Abstract
Siglecs (sialic acid immunoglobulin-like lectins) are members of the immunoglobulin gene family that contain sialoside binding N-terminal domains. They are cell surface proteins found predominantly on cells of the immune system. Among them, Siglec-8 is uniquely expressed by human eosinophils and mast cells, as well as basophils. Engaging this structure with antibodies or glycan ligands results in apoptosis in human eosinophils and inhibition of release of preformed and newly generated mediators from human mast cells without affecting their survival. Pro-apoptotic effects are also seen when its closest functional paralog, Siglec-F, on mouse eosinophils is similarly engaged in vitro, and beneficial effects are observed after administration of Siglec-F antibody using models of eosinophilic pulmonary and gastrointestinal inflammation in vivo. Siglec-8 targeting may thus provide a means to specifically inhibit or deplete these cell types. Cell-directed therapies are increasingly sought after by the pharmaceutical industry for their potential to reduce side effects and increase safety. The challenge is to identify suitable targets on the cell type of interest, and selectively deliver a therapeutic agent. By targeting Siglec-8, monoclonal antibodies and glycan ligand-conjugated nanoparticles may be ideally suited for treatment of eosinophil and mast cell-related diseases, such as asthma, chronic rhinosinusitis, chronic urticaria, hypereosinophilic syndromes, mast cell and eosinophil malignancies and eosinophilic gastrointestinal disorders.
Keywords: Siglec-8, Siglec-F, Glycan ligands, Allergic diseases, Apoptosis, Liposomes
1. Introduction
Siglecs (sialic acid immunoglobulin-like lectins) are members of the immunoglobulin (Ig) gene family that contain sialoside binding N-terminal domains (Crocker et al., 1998). They are cell surface proteins found predominantly on cells of the immune system. Siglec-8 was originally identified as being uniquely expressed on human eosinophils, mast cells and weakly on basophils (Floyd et al., 2000; Kikly et al., 2000). These cells express other siglecs as well (von Gunten & Bochner, 2008; Bochner, 2009; Castro et al., 2011), but it is their selective expression of Siglec-8 that provides a unique opportunity to exploit this molecule for targeting of these cells in diseases in which the biology of these cells is excessive and unwanted, such as in asthma, chronic rhinosinusitis, chronic urticaria, hypereosinophilic syndromes, mast cell and eosinophil malignancies and eosinophilic gastrointestinal disorders.
After presenting an overview of siglecs, their cellular expression patterns and sialylated glycan ligands, the biology of Siglec-8 and its closest functional paralog in the mouse, Siglec-F, will be reviewed, with a focus on mechanisms and cellular consequences that occur following Siglec-8 or Siglec-F engagement on an eosinophil or mast cell (unfortunately nothing is known for the basophil), along with an account of our current understanding of their endogenous tissue ligands. Finally, a description and comparison of glycan and antibody-based approaches occurring via endocytic mechanisms will be discussed as strategies to be used to therapeutically target eosinophils and mast cells via their preferential expression of Siglec-8. The broad concept of therapeutic targeting of siglecs has been the subject of a number of excellent recent publications including those cited here (Varki & Angata, 2006; Crocker et al., 2007; McMillan & Crocker, 2008; von Gunten & Bochner, 2008; O’Reilly & Paulson, 2009; Chen et al., 2010; Magesh et al., 2011; Spergel et al., 2011).
2. Siglec structures and patterns of expression
All siglecs are single-pass transmembrane proteins belonging to the Ig superfamily with I-type lectin domains in their N-terminal regions (Powell & Varki, 1995; Varki & Angata, 2006). Figs. 1 and 2 display the structures of human and mouse siglecs, respectively (reproduced from (S. von Gunten & Bochner, 2008)). Attached to the lectin domain are 1 to 15 Ig domains, a transmembrane domain and a cytosolic tail (Varki & Angata, 2006). Siglecs can be grouped into two types based on evolutionary and sequence similarities: the Siglec-3 (cluster of differentiation [CD]33)-related siglecs (Siglec-5 [CD170], Siglec-6 [CD327], Siglec-7 [CD328], Siglec-8, Siglec-9, Siglec-10, Siglec-11, Siglec-14 and Siglec-16) and all others (Crocker et al., 2007; Varki, 2010). CD33-related siglecs in humans are exceptionally related in structure but are evolving away from other mammalian siglecs (Cao et al., 2009; Varki, 2010). Mouse cells can express CD33 but the murine genome possesses only about half as many CD33-related siglecs (Siglec-E, -F, -G and -H) and they represent paralogs, not true orthologs, of human siglecs. Siglec-H lacks any tyrosine signaling motifs in its cytoplasmic tail; whether it can bind sialic acid remains controversial (Avril et al., 2006; Blasius et al., 2006; Takagi et al., 2011).
