Abstract
Reductions in mobility and cognitive function linked to accrual of brain microvascular disease related white-matter hyperintensities(WMH) on magnetic resonance imaging (MRI) canoccur in older hypertensive patients in as little as 2 years. We have designed a trial evaluating two levels of ambulatory BP control in individuals with normal or mildly impaired mobility and cognition who have detectable cerebrovascular disease (>0.5% WMH fraction of intracranial volume) on functional outcomes. The study is a prospective randomized, open-label trial with blinded endpoints, inpatients ages 75 and older with elevated 24-h systolic BP (≥145 mmHg in the untreated state) who do not have unstable cardiovascular disease, heart failure or stroke. The primary and key secondary outcomes in the trial are: change from baseline in mobility and cognitive function and damage to brain white matter as demonstrated by accrual of WMH volume and changes indiffusion tensor imaging.Approximately 300 patients will be enrolled and 200 randomized to one of two levels of ambulatory BP control (intensive to achieve a goal 24-hour systolic BP of ≤ 130 mmHg or standard to achieve a goal 24-hour systolic BP of ≤ 145 mmHg) for a total of 36 months using similar antihypertensive regimens. The analytical approach provides 85% power to show a clinically meaningful effect in differences in mobility accompanied by quantitative differences in WMH between treatment groups. The INFINITY trial is the first to guide antihypertensive therapy using ambulatory BP monitoring rather than clinic BP to reduce cerebrovascular disease.
Background and Study Rationale
White matter hyperintensities (WMHs), present in the magnetic resonance images (MRI) of older people have been linked to hypertension and other vascular disease risk factors(1). Evidence suggests that WMHs occur as a result of arteriosclerotic changes within the arteriolar wall and have been viewed as the manifestations of microvascular disease (2). Large arterial and microvascular disease of the cerebral circulation share risk factors, (e.g., hypertension, diabetes) and may co-exist in an individual(1-3) although given the differences noted in Table 1, it is unclear if they both produce tissue damage through similar mechanisms (2-4).
Table 1.
Comparison of the characteristics of stroke and microvascular disease
| Characteristic | Stroke (large artery) | Microvascular disease |
|---|---|---|
| Onset/progression | Sudden/brief if any | Ill-defined/gradual over years |
| Manifestations | Focal neurologic deficit | Functional limitations |
| Location | Vascular distribution | Grow from head/tail-lateral ventricles |
| Size | Stroke(cm)→lacune (mm) | <1 mm |
| Vessel | Large to small artery | Arteriolar |
| Pathophysiology | Ischemic | Unclear |
White matter lesions are associated with deterioration of mobility (5, 6), urinary control (7), and cognition (8). Evidence of WMHs within brain pathways known to support mobility, cognition or voiding (9-10) confirms this association. Detail seen on magnetic resonance imaging(MRI) of the brain has allowed localization and quantification of the disseminated WMHs. Cross-sectional and prospective cohort studies have documented the relationships among WMHs and neurologic function in older people and the distinctive nature of the distribution and volume of brain WMHs that are responsible for deterioration of these functions, particularly in older groups. Approximately two-thirds of individuals over 75 years of age have detectable WMH using MRI of the brain (9-11). The lower limit of detection of WMH by experts in neuro-radiology is approximately 0.2-0.3% of intracranial contents and 0.5% is easily visible to the naked eye based on our experience (9-11). It is also known that progression of WMH over time is strongly linked to the initial presence of WMH (9,10).
Blood pressure has been linked to brain WMH although predictors of quantitative WMH progression and their effect on the function of older persons are poorly understood. In our past work in this area, we evaluated the progression of WMH over 2-years in a cohort of 95 patients 75-90 years (mean baseline age, 82 years) who had office and ambulatory BP and volumetric MRI (11). After 2 years, neither clinic BP nor change in clinic BP predicted progression of WMH whereas the 24-hour ambulatory BP and changes in ambulatory BP at 2 years significantly correlated with both WMH volume (p<0.04) and change in WMH (p<0.003). Further analysis demonstrated an association for WMH and mobility indexes with level of systolic BP based on tertiles of the cohort – those in the high (24-hour systolic BP = 144 mmHg) ambulatory BP group showed increases in WMH and slower mobilitycompared to the middle tertile (ambulatory systolic BP = 130 mmHg). Furthermore, gait speed in the higherambulatory BP group decreased 0.15m/sec more than that in the low BP group and while this difference appears small, it represents a between group change after only 2 years of observation. Mobility limitation linked to WMH occurs gradually so that this decrement may be part of a long-term process which compromises gait velocity over 10 or more years. These data suggest that an intervention using mean 24-hour systolic BP as the target could reduce progression of microvascular disease in the elderly and thus favorably impact function.
