total FOG-Q score) and global coordination of steps (mean PCI across all tasks- ‘global PCI’) such that higher FOG-Q scores were associated with higher global PCI (Spearman’s ρ =0.54, p=0.002, Figure 3). Additional analyses confirmed that PCI values for each walking task (forward, backward, large radius turns, and small radius turns) were each statistically significantly related to total FOG-Q score (0.37< Spearman’s ρ <0.46, 0.009< p <0.04, data not shown). These correlations were shown to arise primarily from the PD+FOG group. When global PCI – FOG-Q correlation was run on PD−FOG and PD+FOG groups separately, only the PD+FOG group showed a significant relationship (global PCI vs. FOG-Q for PD+FOG: Spearman’s ρ =0.85, p<0.001; for PD−FOG group: Spearman’s ρ =0.11, p=0.65).
Figure 3.
Scatter plot of mean PCI across tasks (global PCI) and FOG-Q for all subjects with PD. PCI and FOG-Q were significantly correlated (r=0.54; r2=0.29; p=0.002).
DISCUSSION
Previous reports suggest spatial and temporal gait kinematics, including bilateral coordination of stepping, may be altered in individuals who experience freezing [7, 15, 16, 21, 22]. To better understand the relationship between bilateral coordination and FOG, we measured coordination during turning and backward walking, gait tasks associated with freezing. Our three primary results showed that 1) PD+FOG had worse coordination than PD−FOG, 2) gait tasks related to FOG resulted in worse coordination than those that less commonly elicit FOG, and 3) a direct correlation was observed between severity of freezing and coordination, with worse coordination predicting more severe freezing symptoms. Together, these results provide additional support for a relationship between coordination of steps and FOG.
It has been hypothesized that there may be a threshold for gait characteristics (including bilateral coordination of steps) which when crossed, triggers freezing of gait [7]. This threshold may be modulated by numerous factors, including how much one attends to the gait task, environmental stressors (e.g., crowds, doorways), and the individual’s postural stability. In the current study, all groups exhibited worse coordination during tasks associated with FOG with respect to forward walking. Therefore, the higher PCI during turning and backward walking, particularly in PD+FOG, may place one near this hypothetical FOG threshold, potentially contributing to the increased frequency of freezing during these tasks. In addition, correlation analyses showed individuals with PD who exhibit worse coordination experience more severe FOG. This relationship was strongest in the PD+FOG group and suggests the possibility that amongst those who freeze, coordination may modulate freezing severity. However, correlation results must be interpreted with caution, as only 31 individuals with PD (12 with PD+FOG), were analyzed. Future research with larger sample sizes is necessary to better understand this observation.
The reason some tasks elicit worse coordination and frequent freezing is not well understood. It is possible that tasks such as turning pose an increased challenge to lower limb coordination with respect to forward walking, due in part to the inherent asymmetries of inner and outer legs during this task [5, 23]. This increased challenge to coordination posed by turning, along with the already diminished coordination of those with PD [11, 12, 24–26] may bring subjects closer to the hypothetical FOG threshold described above. Indeed, results from the current study show that the higher PCI during turns with respect to forward gait is more pronounced in those with PD than controls (as noted by the task by group interaction), suggesting that individuals with PD who are prone to freezing may have particular difficulty meeting the coordination challenges posed by turning.
We also assessed coordination of steps during backward walking, a task without the inherent step length asymmetry of turning. Similarly to turning, coordination during backward walking was worse than forward walking. Among all tasks presented in this study, prolonged backward gait (i.e., ~10 m) is the most foreign to gait usually performed during daily living conditions. It is therefore suggested that subjects had to invest attention and cognitive resources when confronted with this relatively unfamiliar task. It is possible that the increased cognitive faculties required to spatially orient and plan backward gait may interfere with the autonomous activation of gait. Therefore, the relative novelty and complexity of this gait task may result in less automated gait and reduced bilateral coordination [13].