Fig. 1.
Nomenclature and key structural characteristics of human siglecs. Although 15 are shown, Siglec-13 is present in nonhuman primates but not in man. V structures indicate the arginine-containing V-set domains with lectin activity; these are followed by C2-type Ig repeat domains. U-shaped structures for Siglec-XII indicate the mutated V-set domains missing arginine that have lost their lectin activity. Also shown is DAP12, illustrated as a shorter transmembrane structure co-associating with Siglec-13, Siglec-14, and Siglec-15. See key for symbols representing cytoplasmic signaling motifs. Reproduced from von Gunten and Bochner (2008), with permission.
Fig. 2.
Nomenclature and key structural characteristics of mouse siglecs. V structures indicate the arginine-containing V-set domains with lectin activity; these are followed by C2-type Ig repeat domains. Also shown is DAP12, illustrated as a shorter transmembrane structure co-associating with Siglec-15 and Siglec-H. Whether Siglec-H, shown as having an arginine-containing V-set domains with lectin activity, can bind sialic acid ligands remains controversial but there is no human counterpart. Note that Siglecs-1–4 are conserved with humans, as is Siglec-15 (compare to Fig. 1). While not true orthologs, the closest functional paralog of Siglec-E is Siglec-9, while Siglec-F resembles Siglec-8 and Siglec-G resembles Siglec-10. Reproduced from von Gunten and Bochner (2008), with permission.
In contrast, sialoadhesin (Siglec-1 or CD169), CD22 (Siglec-2), myelin-associated glycoprotein (Siglec-4, found on nerve-related cells rather than leukocytes) and Siglec-15, are the only true orthologs of their human counterparts (Angata et al., 2004; Crocker, 2005; Varki & Angata, 2006; Crocker & Redelinghuys, 2008). The genes encoding for all human siglecs are located on human chromosome 19q13.3–q13.4, except for sialoadhesin (chromosome 20) and Siglec-15 (chromosome 18). Siglec-12 in humans cannot bind sialic acid and thus is not a true siglec, so it has been given the designation Siglec-XII. Siglec-13 does not exist on human leukocytes but can be identified on baboon and chimpanzee cells (Angata et al., 2004; Cao et al., 2009).
Most siglecs have immunoreceptor tyrosine-based motifs (ITIMs) located in their intracellular domain, suggesting that these receptors are involved in negative cell signaling (Crocker, 2002, 2005; Varki & Angata, 2006; Crocker et al., 2007; Varki, 2007; von Gunten & Bochner, 2008, 2009). The best studied is the conserved membrane proximal ITIM motif containing the amino acid sequence (I/L/V) xYxx(L/V) (Vely & Vivier, 1997). ITIMs in various siglecs can recruit Src homology 2 domain-containing phosphatase (SHP)-1 and others when phosphorylated (Ikehara et al., 2004), and inhibit cell function when cross-linked (Avril et al., 2006). It is therefore assumed that engagement of these ITIM-containing siglecs results in the delivery of a negative regulatory signal and in some cases this signal is apoptosis. Exceptions to the cytoplasmic ITIM paradigm include Siglec-1 and Siglec-4, where no such ITIMs exists, and Siglecs 14 and 15, where no such ITIMs exists but instead these siglecs co-associate with DAP-12 and therefore have activating activity. CD33-related siglecs also possess a second membrane-distal tyrosine motif. Although little is know about its function, these appear to be immunoreceptor tyrosine-based switch motifs that may recruit so-called adaptor molecules like signaling lymphocytic activation molecule-associated protein or other molecules (Munitz & Levi-Schaffer, 2007; Shik & Munitz, 2010). More work is needed to explore this further.