Data from Hypertension in the Very Elderly Trial (HYVET) demonstrate antihypertensive therapy decreases stroke mortality even in patients in their mid-80s (12). In HYVET, the goal of therapy was to reduce systolic BP to < 150 mmHg and this did result in a 39% reduction in stroke mortality linked to a 15 mmHg difference in systolic BP between active and placebo groups. To our knowledge, there is no other information on level of systolic BP and outcomes in a hypertensive population over the age of 80 years. Thus, the goal of the standard of care systolic BP in the clinic in this age group isabout 150 mmHg. Further, no clinical trial in hypertensive patients has used ambulatory BP to guide therapy and to assess cerebrovascular outcomes. Our study has been designed to evaluate the functional impact of a clinically relevant separation in ambulatory systolic BP in an older population (that is, <130 mmHg versus <145 mmHg).
STUDY DESIGNand CONDUCT
The study is a prospective, randomized, parallel group, open-label trial with blinded endpoints (PROBE), in older patients who have untreated 24-hour systolic BPs between 150 and 180 mmHg mmHg) and evidence of at least 0.5% WMH volume on MRI. The key objectives in the trial are to evaluate the effects of 2 levels of ambulatory BP control (standard versus intensive) on the changes from baseline in mobility parameters (primarily times) and cognitive function (executive function, processing speed) and their association with changes in accrual of WMH. We hypothesize that change in WMH provides the mechanism that mediates the functional decline. The general inclusion and exclusion criteria for the trial are outlined in Table II.
Table II.
Intensive versus Standard Ambulatory BP Control to Lessen Functional Decline Inclusion and Exclusion Criteria
| Inclusion Criteria |
| 1. 75 years of age or older |
| 2. Seated clinic systolic BP >150 mmHg in the untreated state |
| 3. At risk for cerebrovascular disease (history of smoking, dyslipidemia, type 2 diabetes, longstanding hypertension, family history). Patients must have visible (0.5% WMH or more) white-matter hyperintensity lesions on screening magnetic resonance imaging of the brain. |
| 4. To achieve success in maintaining a 24-hour systolic BP of <145 mmHg in the standard treatment group or a systolic BP <130 mmHg in the intensive treatment group, patients will be eligible for inclusion if (1) their clinic systolic BP is 150-170 mmHg, and they are taking 0 to 2 antihypertensives, (2) their systolic BP is > 170 mmHg and they are taking 0 to 1 antihypertensive. |
| Exclusion Criteria |
| 1. Uncontrolled diabetes mellitus (HBA1c >10%) |
| 2. History of stroke, dementia or clinically impaired gait (Mini-mental status exam score (MMSE) <24, Short Physical Performance Battery for gait (SPPB) < 9) |
| 3. Body Mass Index > 45 kg/m2 and/or arm circumference > 44 cm) |
| 4. Poor kidney function (defined as estimated GFR <25 ml/minute) |
| 5. Active liver disease or serum transaminases >3 times the upper limit of normal |
| 6. Major cardiovascular event (e.g. myocardial infarction) or procedure (e.g. cardiac bypass surgery) in past 3 months, uncompensated congestive heart failure (NYHA class III or IV or documented ejection fraction <30%) |
| 7. Chronic atrial fibrillation that disallows ambulatory BP monitoring to be successfully performed |
| 8. Medical conditions that limit survival to < 3 years |
| 9. MRI contraindications (including MRI-incompatible implants, severe claustrophobia). |
General Study Conduct