Results from a recent report show that freezers turn with a wider arc than non-freezers [27]. In addition, walking in large arc circles is a technique used in the clinic to improve turning in freezers [19]. In the current study, coordination of steps was significantly better during large turns than during small turns. The improved coordination during large radius turning with respect to small radius turning may, in part, drive the strategy observed in PD+FOG to walk in large radius circles while turning. This improvement in coordination could pull individuals further away from a FOG threshold [7] and may partially explain why large turns seem to reduce FOG during turning.
Other factors have been shown to increase the prevalence of FOG. For example, if the individual is stressed, or distracted from the gait task as with dual tasking, freezing is more common [28]. Other external stimuli such as transitions in flooring or doorways can pull attention from gait and elicit freezing [29, 30]. Walking with split attention also results in worse coordination [13], exemplifying a covariance of coordination and FOG. Gait initiation, like turning, may be considered an instance of high temporal asymmetry of steps, needing a large degree of lower limb coordination to be completed effectively. The increased coordination needs during asymmetric tasks such as turning and gait initiation may be related to the high incidence of FOG during these tasks. These studies, in conjunction with results from the current investigation further support the relationship between bilateral coordination of steps and FOG.
CONCLUSION
Previous literature suggests that impaired coordination of steps during gait may be related to, or even on the causal pathway of FOG. The present study provides support for a relationship between these variables in three ways. Coordination of steps was 1) worse in those who experience freezing compared to those who do not, 2) worse during tasks associated with freezing, and 3) directly correlated to freezing severity. Impaired coordination of steps likely contributes to FOG.
Table 2.
PCI, ϕCV, and PϕABS for all subjects and across tasks.
| Forward | Large Radius Turns | Backward | Small Radius Turns | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Con | PD− FOG | PD+FOG | Con | PD− FOG | PD+FOG | Con | PD−FOG | PD+FOG | Con | PD−FOG | PD+FOG | |
| PCI* | 4.3 (1.3) | 6.1 (2.5) | 7.3 (2.5) | 4.7 (1.0) | 6.8 (2.2) | 9.0 (3.9) | 7.3 (2.7) | 10.9 (3.8) | 13.9 (3.9) | 9.1 (2.4) | 13.5 (3.5) | 17.7 (4.7) |
| ϕCV* | 2.0 (0.5) | 3.0 (1.2) | 3.7 (1.2) | 2.4 (0.5) | 3.4 (1.2) | 4.4 (1.9) | 3.7 (1.1) | 5.6 (1.9) | 6.4 (1.4) | 4.4 (1.2) | 6.5 (1.8) | 8.3 (2.0) |
| PϕABS* | 2.3 (1.1) | 3.2 (1.4) | 3.7 (1.5) | 2.3 (0.8) | 3.5 (1.2) | 4.6 (2.2) | 3.6 (1.6) | 5.3 (2.1) | 7.4 (3.2) | 4.7 (1.5) | 7.0 (2.2) | 9.4 (2.2) |
Significant group and task effects
Acknowledgments
Funding for this work was provided by: NIH- TL1RR024995; NIH- RO1HD056051-01; NIH- 2T32HD007434-18A; Parkinson’s Disease Foundation; American Parkinson Disease Association Center for Advanced PD Research at Washington University.