Many human cells express siglecs and each siglec has a unique pattern of expression as shown in Table 1. Other than Siglec-4, found on cells of the nervous system, Siglec-6, found on placental trophoblasts (Brinkman-Van der Linden et al., 2007), and Siglec-XII, found on epithelial and other cells (Angata et al., 2001b), all are expressed by various leukocyte subsets and mast cells. Some siglecs are more distinctively expressed than others. For instance, macrophages uniquely express sialoadesin (Siglec-1, true in the mouse too) (Hartnell et al., 2001), B cells uniquely express CD22 (Siglec-2, also true in mice), and eosinophils and mast cells uniquely express Siglec-8, although for mast cells, Siglec-6 is particularly prominent in its expression (Florian et al., 2006; Yokoi et al., 2006). Among the CD33-related siglecs, Siglec-E is found on mouse neutrophils, monocytes, and dendritic cells and closely resembles Siglec-9, whereas Siglec-F and Siglec-G are considered the closest functional paralogs of Siglec-8 and Siglec-10, respectively (Aizawa et al., 2003; Zhang et al., 2004; Tateno et al., 2005; Hoffmann et al., 2007). Siglec-H is found on plasmacytoid dendritic cells but to date has no known human counterpart (Blasius et al., 2006; Zhang et al., 2006). CD33-related siglecs are roughly 50% identical, yet cellular expression patterns are not necessarily the same from species to species (e.g., see below regarding patterns of Siglec-8 versus Siglec-F expression). Because human CD4+ lymphocytes have evolved to lack them (Nguyen et al., 2006), siglecs are often thought of as innate immune receptors (Crocker, 2005), although CD8+ T cells can express Siglec-7 and Siglec-9 (Ikehara et al., 2004), and under allergic inflammatory conditions, Siglec-F can reportedly be detected at low levels on CD4+ T cells (Zhang et al., 2007).
Table 1.
Patterns of expression of siglecs on human cells.
| Cell | Siglec
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | XII | 14 | 15 | ||
| B cell | + | ± | ± | + | ± | ||||||||||
| Basophil | ± | + | + | + | ± | + | |||||||||
| CD4 T cell | None | ||||||||||||||
| CD8 T cell | + | + | |||||||||||||
| CD34+ cell | + | + | + | + | + | ||||||||||
| Dendritic cell | + | + | + | + | |||||||||||
| Eosinophil | + | + | ± | ||||||||||||
| Epithelial cell | + | ||||||||||||||
| Macrophage | + | + | + | + | + | + | |||||||||
| Mast cell | + | + | + | + | + | ||||||||||
| Microglial cell | + | ||||||||||||||
| Monocyte | + | + | + | + | + | + | + | + | |||||||
| Neutrophil | + | + | + | + | |||||||||||
| NK cell | + | + | ± | + | |||||||||||
| Oligodendrocyte | + | ||||||||||||||
| Placental trophoblast | + | ||||||||||||||
| Schwann cell | + | ||||||||||||||
Modified from von Gunten and Bochner (2008), with permission.
3. Expression patterns of Siglec-8 and Siglec-F during hematopoiesis, across various species and in disease states
Siglec-8, originally discovered by two laboratories using the same eosinophil cDNA library generated from a patient with marked peripheral blood eosinophilia, was initially described as having a short cytoplasmic domain lacking the tyrosine motifs found in other CD33-related siglecs (Floyd et al., 2000; Kikly et al., 2000). In its original description it was also referred to as SAF-2 or sialoadhesin factor 2. It soon became clear that mast cells and basophils also expressed Siglec-8, and this was followed by the observation that Siglec-8 could undergo alternative splicing to yield both a “short form” as originally described, and a “long form” containing two tyrosine cytoplasmic motifs consistent with other CD33-related siglecs (Foussias et al., 2000; Aizawa et al., 2002). Since then, the term Siglec-8 is used to describe the “long form”. Additional studies examining leukocyte transcriptomes in an unbiased fashion confirmed the highly selective expression of Siglec-8 by eosinophils (Liu et al., 2006).
Relatively little is known about Siglec-8 expression during inflammation and disease states, and what is known pertains only to eosinophils as nothing is known about mast cells on this topic. Eosinophils obtained by bronchoalveolar lavage from asthmatic subjects have similar levels of surface Siglec-8 when compared to their blood counterparts (unpublished observations). In one patient with hypereosinophilic syndrome, both the levels and function of Siglec-8 on their eosinophils appeared normal despite the presence of a point mutation near the putative glycan binding site (Gao et al., 2010). A limited analysis of various human blood samples revealed that Siglec-8 is expressed at normal levels on eosinophils and basophils from subjects with chronic eosinophilic and chronic myelogenous leukemia, and on normal bone marrow mast cells and mast cells from subjects with indolent systemic mastocytosis (Hudson et al., 2011). As predicted by genomic analyses (Angata et al., 2004; Cao et al., 2009), Siglec-8 is maintained in chimpanzees but is not detected on baboon, rhesus and cynomolgus monkey eosinophils or dog mastocytoma cells when analyzed by indirect immunofluorescence and flow cytometry (Hudson et al., 2011). So far, there is no known eosinophil-like cell line that expresses Siglec-8 (Kikly et al., 2000; Hudson et al., 2011), but several mast cell lines, such as LAD2, LUVA, and HMC1.2 (but not HMC1.1) do express Siglec-8 (Yokoi et al., 2006; Hudson et al., 2011; Salicru et al., 2012) (Table 2).