The trial complies with the Declaration of Helsinki and subsequent revisions and follows Good Clinical Practice guidelines. Our institution has obtained approval for study conduct by the Institutional Review Boardof the University of Connecticut School of Medicine. Study patients must review and sign informed consent prior to any study related procedure. INFINITYis sponsored by the National Institute of Aging. Additionally, the only source of funding used to support the research and creation of the paper is from the National Institutes of Health. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
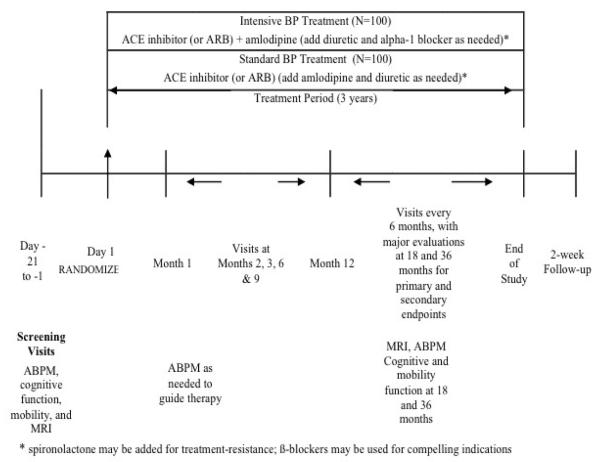
After baseline evaluation, patients will be randomized to the intensive group (goal 24-hour systolic BP < 130 mmHg) or to the standard group (goal 24-hour systolic BP < 145 mmHg). Assessments for the outcomes will be made at baseline and at 18 and 36 months post-randomization. Adverse events, tolerability, and health-related quality of life will be evaluated outcomes as well. Complete assessments will be performed at baseline and after 18 and 36 months (Figure 1). The study has been registered on Clinicaltrials.gov(NCT01650402).
Figure 1. Design of the INFINITY Trial.
–Intensive BP Treatment – goal 24-hour ambulatory systolic BP ≤ 130 mmHg ; Standard BP Treatment – goal 24-hour systolic BP ≤ 145 mmHg
Study Treatment and Procedures
Regimens, Administration, and Duration
The same classes of antihypertensive regimens will be used in both the intensive and standard treatment groups (Figure 1). As noted above, the BP goal in the intensive group is a 24-hour systolic BP mean of <130 mmHg and in the standard treatment group a 24-hour systolic BP mean of <145 mmHg. The types of therapy chosen for this trial are evidenced based– recent results from the HYVET study (12), a 2 year trial in hypertensive patients >80 years demonstrated that diuretic and ACE inhibitor treatment reduced the risk of stroke using 150 mmHg as the treatment goal. But in addition to efficacy and tolerability, ease of dosing plays a role in treatment adherence. Consideration of these issues as well as the finding from the ACCOMPLISH (13) and ASCOT (14) trials that use of an ACE inhibitor with a calcium antagonist were superior to a diuretic and ACE inhibitor are also central to ourtreatment algorithms. At the baseline, post-screening visit, an urn randomization procedure (15)will be used to assign subjects to treatment conditions described below.
Treatment strategy for the intensive therapy group
Patients in the intensive group will receive a low dose dihyropyridine calcium antagonist (amlodipine, 5 mg daily) and a low dose ACE inhibitor (lisinopril 10 mg daily) at the time of randomization. If there is a history of ACE inhibitor intolerance (e.g. angioedema or cough), an angiotensin receptor blocker (ARB) (e.g. losartan 50 mg daily) may be substituted. Intensive group patients will be seen monthly for titration of the 2 agents (maximal doses: amlodipine 10 mg and/or lisinopril 40 mg once daily) until achieving a clinic systolic BP of <135 mmHg – at that time (average time, 3 months), we will verify the 24-hour mean systolic BP is ≤130 mmHg. If the systolic BP has not reached goal on maximally tolerated doses of a calcium antagonist and an ACEi or ARB, low dose thiazide diuretic will be added (hydrochlorothiazide, 12.5 mg daily). If the BP goal is not achieved with 3 drugs, addition of daily dosing of one of the following agents is allowed: 1) beta-adrenergic blocker (first choice in a patient with known coronary disease), 2) alpha-adrenergic blocker (first choice in men with benign prostatic hyperplasia), 3) aldosterone antagonist (first choice in patients with low serum potassium levels). A loop diuretic may be substituted for a thiazide in patients whose estimated GFR is <50 ml/minute. In subjects who had not reached the ABP goal at 3 months and who have further drug titration, the ABP will be repeated at 4 to 6 months to confirm that the 24-hour systolic BP goal has been achieved.
