Abstract
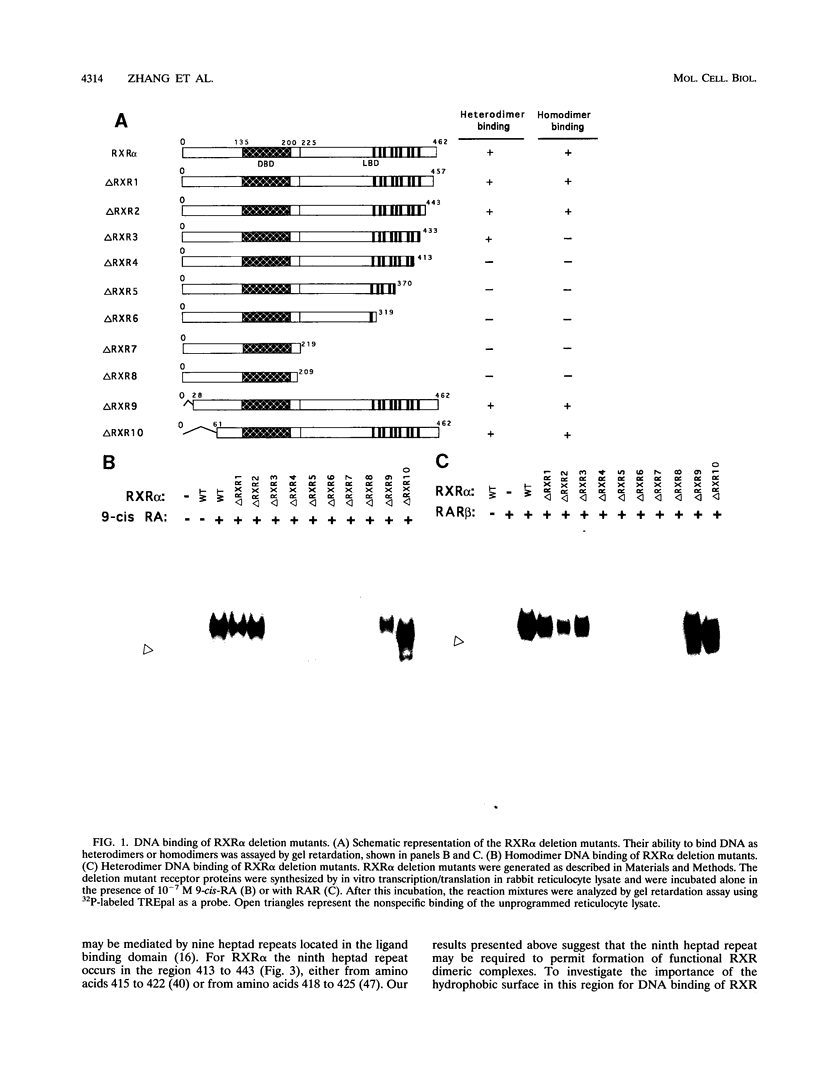
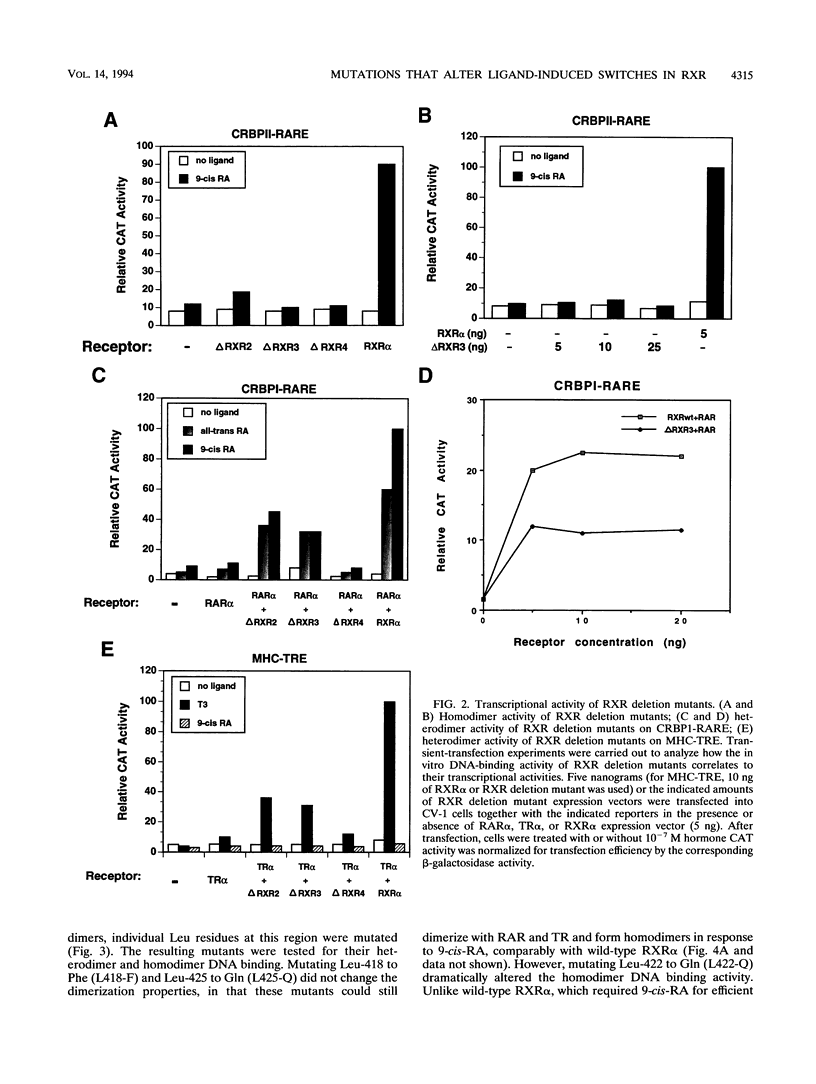
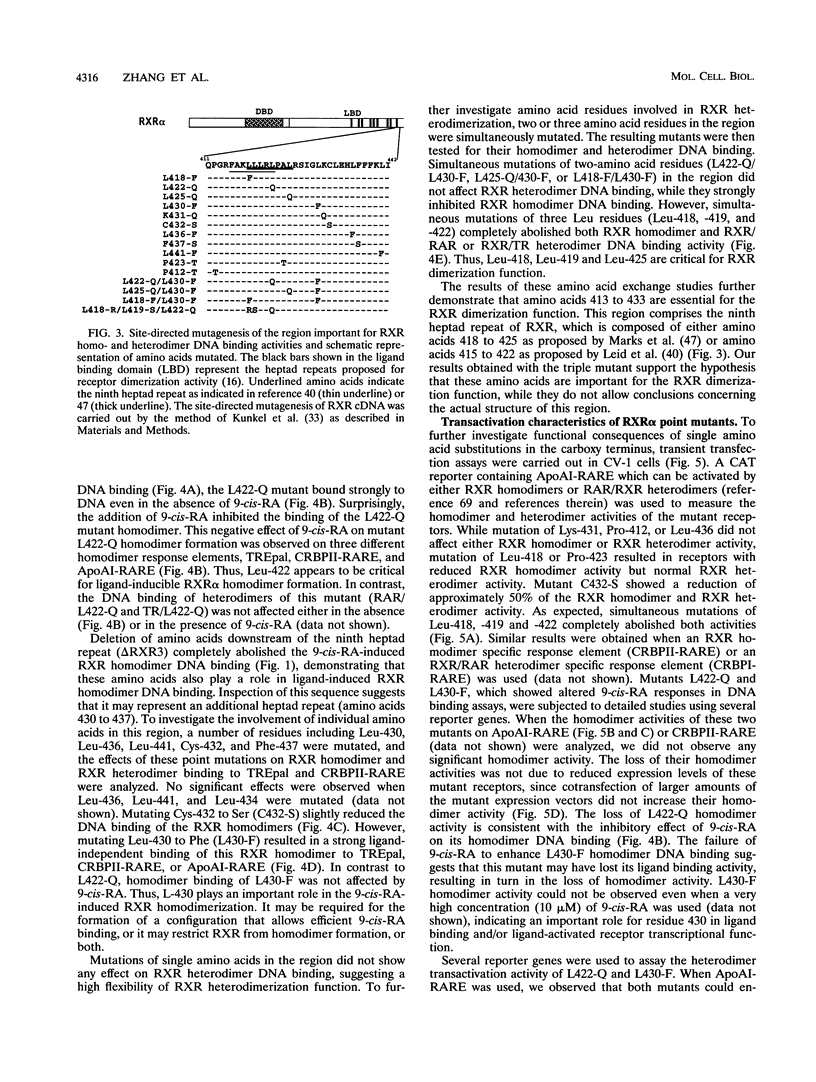
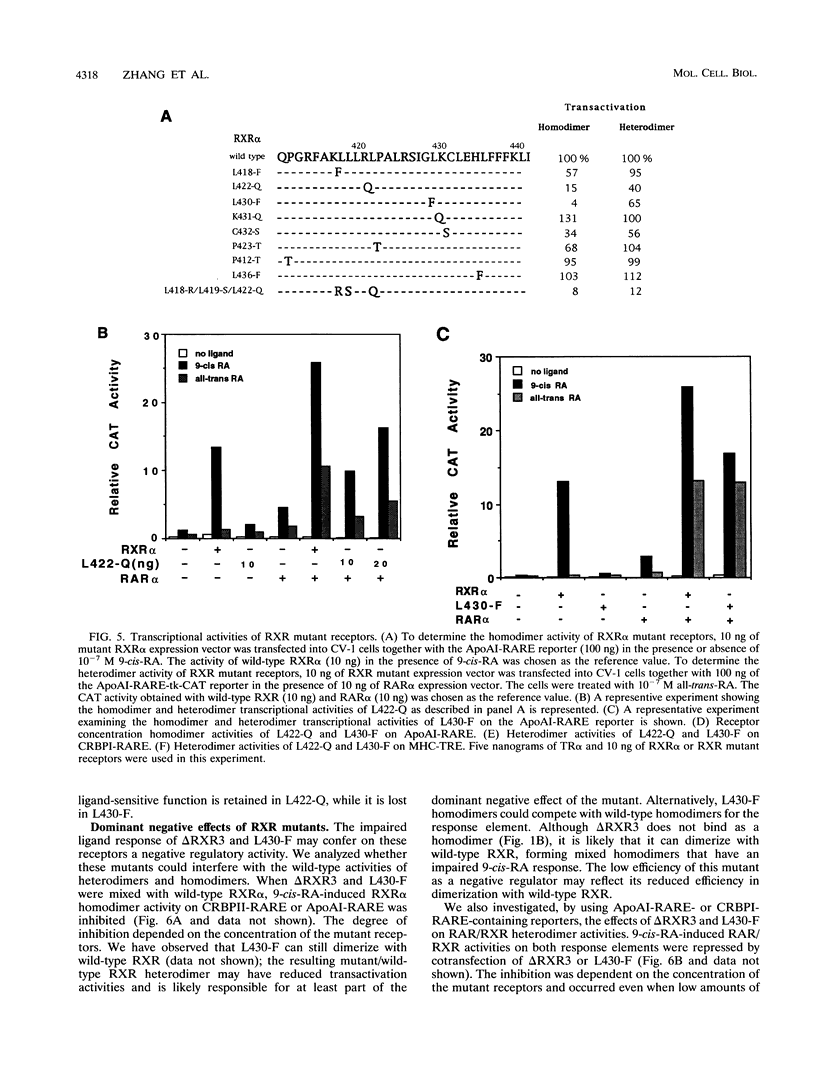
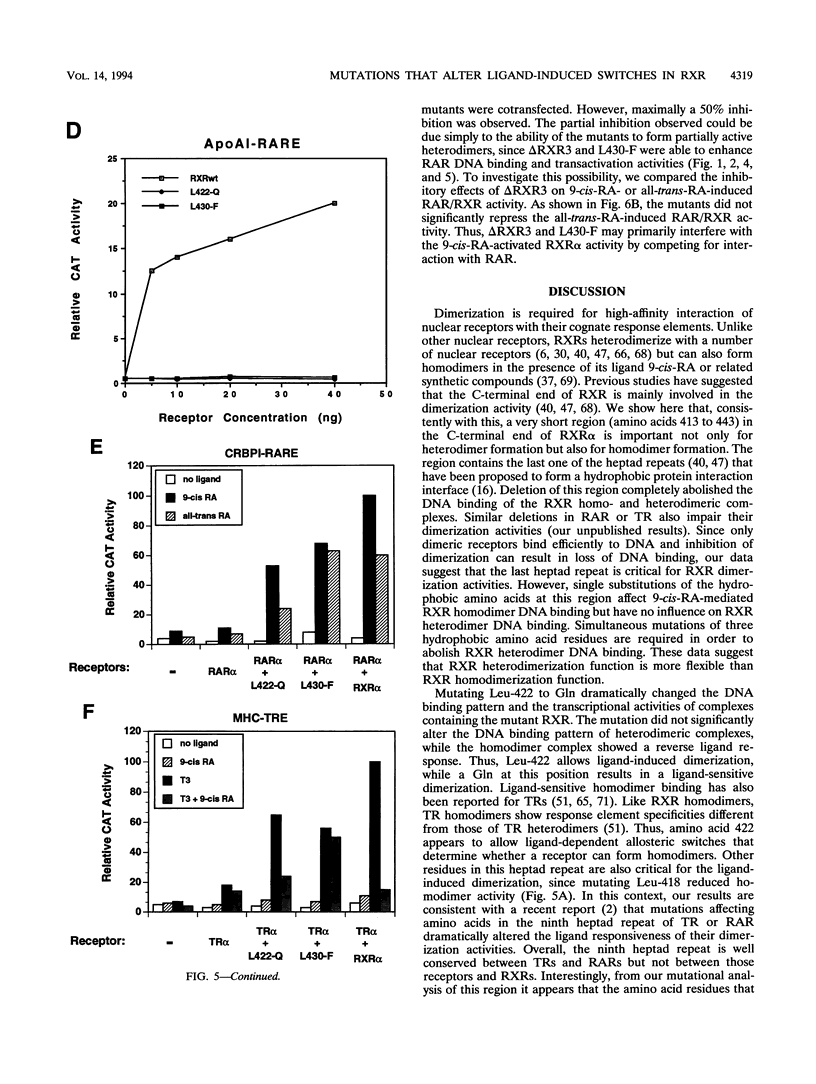
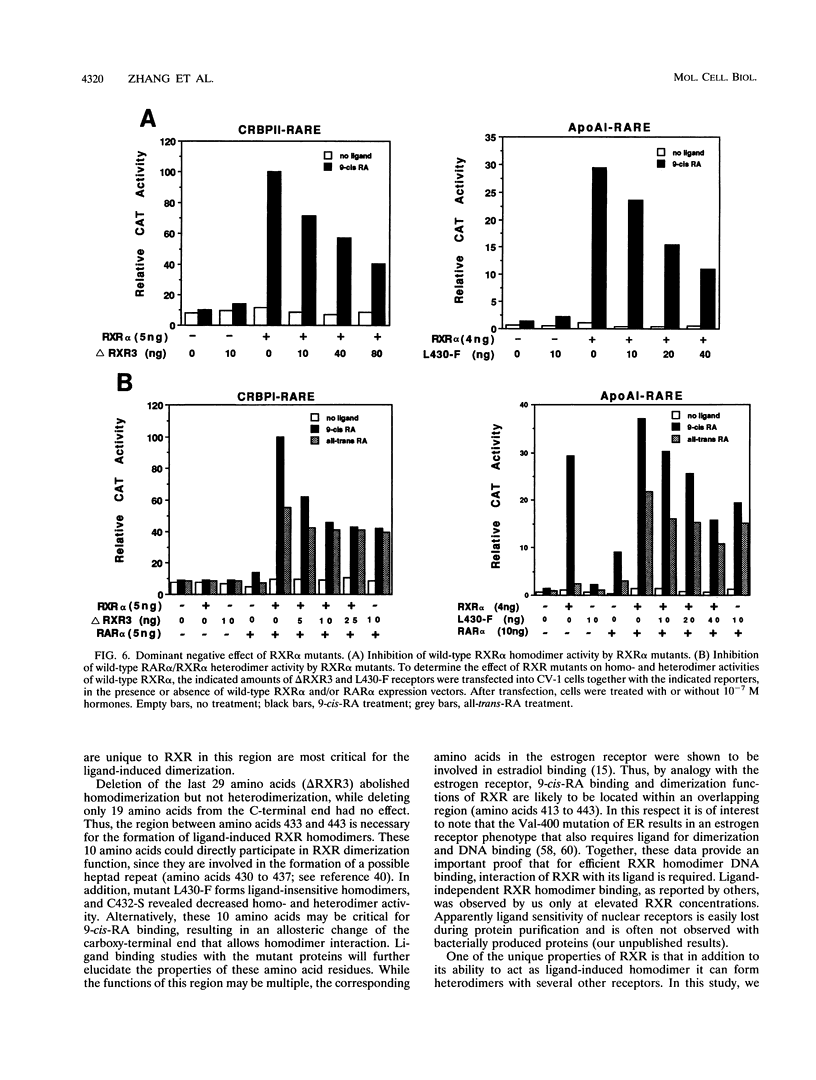
The retinoid X receptor (RXR) heterodimerizes with a variety of nuclear receptors. In addition, RXR forms homodimers in the presence of its ligand, 9-cis-retinoic acid. From deletion and point mutation analysis we present evidence that a short region (amino acids 413 to 443) in the carboxy terminus of RXR alpha is critical for both homo- and heterodimeric interactions as well as for diverse functional activities. In addition, we present evidence that homo- and heterodimer functions can be separated. The deletion of 19 amino acids from the C-terminal end of RXR dramatically reduced the transcriptional activation function of RXR. The removal of 10 additional amino acids resulted in a receptor (delta RXR3) that had completely lost its ligand-dependent homodimer function but retained its heterodimer activities. Heterodimer function was abolished by the deletion of an additional 20 amino acids. Single amino acid substitutions in the region generated receptors with altered RXR homodimer DNA binding, while simultaneous mutation of three Leu residues (Leu-418, -419 and -422) completely abolished both RXR homodimer and heterodimer DNA binding activities. Mutation of Leu-430 to Phe (L430-F) resulted in a receptor that bound to DNA strongly as homodimers in a ligand-independent manner, while another single amino acid exchange (L422-Q) led to a mutant that behaved in a manner exactly opposite to that of wild-type RXR in that the homodimerization of the mutant occurred in the absence of ligand and was inhibited by 9-cis-retinoic acid. In transfection assays, both L422-Q and L430-F failed to act as homodimers but retained their heterodimer function. Our studies demonstrate the unique properties of the RXR ligand binding domain and point to specific residues that mediate homo- and heterodimer activities and ligand-induced conformational switches.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allenby G., Bocquel M. T., Saunders M., Kazmer S., Speck J., Rosenberger M., Lovey A., Kastner P., Grippo J. F., Chambon P. Retinoic acid receptors and retinoid X receptors: interactions with endogenous retinoic acids. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):30–34. doi: 10.1073/pnas.90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arluke A. The ethical thinking of animal researchers: problems and prospects. New Biol. 1991 Jan;3(1):1–2. [PubMed] [Google Scholar]