Footnotes
Financial disclosure/Conflict of Interest: The authors declare that they have no conflict of interest, financial or otherwise, related to the submitted manuscript or the associated research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Giladi N, Nieuwboer A. Understanding and treating freezing of gait in parkinsonism, proposed working definition, and setting the stage. Mov Disord [Article] 2008;23:S423–S5. doi: 10.1002/mds.21927. [DOI] [PubMed] [Google Scholar]
- 2.Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson’s disease: a review of two interconnected, episodic phenomena. Mov Disord. 2004 Aug;19(8):871–84. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- 3.Backer JH. The symptom experience of patients with Parkinson’s disease. J Neurosci Nurs. 2006 Feb;38(1):51–7. doi: 10.1097/01376517-200602000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Schaafsma JD, Giladi N, Balash Y, Bartels AL, Gurevich T, Hausdorff JM. Gait dynamics in Parkinson’s disease: relationship to Parkinsonian features, falls and response to levodopa. J Neurol Sci. 2003 Aug 15;212(1–2):47–53. doi: 10.1016/s0022-510x(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 5.Courtine G, Schieppati M. Human walking along a curved path. I. Body trajectory, segment orientation and the effect of vision. Eur J Neurosci. 2003 Jul;18(1):177–90. doi: 10.1046/j.1460-9568.2003.02736.x. [DOI] [PubMed] [Google Scholar]
- 6.Fasano A, Herzog J, Seifert E, Stolze H, Falk D, Reese R, et al. Modulation of gait coordination by subthalamic stimulation improves freezing of gait. Mov Disord. 2011 Apr;26(5):844–51. doi: 10.1002/mds.23583. [DOI] [PubMed] [Google Scholar]
- 7.Plotnik M, Hausdorff JM. The role of gait rhythmicity and bilateral coordination of stepping in the pathophysiology of freezing of gait in Parkinson’s disease. Mov Disord. 2008;23 (Suppl 2):S444–50. doi: 10.1002/mds.21984. [DOI] [PubMed] [Google Scholar]
- 8.Nelson A. Appendix A: Functional Ampulation Profile Score. In: Nelson A, editor. GAITRite Operating manual. Havertown, PA: CIR Systems; 2008. [Google Scholar]
- 9.Hackney ME, Earhart GM. Backward walking in Parkinson’s disease. Mov Disord. 2009 Jan 30;24(2):218–23. doi: 10.1002/mds.22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plotnik M, Giladi N, Balash Y, Peretz C, Hausdorff JM. Is freezing of gait in Parkinson’s disease related to asymmetric motor function? Ann Neurol. 2005 May;57(5):656–63. doi: 10.1002/ana.20452. [DOI] [PubMed] [Google Scholar]
- 11.Plotnik M, Giladi N, Hausdorff JM. A new measure for quantifying the bilateral coordination of human gait: effects of aging and Parkinson’s disease. Experimental Brain Research. 2007 Aug;181(4):561–70. doi: 10.1007/s00221-007-0955-7. [DOI] [PubMed] [Google Scholar]
- 12.Plotnik M, Giladi N, Hausdorff JM. Bilateral coordination of walking and freezing of gait in Parkinson’s disease. Eur J Neurosci. 2008 Apr;27(8):1999–2006. doi: 10.1111/j.1460-9568.2008.06167.x. [DOI] [PubMed] [Google Scholar]
- 13.Plotnik M, Giladi N, Hausdorff JM. Bilateral coordination of gait and Parkinson’s disease: the effects of dual tasking. J Neurol Neurosur Ps. 2009 Mar;80(3):347–50. doi: 10.1136/jnnp.2008.157362. [DOI] [PubMed] [Google Scholar]
- 14.Plotnik M, Herman T, Shaviv E, Brozgol M, Giladi N, Hausdorff JM. Impaired bilateral coordination of gait and upper extremity rhythmic movements in Parkinson’s disease: Association with freezing of gait. Mov Disord. 2009;24:S341-S. [Google Scholar]