Treatment strategy for the standard care group
Treatment in this group will start with the ACEi lisinopril (initial dose 10 mg daily and maximal dose of 40 mg once daily) (or ARB if there is a history of ACEi intolerance). Subjects will be seen monthly until achieving a clinic systolic BP <150 mmHg with subsequent confirmation that the 24-hour BP goal of <145 mmHg has been achieved. If systolic BP does not reach the goal on maximally tolerated doses of an ACEi or ARB, a dihydropyridine calcium antagonist (e.g., amlodipine 5 mg up to 10 mg qd) will be added. Similarly, if the 24-hour systolic BP goal of <145 mmHg isn‘t achieved with this regimen, a thiazide diuretic will be added (hydrochlorothiazide 12.5 mg daily). Similarly to the intensive BP group algorithm, a loop diuretic may be substituted for a thiazide in patients with chronic kidney disease. Finally, if goal BP is not achieved on 3 drugs, daily dosing of: 1) beta-adrenergic blocker, 2) alpha-adrenergic blocker, 3) aldosterone antagonist may be added. As in the intensive group, subjects not reaching their 24-hour systolic BP goal at 3 months and who have drug titration will have an ABP monitor performed at 6 months to confirm that goal 24-hour systolic BP has been reached.
Down-titration of antihypertensive therapy
Since it is unknown if lowering 24-hour systolic BP to the more intensive goal of ≤130 mmHg is beneficial in this age group, a reduction in the dose or number of antihypertensive drugs is allowed for patients in both groups but is likely to be more common in the standard group. The criterion that permits down-titration of medications in the standard care group is a clinic systolic BP <140 mmHg at 2 successive clinic visits or systolic BP <135 mmHg at a single visit accompanied by a 24-hour systolic BP value of <140 mmHg. The goal of down-titration is to produce a usual care group systolic BP of 140-145 mmHg thus producing at least a 10-15 mmHg difference in ABP levels between the intensive and standard care groups.
Study procedures
Blood pressure measurements
Clinic BP will be taken twice, 2-3 minutes apart in the non-dominant arm and averaged using a digital device (TM -247, Suntech Medical Instruments, Morrisville, NC). BP readings will be taken between 8 and 11 am, before taking antihypertensive medication. Home BP will be performed for 1 week obtaining duplicate readings in the morning and evening prior to medication administration at baseline, 18 months, and 36 months using an Omron 10 series self-BP device (Omron Healthcare, Vernon Hills, IL). The 24-hour ambulatory BP Monitoring (ABPM) will be conducted using the Oscar II BP device (Suntech Medical Instruments, Morrisville, NC) Eighty BP readings will be programmed during the 24-h study. Monitors will measure BP and heart rate every 15 min from 6 am to 10 pm, and every 30 min from 10 pm to 6 am. Follow-up ambulatory BP recordings will be made at 3 months to evaluate response to therapy and at 6 months if medications were adjusted at 3 months to ascertain if systolic BP goals were achieved.
Mobility Measures
At baseline and following 18 and 36 months, standardized tests will be used to document patients‘ ability to maintain stance (side by side, semi-tandem, tandem, unipedal) and to change position (supine-to-sit, sit-to-stand, forward reach, 8 meter walk, ascend and descend 4 steps) (11,16). Excepting functional reach, documentation will involve timing with a digital stopwatch.
Neuropsychological test battery
At baseline, participants with a high school education must achieve a score of 25 or higher on the Mini-Mental State Exam (MMSE) to be included. Measures of executive functioning and processing speed include the Trail Making Test, Symbol Digit Modalities Test, Controlled Word Association Test, the Stroop Color and Word Test, and 3 subtests from the California Computerized Assessment Package (CalCAP), Simple Reaction Time (SRT), Choice Reaction Time (CRT), and Sequential reaction time (SeqRT). The Trail Making Test evaluates the speed of visual search, attention, mental flexibility and motor function (17). The Stroop Color and Word assesses how well an individual suppresses a habitual response in favor of an unusual one thus assessing complex processing speed. Slower Stroop performance has been associated with greater WMH (18). The SRT measures the time it takes an individual to respond a visually presented stimulus. The CRT adds the element of memory to the task. The SeqRT adds the element of working memory.