- Au-Fliegner M., Helmer E., Casanova J., Raaka B. M., Samuels H. H. The conserved ninth C-terminal heptad in thyroid hormone and retinoic acid receptors mediates diverse responses by affecting heterodimer but not homodimer formation. Mol Cell Biol. 1993 Sep;13(9):5725–5737. doi: 10.1128/mcb.13.9.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniahmad A., Tsai S. Y., O'Malley B. W., Tsai M. J. Kindred S thyroid hormone receptor is an active and constitutive silencer and a repressor for thyroid hormone and retinoic acid responses. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10633–10637. doi: 10.1073/pnas.89.22.10633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrook D., Lernhardt E., Pfahl M. A new retinoic acid receptor identified from a hepatocellular carcinoma. Nature. 1988 Jun 16;333(6174):669–672. doi: 10.1038/333669a0. [DOI] [PubMed] [Google Scholar]
- Brand N., Petkovich M., Krust A., Chambon P., de Thé H., Marchio A., Tiollais P., Dejean A. Identification of a second human retinoic acid receptor. Nature. 1988 Apr 28;332(6167):850–853. doi: 10.1038/332850a0. [DOI] [PubMed] [Google Scholar]
- Bugge T. H., Pohl J., Lonnoy O., Stunnenberg H. G. RXR alpha, a promiscuous partner of retinoic acid and thyroid hormone receptors. EMBO J. 1992 Apr;11(4):1409–1418. doi: 10.1002/j.1460-2075.1992.tb05186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee V. K., Nagaya T., Madison L. D., Datta S., Rentoumis A., Jameson J. L. Thyroid hormone resistance syndrome. Inhibition of normal receptor function by mutant thyroid hormone receptors. J Clin Invest. 1991 Jun;87(6):1977–1984. doi: 10.1172/JCI115225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm K., Heyman R. A., Umesono K., Evans R. M. Functional inhibition of retinoic acid response by dominant negative retinoic acid receptor mutants. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2989–2993. doi: 10.1073/pnas.90.7.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm K., Thompson C. C., Evans R. M. Protein encoded by v-erbA functions as a thyroid-hormone receptor antagonist. Nature. 1989 Jun 22;339(6226):593–597. doi: 10.1038/339593a0. [DOI] [PubMed] [Google Scholar]