Table 2.
Comparison of Siglec-8 and Siglec-F surface expression on primary cells and cell lines as determined by flow cytometry.
| Cell type | Siglec-8 | Siglec-F |
|---|---|---|
| Primary human cells | ||
| Alveolar macrophages | − | ++ |
| B cells | − | − |
| Basophils | + | ND |
| Eosinophils | +++ | +++ |
| Eosinophils grown from CD34+ cells | +++ | +++ |
| Stem cells | − | − |
| Mast cells | ++ | − |
| Mast cells grown from progenitor cells | ++ | − |
| Monocytes | − | − |
| Neutrophils | − | ± |
| NK cells | − | − |
| T cells | − | ± |
| Primary baboon blood cells | ||
| All leukocytes including eosinophils | − | ND |
| Primary cynomolgus monkey blood cells | ||
| All leukocytes including eosinophils | − | ND |
| Primary rhesus monkey blood cells | ||
| All leukocytes including eosinophils | − | ND |
| Primary dog blood cells | ||
| All leukocytes including eosinophils | − | ND |
| Cell lines | ||
| Human eosinophil-like | ||
| HL60, AML14, AML14.3D10, EOL-1 | −* | ND |
| Human mast cell-like | ||
| HMC-1.2, LAD2, LUVA | ++ | ND |
| HMC-1.1, KU812 | − | ND |
| Dog C2 mastocytoma | − | ND |
−: not expressed.
±: Expressed weakly or only under inflammatory conditions.
+, ++, +++: low, moderate or high expression.
ND: not determined.
Siglec-8 mRNA expression was detected in eosinophil-committed AML14 and AML14.3D10 cells but no protein expression was detected.
Neither Siglec-8 nor Siglec-F is expressed on stem cells, and based on studies where cells are grown in vitro from precursors, Siglec-8 appears late in mast cell and eosinophil maturation, as is the case for Siglec-F on mouse eosinophils (Kikly et al., 2000; Yokoi et al., 2006; Dyer et al., 2008; Hudson et al., 2011). The cellular expression pattern of Siglec-F is slightly different from that of Siglec-8. Siglec-F was initially identified in immature cells of the myelomonocytic lineage and in a subset of CD11b-positive cells in some tissues (Angata et al., 2001a). Using interleukin (IL)-5 transgenic mice and Northern blotting analyses, mRNA levels of Siglec-F were found to be present in mouse eosinophils (Aizawa et al., 2003). Additional studies using antibodies, Siglec-F null mice and other molecular analyses have confirmed selective Siglec-F expression by eosinophils (Zhang et al., 2004; Tateno et al., 2005; Zhang et al., 2007). Because the sequence of Siglec-F is more similar to that of human Siglec-5, which is not expressed by human eosinophils, this was rather unexpected (Aizawa et al., 2003). Subsequent studies showed that Siglec-F is expressed on a wider range of cells than Siglec-8, including mouse alveolar macrophages, some T cells, and weakly on neutrophils (see Table 2) (Stevens et al., 2007; Tateno et al., 2007; Zhang et al., 2007).
4. Glycan ligands for Siglec-F and Siglec-8
As implied by their name, siglecs recognize sialylated glycans as ligands. Numerous groups have investigated the sialic acid-dependent binding of siglecs, and have demonstrated that each siglec exhibits preference for one or several of the diverse glycan structures found on glycans of glycoproteins and glycolipids (Campanero-Rhodes et al., 2006; Crocker et al., 2007; Varki, 2009; Paulson et al., 2012). Although there is an extensive literature on siglec ligands, a family-wide comparison is difficult because of the diverse assays used to analyze ligand specificity. Nonetheless, it is abundantly clear that siglecs exhibit divergent specificities for their glycan ligands, which play a role in their biology through their interactions with ligands expressed on the same cell in cis and on other cells and/or soluble glycoconjugates in trans.