Brain Magnetic Resonance Imaging (MRI) and analysis
The brain will be imaged in a 1.5-Tesla Siemens Avanto scanner (Erlangen, Germany) with the following sequences: T1-weighted magnetization prepared rapid gradient echo (MPRAGE), T2-weighted 3D-Fast Spin Echo (T2), fluid attenuated inversion recovery (FLAIR), diffusion tensor imaging (DTI) using a standard EPI sequence and 12-directions. All MR imaging analyses will be performed blinded to clinical and gait laboratory data. White matter lesions will be quantified with a modified protocol based on that described previously (19). The MPRAGE series will be used as input in the FreeSurfer application (www.fmrib.ox.ac.uk/fsl/) to obtain a brain tissue segmentation map (FS-map). We will identify the intracranial cavity volume (ICV) from the T2 series in Matlab (Matworks Inc., USA) using an in-house algorithm. In Matlab we will use the FLAIR series to map areas with signal above a defined threshold (WMH). This preliminary map will then be filtered using FS-map and ICV to exclude all the WMHs outside of the WM and smaller than 4 pixels. Aneuroimaging expert will review and manually edit when necessary the ICV and WMH maps. To normalize for head size differences the volume of WMH will be expressed as percent of ICV. Integrity of brain WM microstructure will be evaluated using DTI (20). We will derive measures of diffuse tissue damage,i.e. fractional anisotropy (FA) and mean diffusivity (MD) using the Slicer 3D application (www.slicer.org). These measures together with WMH burden will provide a more complete assessment that captures disease progress at different stages.
The investigators performing mobility testing, cognitive testing, voiding function studies, and volumetric MRI will be blinded to treatment assignment until the 36 month data are finalized and data lock has occurred.
SAFETY MONITORING
All research staff involved with INFINITY are trained in adverse event reporting and understand that the responsibility is to document and report adverse events reported by study participants, independent of determinations made at the time or later of the relationship between the event and participation in the study. Serious and non-serious adverse eventsare reported on a regular basis to an independent chartered Data Safety Monitoring Committee (DSMC). Reports of aggregate data submitted to the DSMC members contain baseline demographics, retention data, adverse events data, including laboratory data and events of special interest (falls, orthostatic hypotension, syncope and any CV event) and any other data that will help in the assessment of the clinical trial. At their meetings, the DSMC will vote on whether the study should: 1) continue recruitment unchanged; 2) continue with a protocol amendment; 3) stop recruiting pending further investigation. If, after a DSMC meeting, the vote is to stop recruitment or to request a protocol modification, the IRB will be informed.
All deaths and unexpected or study related serious adverse events will be reported immediately to the PI and DSMC members, and within 48 hours of discovery to the Institutional Review Boards (IRBs) and NIA project officer.
STATISTICAL CONSIDERATIONS
Hypotheses and Statistical Analyses
Several hypotheses with related outcomes in the trial are shown in Figure 2. The pathways on the diagram delineate the relations among the various hypotheses and outcome measurements according to treatment considerations. The key hypotheses are stated below:
Figure 2. Hypothesis Flow Path.
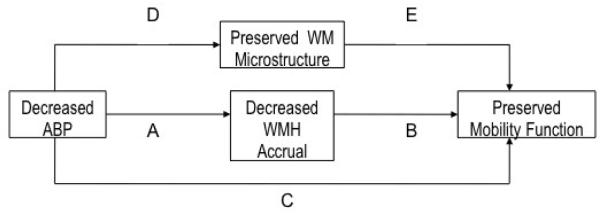
Graphic representation of how our hypotheses relate to one another. Hypothesis 1 tests the combined effects of paths A, B, C, D & E i.e., the total effect of BP on mobility. Hypothesis 2 tests path A, Hypothesis 3 tests path D, The secondary hypotheses test path B+E; together these test the effect of BP on mobility mediated through BP’s effects on WMH and WM microstructure. An additional secondary hypothesis tests the relative importance of paths A and B to that of path C, i.e., the degree to which the effect of BP on mobility is explained by WMH. The final secondary hypothesis tests the relative importance of paths D and E to that of path C, i.e., the degree to which the effect of BP on mobility is explained by changes in WM microstructure.