- Danielian P. S., White R., Lees J. A., Parker M. G. Identification of a conserved region required for hormone dependent transcriptional activation by steroid hormone receptors. EMBO J. 1992 Mar;11(3):1025–1033. doi: 10.1002/j.1460-2075.1992.tb05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca L. M. Retinoids and their receptors in differentiation, embryogenesis, and neoplasia. FASEB J. 1991 Nov;5(14):2924–2933. [PubMed] [Google Scholar]
- Durand B., Saunders M., Leroy P., Leid M., Chambon P. All-trans and 9-cis retinoic acid induction of CRABPII transcription is mediated by RAR-RXR heterodimers bound to DR1 and DR2 repeated motifs. Cell. 1992 Oct 2;71(1):73–85. doi: 10.1016/0092-8674(92)90267-g. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawell S. E., Lees J. A., White R., Parker M. G. Characterization and colocalization of steroid binding and dimerization activities in the mouse estrogen receptor. Cell. 1990 Mar 23;60(6):953–962. doi: 10.1016/0092-8674(90)90343-d. [DOI] [PubMed] [Google Scholar]
- Forman B. M., Samuels H. H. Interactions among a subfamily of nuclear hormone receptors: the regulatory zipper model. Mol Endocrinol. 1990 Sep;4(9):1293–1301. doi: 10.1210/mend-4-9-1293. [DOI] [PubMed] [Google Scholar]
- Gearing K. L., Göttlicher M., Teboul M., Widmark E., Gustafsson J. A. Interaction of the peroxisome-proliferator-activated receptor and retinoid X receptor. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1440–1444. doi: 10.1073/pnas.90.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguere V., Ong E. S., Segui P., Evans R. M. Identification of a receptor for the morphogen retinoic acid. Nature. 1987 Dec 17;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Holloway J. M., Devary O. V., Rosenfeld M. G. The thyroid hormone receptor binds with opposite transcriptional effects to a common sequence motif in thyroid hormone and estrogen response elements. Cell. 1988 Jul 29;54(3):313–323. doi: 10.1016/0092-8674(88)90194-8. [DOI] [PubMed] [Google Scholar]
- Graupner G., Wills K. N., Tzukerman M., Zhang X. K., Pfahl M. Dual regulatory role for thyroid-hormone receptors allows control of retinoic-acid receptor activity. Nature. 1989 Aug 24;340(6235):653–656. doi: 10.1038/340653a0. [DOI] [PubMed] [Google Scholar]