- 15.Nieuwboer A, Dom R, De Weerdt W, Desloovere K, Fieuws S, Broens-Kaucsik E. Abnormalities of the spatiotemporal characteristics of gait at the onset of freezing in Parkinson’s disease. Mov Disord. 2001 Nov;16(6):1066–75. doi: 10.1002/mds.1206. [DOI] [PubMed] [Google Scholar]
- 16.Iansek R, Huxham F, McGinley J. The sequence effect and gait festination in Parkinson disease: contributors to freezing of gait? Mov Disord. 2006 Sep;21(9):1419–24. doi: 10.1002/mds.20998. [DOI] [PubMed] [Google Scholar]
- 17.Giladi N, Shabtai H, Simon ES, Biran S, Tal J, Korczyn AD. Construction of freezing of gait questionnaire for patients with Parkinsonism. Parkinsonism Relat Disord. 2000 Jul 1;6(3):165–70. doi: 10.1016/s1353-8020(99)00062-0. [DOI] [PubMed] [Google Scholar]
- 18.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967 May;17(5):427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 19.Morris ME. Locomotor training in people with Parkinson disease. Phys Ther. 2006 Oct;86(10):1426–35. doi: 10.2522/ptj.20050277. [DOI] [PubMed] [Google Scholar]
- 20.Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011 Aug;10(8):734–44. doi: 10.1016/S1474-4422(11)70143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nieuwboer A, Chavret F, Willems AM, Desloovere K. Does freezing in Parkinson’s disease change limb coordination? A kinematic analysis. J Neurol. 2007 Sep;254(9):1268–77. doi: 10.1007/s00415-006-0514-3. [DOI] [PubMed] [Google Scholar]
- 22.Nieuwboer A, Dom R, De Weerdt W, Desloovere K, Janssens L, Stijn V. Electromyographic profiles of gait prior to onset of freezing episodes in patients with Parkinson’s disease. Brain. 2004 Jul;127(Pt 7):1650–60. doi: 10.1093/brain/awh189. [DOI] [PubMed] [Google Scholar]
- 23.Courtine G, Schieppati M. Human walking along a curved path. II. Gait features and EMG patterns. Eur J Neurosci. 2003 Jul;18(1):191–205. doi: 10.1046/j.1460-9568.2003.02737.x. [DOI] [PubMed] [Google Scholar]
- 24.Almeida QJ, Wishart LR, Lee TD. Bimanual coordination deficits with Parkinson’s disease: the influence of movement speed and external cueing. Mov Disord. 2002 Jan;17(1):30–7. doi: 10.1002/mds.10030. [DOI] [PubMed] [Google Scholar]
- 25.Wu T, Wang L, Hallett M, Li K, Chan P. Neural correlates of bimanual anti-phase and in-phase movements in Parkinson’s disease. Brain. 2010 Aug;133(Pt 8):2394–409. doi: 10.1093/brain/awq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abe K, Asai Y, Matsuo Y, Nomura T, Sato S, Inoue S, et al. Classifying lower limb dynamics in Parkinson’s disease. Brain Res Bull. 2003 Jul 15;61(2):219–26. doi: 10.1016/s0361-9230(03)00119-9. [DOI] [PubMed] [Google Scholar]
- 27.Willems AM, Nieuwboer A, Chavret F, Desloovere K, Dom R, Rochester L, et al. Turning in Parkinson’s disease patients and controls: the effect of auditory cues. Mov Disord. 2007 Oct 15;22(13):1871–8. doi: 10.1002/mds.21445. [DOI] [PubMed] [Google Scholar]
- 28.Camicioli R, Oken BS, Sexton G, Kaye JA, Nutt JG. Verbal fluency task affects gait in Parkinson’s disease with motor freezing. J Geriatr Psychiatry Neurol. 1998 Winter;11(4):181–5. doi: 10.1177/089198879901100403. [DOI] [PubMed] [Google Scholar]
- 29.Almeida QJ, Lebold CA. Freezing of gait in Parkinson’s disease: a perceptual cause for a motor impairment? J Neurol Neurosurg Psychiatry. 2010 May;81(5):513–8. doi: 10.1136/jnnp.2008.160580. [DOI] [PubMed] [Google Scholar]
- 30.Cowie D, Limousin P, Peters A, Day BL. Insights into the neural control of locomotion from walking through doorways in Parkinson’s disease. Neuropsychologia. 2010 Jul;48(9):2750–7. doi: 10.1016/j.neuropsychologia.2010.05.022. [DOI] [PubMed] [Google Scholar]