Hypothesis 1
Intensively treating 24-h systolic BP to a goal of <130 mmHg versus standard BP control of <145 mmHg will lead to faster walking speeds at 36 months.
Hypothesis 2
Intensively treating 24-h systolic BP to a goal of <130 mmHg versus standard BP control <145 mmHg will lead to smaller total WMH volume.
Hypothesis 3
Intensively treating 24-h systolic BP to a goal of <130 mmHg versus standard BP control <145 mmHg will stabilize brain WMmicrostructural integrity at 36 months.
Multiple linear regression will be performed with treatment group (intensive vs. standard) as the predictor of interest. Gait velocity at 36 months is the primary outcome for hypothesis 1, WMH at 36 months is the primary outcome for hypothesis 2, and DTI indices are the primary outcomes for hypothesis 3. The analysis will be intention-to-treat. Age, sex, and 24 hour SBP at baseline will be included as covariates, along with other demographic factors for which the treatment groups are not comparable. The analysis for hypothesis 1 is the proposal‘s primary efficacy analysis and thus the proposal is powered to address this analysis (see determine of sample size).
In addition, we will perform preliminary analyses on the outcomes at 18 months, and will examine the progression of mobility and WMH changes using a longitudinal model using methods adapted from Diggle (21) to compare rates of progression during the two time intervals. Other mobility measures will also be examined as secondary outcomes in models otherwise identical to the above-mentioned analyses. The analyses will be repeated for cognition, with the speed obtained on Trailmaking B at 36 months as the primary outcome and other cognitive measures as secondary outcomes.
Secondary analyses
Other hypotheses to be analyzed include 1) reduced total WMH and decreased markers of tissue damage on DTI at baseline and 36 months are linked to better mobility at 36 months, 2) the total WMH at baseline and 36 months mediate the effect of the treatment group, showing that the mechanism by which reduced ambulatory BP is linked to better mobility occurs by reducing total WMH and 3) diffusion tensor imaging indices at baseline and 36 months will mediate the effect of the treatment group, showing that the mechanism by which reduced ambulatory BP is linked to better mobility occurs by preserving brain white matter microstructural integrity.
Determination of Sample Size
We have conservatively estimated that at least 140 of the original 200 participants will complete assessments at 36 months. We analyzed a subset of participants in our earlier observational study (11) in order to mimic the results of the 2 treatment groups we expect to see in the intervention study. For a standard care arm, we selected participants in the observational study who had 24 hour systolic BP between 140 and 150 mmHg, inclusive, at follow-up, and for the intensive treatment arm, we selected participants with 24 hour systolic BP between 120 and 130 mmHg, inclusive, at follow-up. A regression of gait velocity over 8 feet on our covariates age, sex, and 24 hour systolic BP at baseline showed an r2 of 0.1267. If similar results are observed in the intervention study, 140 participants (70 in each group) will give us 85% power to observe an increase in r2 of 0.0533 to 0.1800.
Missing Data
Death and study attrition are associated with age-related diseases. Failure to account for this informative censoring may result in biased estimates of dementia risk and cognitive decline (22). It is likely that the same is true for mobility impairments as well, as persons with limited mobility are probably more likely to fail to present for scheduled clinic assessments. Hence, we will administer part of the Telephone Mobility Assessment Questionnaire (TMAQ) (23), and the Memory Impairment Screen for Telephone, a test of semantic memory (MIS-T) (24) to all participants on the telephone prior to each clinic visit, including any study participants who are unable to present for their clinic visit. These will be used as auxiliary data with which to assess the degree to which estimates of mobility limitation and cognitive change may be affected by informative loss to follow-up, using multiple imputation and joint modeling. While the primary efficacy outcome analysis will be limited to participants who complete the study, this will be the first clinical trial in aging with the potential to assess the impact of informative dropout beyond usual sensitivity analyses.
Discussion
The INFINITY trial represents the first randomized clinical trial that will evaluate the effects of antihypertensive treatment guided by ambulatory BP on cerebrovascular disease outcomes. The primary outcomes of this trial will determine the potential benefit of intensive reduction of BP on functional outcomes in older persons with hypertension. The study population will include patients at elevated vascular risk to reach these functionalendpoints. Consequently, the study population has been enriched with patients with hypertension and cerebrovascular disease based on screening MRI and represents patients who are likely candidates for this potential treatment strategy in clinical practice.