- Green S., Chambon P. Nuclear receptors enhance our understanding of transcription regulation. Trends Genet. 1988 Nov;4(11):309–314. doi: 10.1016/0168-9525(88)90108-4. [DOI] [PubMed] [Google Scholar]
- Hamada K., Gleason S. L., Levi B. Z., Hirschfeld S., Appella E., Ozato K. H-2RIIBP, a member of the nuclear hormone receptor superfamily that binds to both the regulatory element of major histocompatibility class I genes and the estrogen response element. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8289–8293. doi: 10.1073/pnas.86.21.8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann T., Hoffmann B., Piedrafita F. J., Zhang X. K., Pfahl M. V-erbA requires auxiliary proteins for dominant negative activity. Oncogene. 1993 Jan;8(1):55–65. [PubMed] [Google Scholar]
- Hermann T., Hoffmann B., Zhang X. K., Tran P., Pfahl M. Heterodimeric receptor complexes determine 3,5,3'-triiodothyronine and retinoid signaling specificities. Mol Endocrinol. 1992 Jul;6(7):1153–1162. doi: 10.1210/mend.6.7.1324421. [DOI] [PubMed] [Google Scholar]
- Hermann T., Zhang X. K., Tzukerman M., Wills K. N., Graupner G., Pfahl M. Regulatory functions of a non-ligand-binding thyroid hormone receptor isoform. Cell Regul. 1991 Jul;2(7):565–574. doi: 10.1091/mbc.2.7.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman R. A., Mangelsdorf D. J., Dyck J. A., Stein R. B., Eichele G., Evans R. M., Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992 Jan 24;68(2):397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- Husmann M., Hoffmann B., Stump D. G., Chytil F., Pfahl M. A retinoic acid response element from the rat CRBPI promoter is activated by an RAR/RXR heterodimer. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1558–1564. doi: 10.1016/0006-291x(92)90480-9. [DOI] [PubMed] [Google Scholar]
- Keller H., Dreyer C., Medin J., Mahfoudi A., Ozato K., Wahli W. Fatty acids and retinoids control lipid metabolism through activation of peroxisome proliferator-activated receptor-retinoid X receptor heterodimers. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2160–2164. doi: 10.1073/pnas.90.6.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Mangelsdorf D. J., Evans R. M. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992 Jan 30;355(6359):446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Noonan D. J., Heyman R. A., Evans R. M. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992 Aug 27;358(6389):771–774. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krust A., Kastner P., Petkovich M., Zelent A., Chambon P. A third human retinoic acid receptor, hRAR-gamma. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5310–5314. doi: 10.1073/pnas.86.14.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lazar M. A., Hodin R. A., Chin W. W. Human carboxyl-terminal variant of alpha-type c-erbA inhibits trans-activation by thyroid hormone receptors without binding thyroid hormone. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7771–7774. doi: 10.1073/pnas.86.20.7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. S., Kliewer S. A., Provencal J., Wright P. E., Evans R. M. Structure of the retinoid X receptor alpha DNA binding domain: a helix required for homodimeric DNA binding. Science. 1993 May 21;260(5111):1117–1121. doi: 10.1126/science.8388124. [DOI] [PubMed] [Google Scholar]
- Lehmann J. M., Jong L., Fanjul A., Cameron J. F., Lu X. P., Haefner P., Dawson M. I., Pfahl M. Retinoids selective for retinoid X receptor response pathways. Science. 1992 Dec 18;258(5090):1944–1946. doi: 10.1126/science.1335166. [DOI] [PubMed] [Google Scholar]
- Lehmann J. M., Zhang X. K., Graupner G., Lee M. O., Hermann T., Hoffmann B., Pfahl M. Formation of retinoid X receptor homodimers leads to repression of T3 response: hormonal cross talk by ligand-induced squelching. Mol Cell Biol. 1993 Dec;13(12):7698–7707. doi: 10.1128/mcb.13.12.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J. M., Zhang X. K., Pfahl M. RAR gamma 2 expression is regulated through a retinoic acid response element embedded in Sp1 sites. Mol Cell Biol. 1992 Jul;12(7):2976–2985. doi: 10.1128/mcb.12.7.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leid M., Kastner P., Lyons R., Nakshatri H., Saunders M., Zacharewski T., Chen J. Y., Staub A., Garnier J. M., Mader S. Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently. Cell. 1992 Jan 24;68(2):377–395. doi: 10.1016/0092-8674(92)90478-u. [DOI] [PubMed] [Google Scholar]
- Leroy P., Krust A., Zelent A., Mendelsohn C., Garnier J. M., Kastner P., Dierich A., Chambon P. Multiple isoforms of the mouse retinoic acid receptor alpha are generated by alternative splicing and differential induction by retinoic acid. EMBO J. 1991 Jan;10(1):59–69. doi: 10.1002/j.1460-2075.1991.tb07921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin A. A., Sturzenbecker L. J., Kazmer S., Bosakowski T., Huselton C., Allenby G., Speck J., Kratzeisen C., Rosenberger M., Lovey A. 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR alpha. Nature. 1992 Jan 23;355(6358):359–361. doi: 10.1038/355359a0. [DOI] [PubMed] [Google Scholar]