The standard means for treating hypertension in older people is to titrate drugs based on measurement of BP in the doctor‘s office. However, we have shown that the reproducibility of clinic BP is fairly poor in this setting and hence, less reliable (25). Use of ambulatory BP monitoring rather than the clinic BP to guide antihypertensive therapy reduces the BP variance over time by 50% and allows for a better understanding of BP control with a smaller sample. The requirement that patients demonstrate MRI evidence of WMH as an inclusion criterion also serves to reduce the sample size of the trial as there is strong evidence to support that patients with existing WMH are more likely to accrue more WMH over time than those lacking WMH (16, 18, 19). Therefore, enrollment of patients with elevated cerebrovascular risk in INFINITY increases the likelihood of demonstrating the value of lower ambulatory BP and will aid in establishing the treatment goal relative to standard levels of systolic BP control in older people.
There are a number of distinctiveaspects of the INFINITY trial. First, the trial is attempting to determine a benefit in functional activities following 3 years of treatment in the study population based on accrual of WMH. Cognition and mobility, two critical functions in older persons, supported by quantitative MRI measurement of WMH accrual are important and precise end-points for this population. Additionally, use of ambulatory BP measurement guided therapy in conjunction with the high cerebrovascular risk profile of the study population maximizes the possibility that even with a moderate sample-size the study will answer the questions raised in this trial.
Conclusions
INFINITYis an important and novel trial for establishing clinical benefit of antihypertensive therapy in older patients with systolic hypertension and increased cerebrovascular risk. The study has been ongoing since early 2012 and has randomized approximately one-fourth of the study population, many of whom are > 85 years of age. The results of this trial could allow for improved management of systolic hypertension that would eventually lessen functional decline in older patients at increased risk of microvascular disease of the brain.
Acknowledgments
Supported by: The National Institute on Aging - R01AG022092
Appendix. INFINITY Steering Committee and Data Safety Monitoring Committee
Steering Committee:
William B. White, M.D., Farmington, CT (Principal Investigator)
Leslie Wolfson, M.D., Farmington, Connecticut (Principal Investigator),
Richard F. Kaplan, Ph.D. , Farmington, CT (investigator - neuropsychology);
Richard Bohannon, Ph.D., Storrs, CT (investigator - mobility);
Charles B. Hall, Ph.D., Bronx, NY (investigator - biostatistics)’
Charles R. Guttmann, M.D., Boston, MA (investigator - imaging)
Data Safety Monitoring Committee:
Aldo Peixoto, M.D., New Haven, CT (Chair),
Joseph Vita, MD, Boston, MA,
David Greer, MD, New Haven, CT,
Neelum Aggarwal, MD, Chicago, IL,
Peter van Ness, Ph.D., New Haven, CT
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Vermeer SEDHT, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Incidence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke. 2003;34:392–396. doi: 10.1161/01.str.0000052631.98405.15. [DOI] [PubMed] [Google Scholar]
- 2.Wolfson L. Microalbuminurea as an index of brain microvascular dysfunction. J Neurol Sci. 2008;272:34–35. doi: 10.1016/j.jns.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 3.Kuller LHLW, Jr., Arnold AM, Bernick C, Bryan RN, Beauchamp NJ., Jr. White matter hyperintensity on cranial magnetic resonance imaging: a predictor of stroke. Stroke. 2004;35:1821–1825. doi: 10.1161/01.STR.0000132193.35955.69. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg G. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol. 2009;8:205–216. doi: 10.1016/S1474-4422(09)70016-X. [DOI] [PubMed] [Google Scholar]
- 5.Benson RGC, Wei X, et al. Older people with impaired mobility have specific loci of perventricular abnormality on MRI. Neurology. 2002;58:48–55. doi: 10.1212/wnl.58.1.48. [DOI] [PubMed] [Google Scholar]