- Lotan R. Effects of vitamin A and its analogs (retinoids) on normal and neoplastic cells. Biochim Biophys Acta. 1980 Mar 12;605(1):33–91. doi: 10.1016/0304-419x(80)90021-9. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Borgmeyer U., Heyman R. A., Zhou J. Y., Ong E. S., Oro A. E., Kakizuka A., Evans R. M. Characterization of three RXR genes that mediate the action of 9-cis retinoic acid. Genes Dev. 1992 Mar;6(3):329–344. doi: 10.1101/gad.6.3.329. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Ong E. S., Dyck J. A., Evans R. M. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature. 1990 May 17;345(6272):224–229. doi: 10.1038/345224a0. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Umesono K., Kliewer S. A., Borgmeyer U., Ong E. S., Evans R. M. A direct repeat in the cellular retinol-binding protein type II gene confers differential regulation by RXR and RAR. Cell. 1991 Aug 9;66(3):555–561. doi: 10.1016/0092-8674(81)90018-0. [DOI] [PubMed] [Google Scholar]
- Marks M. S., Hallenbeck P. L., Nagata T., Segars J. H., Appella E., Nikodem V. M., Ozato K. H-2RIIBP (RXR beta) heterodimerization provides a mechanism for combinatorial diversity in the regulation of retinoic acid and thyroid hormone responsive genes. EMBO J. 1992 Apr;11(4):1419–1435. doi: 10.1002/j.1460-2075.1992.tb05187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- När A. M., Boutin J. M., Lipkin S. M., Yu V. C., Holloway J. M., Glass C. K., Rosenfeld M. G. The orientation and spacing of core DNA-binding motifs dictate selective transcriptional responses to three nuclear receptors. Cell. 1991 Jun 28;65(7):1267–1279. doi: 10.1016/0092-8674(91)90021-p. [DOI] [PubMed] [Google Scholar]
- Petkovich M., Brand N. J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987 Dec 3;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Pfahl M., Tzukerman M., Zhang X. K., Lehmann J. M., Hermann T., Wills K. N., Graupner G. Nuclear retinoic acid receptors: cloning, analysis, and function. Methods Enzymol. 1990;189:256–270. doi: 10.1016/0076-6879(90)89297-u. [DOI] [PubMed] [Google Scholar]
- Rottman J. N., Widom R. L., Nadal-Ginard B., Mahdavi V., Karathanasis S. K. A retinoic acid-responsive element in the apolipoprotein AI gene distinguishes between two different retinoic acid response pathways. Mol Cell Biol. 1991 Jul;11(7):3814–3820. doi: 10.1128/mcb.11.7.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmi S., Samuels H. H. Thyroid hormone receptor/and v-erbA. A single amino acid difference in the C-terminal region influences dominant negative activity and receptor dimer formation. J Biol Chem. 1991 Jun 25;266(18):11589–11593. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Role of retinoids in differentiation and carcinogenesis. Cancer Res. 1983 Jul;43(7):3034–3040. [PubMed] [Google Scholar]
- Thomas H. E., Stunnenberg H. G., Stewart A. F. Heterodimerization of the Drosophila ecdysone receptor with retinoid X receptor and ultraspiracle. Nature. 1993 Apr 1;362(6419):471–475. doi: 10.1038/362471a0. [DOI] [PubMed] [Google Scholar]
- Tini M., Otulakowski G., Breitman M. L., Tsui L. C., Giguère V. An everted repeat mediates retinoic acid induction of the gamma F-crystallin gene: evidence of a direct role for retinoids in lens development. Genes Dev. 1993 Feb;7(2):295–307. doi: 10.1101/gad.7.2.295. [DOI] [PubMed] [Google Scholar]
- Tora L., Mullick A., Metzger D., Ponglikitmongkol M., Park I., Chambon P. The cloned human oestrogen receptor contains a mutation which alters its hormone binding properties. EMBO J. 1989 Jul;8(7):1981–1986. doi: 10.1002/j.1460-2075.1989.tb03604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran P., Zhang X. K., Salbert G., Hermann T., Lehmann J. M., Pfahl M. COUP orphan receptors are negative regulators of retinoic acid response pathways. Mol Cell Biol. 1992 Oct;12(10):4666–4676. doi: 10.1128/mcb.12.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzukerman M., Zhang X. K., Hermann T., Wills K. N., Graupner G., Pfahl M. The human estrogen receptor has transcriptional activator and repressor functions in the absence of ligand. New Biol. 1990 Jul;2(7):613–620. [PubMed] [Google Scholar]
- Umesono K., Giguere V., Glass C. K., Rosenfeld M. G., Evans R. M. Retinoic acid and thyroid hormone induce gene expression through a common responsive element. Nature. 1988 Nov 17;336(6196):262–265. doi: 10.1038/336262a0. [DOI] [PubMed] [Google Scholar]