- 6.Baloh RWYQ, Socotch TM, Jacobson KM. White matter lesions and disequilibrium in older people. I. Case-control comparison. Arch Neurol. 1995;52:970–974. doi: 10.1001/archneur.1995.00540340062013. [DOI] [PubMed] [Google Scholar]
- 7.Sakakibara RHT, Uchiyama T, Yamanishi T. Urinary function in elderly people with and without leukoaraiosis: relation to cognitive and gait function. J Neurol Neurosurg Psychiatry. 1999;67:658–660. doi: 10.1136/jnnp.67.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Junque CPJ, Vendrell P, et al. Leuko-araiosis on magnetic resonance imaging and speed of mental processing. Arch Neurol. 1990;47:151–156. doi: 10.1001/archneur.1990.00530020047013. [DOI] [PubMed] [Google Scholar]
- 9.Kuchel GAMN, Guttmann CR, et al. Localization of brain white matter hyperintensities and urinary incontinence in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2009;64:902–909. doi: 10.1093/gerona/glp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan RF, Cohen RA, Moscufo N, et al. Demographic and biological influences on cognitive reserve. J Clin Exp Neuropsychol. 2009;31:868–76. doi: 10.1080/13803390802635174. [DOI] [PubMed] [Google Scholar]
- 11.White WBWL, Wakefield DB, Hall CB, Campbell P, Moscufo N, Schmidt J, Kaplan RF, Pearlson GP, Guttmann CR. Average daily blood pressure, not office blood pressure, predicts progression of cerebrovascular disease and functional decline in older people. Circulation. 2011;124:2312–9. doi: 10.1161/CIRCULATIONAHA.111.037036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beckett NSPR, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 13.Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417–2428. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- 14.Dahlof B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an anti-hypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandanavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm: a multicentre randomised controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 15.Schulz KFGD. Generation of allocation sequences in randomised trials: chance, not choice. Lancet. 2002;359:515–519. doi: 10.1016/S0140-6736(02)07683-3. [DOI] [PubMed] [Google Scholar]
- 16.Simonsick EM, Newman AB, Nevitt MC, et al. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001;56:M644–9. doi: 10.1093/gerona/56.10.m644. [DOI] [PubMed] [Google Scholar]
- 17.Strauss E, Sherman MS, Strauss . A compendium of neuropsychological tests. 3rd ed Oxford; New York: 2006. [Google Scholar]
- 18.Ylikoski R, Ylikoski A, Erkinjuntti T, Sulkava R, Raininko R, Tilvis R. White matter changes in healthy elderly persons correlate with attention and speed of mental processing. Arch Neurol. 1993;50:818–824. doi: 10.1001/archneur.1993.00540080029009. [DOI] [PubMed] [Google Scholar]
- 19.Moscufo N, Wolfson L, Meier D, et al. Mobility decline in the elderly relates to lesion accrual in the splenium of the corpus callosum. Age (Dordr) 2012;34:405–414. doi: 10.1007/s11357-011-9242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierpaoli CJP, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- 21.Diggle PJ. An approach to the analysis of repeated measurements. Biometrics. 1988;44:959–971. [PubMed] [Google Scholar]
- 22.Sliwinski MJHS, Hall C, Buschke H, Lipton RB. Modeling memory decline in older adults: the importance of preclinical dementia, attrition, and chronological age. Psychol Aging. 2003;18:658–671. doi: 10.1037/0882-7974.18.4.658. [DOI] [PubMed] [Google Scholar]
- 23.Verghese J, Katz MJ, Derby CA, Kuslansky G, Hall CB, Lipton RB. Reliability and validity of a telephone-based mobility assessment questionnaire. Age Ageing. 2004;33:628–632. doi: 10.1093/ageing/afh210. [DOI] [PubMed] [Google Scholar]
- 24.Lipton RB, Katz MJ, Kuslansky G, Sliwinski MJ, Stewart WF, Verghese J, Crystal HA, Buschke H. Screening for dementia by telephone using the memory impairment screen. J Am Geriatr Soc. 2003;51:1382–90. doi: 10.1046/j.1532-5415.2003.51455.x. [DOI] [PubMed] [Google Scholar]
- 25.Campbell PGNWD, Wolfson L, White WB. Long-term reproducibility of ambulatory blood pressure is superior to office pressure in the very elderly. J Hum Hypertens. 2010;24:749–54. doi: 10.1038/jhh.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]