- Umesono K., Murakami K. K., Thompson C. C., Evans R. M. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991 Jun 28;65(7):1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills K. N., Zhang X. K., Pfahl M. Coordinate expression of functionally distinct thyroid hormone receptor alpha isoforms during neonatal brain development. Mol Endocrinol. 1991 Aug;5(8):1109–1119. doi: 10.1210/mend-5-8-1109. [DOI] [PubMed] [Google Scholar]
- Yao T. P., Segraves W. A., Oro A. E., McKeown M., Evans R. M. Drosophila ultraspiracle modulates ecdysone receptor function via heterodimer formation. Cell. 1992 Oct 2;71(1):63–72. doi: 10.1016/0092-8674(92)90266-f. [DOI] [PubMed] [Google Scholar]
- Yen P. M., Darling D. S., Carter R. L., Forgione M., Umeda P. K., Chin W. W. Triiodothyronine (T3) decreases binding to DNA by T3-receptor homodimers but not receptor-auxiliary protein heterodimers. J Biol Chem. 1992 Feb 25;267(6):3565–3568. [PubMed] [Google Scholar]
- Yu V. C., Delsert C., Andersen B., Holloway J. M., Devary O. V., När A. M., Kim S. Y., Boutin J. M., Glass C. K., Rosenfeld M. G. RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell. 1991 Dec 20;67(6):1251–1266. doi: 10.1016/0092-8674(91)90301-e. [DOI] [PubMed] [Google Scholar]
- Zelent A., Krust A., Petkovich M., Kastner P., Chambon P. Cloning of murine alpha and beta retinoic acid receptors and a novel receptor gamma predominantly expressed in skin. Nature. 1989 Jun 29;339(6227):714–717. doi: 10.1038/339714a0. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Hoffmann B., Tran P. B., Graupner G., Pfahl M. Retinoid X receptor is an auxiliary protein for thyroid hormone and retinoic acid receptors. Nature. 1992 Jan 30;355(6359):441–446. doi: 10.1038/355441a0. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Lehmann J., Hoffmann B., Dawson M. I., Cameron J., Graupner G., Hermann T., Tran P., Pfahl M. Homodimer formation of retinoid X receptor induced by 9-cis retinoic acid. Nature. 1992 Aug 13;358(6387):587–591. doi: 10.1038/358587a0. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Tran P. B., Pfahl M. DNA binding and dimerization determinants for thyroid hormone receptor alpha and its interaction with a nuclear protein. Mol Endocrinol. 1991 Dec;5(12):1909–1920. doi: 10.1210/mend-5-12-1909. [DOI] [PubMed] [Google Scholar]
- de Thé H., Vivanco-Ruiz M. M., Tiollais P., Stunnenberg H., Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature. 1990 Jan 11;343(6254):177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]