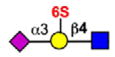
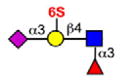
Although some siglecs exhibit quite broad specificity for sialosides commonly found on glycans of glycoproteins and glycolipids, others exhibit a strong preference for one or several sialoside structures. Siglec-8 and the murine paralog Siglec-F are among the most specific (Bochner et al., 2005; Tateno et al., 2005). As illustrated in Table 3, Siglec-8 and Siglec-F bind with highest affinity to two sialosides that have in common a terminal epitope with both sialic acid and sulfate attached to the same galactose residue (NeuAcα2-3(6S) Galβ1-4(±Fucα1-3)GlcNAc-). Of the two, Siglec-8 is the most specific, while Siglec-F exhibits weaker affinity for non-sulfated glycans. No other human or mouse siglec exhibits a strong preference for this ligand, as illustrated by the exemplary siglecs in Table 3. However, Rinaldi and coworkers, using arrays of glycolipids, immobilized either individually or in complexes on either a polystyrene surface or polyvinyldifluoride membrane, reported that Siglec-F-Fc, a fusion protein containing two extracellular Siglec-F domains linked to the human Fc (fragment crystalizable) region of IgG) unexpectedly bound to the ganglioside GM2 and a mixture of GM2:GT1b but only on the polyvinyldifluoride membrane, suggesting that the surface on which a siglec ligand is displayed, and what it is displayed with (i.e., individually or mixed with other glycolipids) can influence binding patterns (Rinaldi et al., 2009). In contrast to Siglec-8, Siglec-9 preferentially recognizes a related sialoside with sulfate on the 6-position of the GlcNAc (Table 3). Similarly, human CD22 preferentially recognizes yet another related sialoside that differs in the sialic acid linkage and position of the sulfate (NeuAcα2-6Galβ1-4(6S)GlcNAc).
Table 3.
Glycan ligand specificities of selected human and murine siglecs.
| Glycan ligand | Siglec specificity† | |||||
|---|---|---|---|---|---|---|
| hCD22 | mCD22 | hSig-8 | mSig-F | Sig-7 | hSig-9 | |
|
|
• | ○ | – | ○ | ○ | ○ |
|
|
• | ● | – | – | ○ | ○ |

|
● | ○ | – | – | – | * |
|
|
– | – | – | ○ | ○ | ○ |
|
|
– | – | – | – | ○ | ○ |
|
– | – | ● | ● | * | * |
|
– | – | ● | ● | • | – |
|
|
– | – | – | ○ | * | * |

|
– | – | – | ○ | • | ● |
|
|
– | – | – | – | ● | – |
Key:
 Gal
Gal
 Fuc
Fuc
 GalNAc
GalNAc
 GlcNAc
GlcNAc
 NeuAc
NeuAc
 NeuGc
NeuGc
Glycan stuctures represent terminal sequences of glycans of mammalian glycoproteins and glycolipids that interact with siglecs. Relative preferences of each siglec are compiled primarily from glycan array data from the Consortium for Functional Glycomics (http://www.functionalglycomics.org). See also reviews on this subject. Relative strengths of interactions are expressed as strong (large filled circle), moderate (small filled circle), weak (small open circle) or not detectable (−). The * symbol indicates not tested.
Knowledge of the ligand specificity of a siglec does not directly inform us about the ligands encountered in situ, but the information provides a concrete basis for experimentation to identify the biologically relevant ligands. The ligands for human and murine CD22 provide good examples of this point. Using an antibody specific for the preferred ligand of human CD22, Kannagi and coworkers showed that this ligand is highly expressed on naïve human B cells, and is the major biologically relevant ligand for CD22 (Kimura et al., 2007), yet germinal center B cells no longer express this ligand, suggesting that there is active regulation of the biosynthesis of the CD22 ligand in vivo. The challenge for the future will be to systematically compare the specificities of all members of the siglec family, including Siglec-8, using the same assay systems, and to determine the extent to which the specificity of each siglec has on the biology it mediates.
5. Endogenous tissue ligands for Siglec-F and Siglec-8
Although the exact composition of natural tissue ligands of Siglec-F and Siglec-8 are still under study, immunohistochemical approaches have begun to reveal the potential tissue distribution of these ligands. For example, Siglec-F-Fc has been used to screen for tissue ligands, and was found to selectively bind mouse lung epithelium and goblet cells with the staining being eliminated by mutating the arginine in the Siglec-F-Fc putative glycan binding domain, or by pretreating the lung tissue with Maackia amurensis lectin (recognizing α2,3-linked sialic acid), sialidase, periodate or proteinase K (Zhang et al., 2007; Patnode et al., 2009; Guo et al., 2011). The staining intensity for this lung epithelial Siglec-F-Fc binding material was increased following allergic lung inflammation in mice and sheep (Zhang et al., 2007; Patnode et al., 2009; Cho et al., 2010). Interestingly, some peribronchial cells, glands and mononuclear leucocytes seem to express Siglec-F-Fc binding molecules under conditions of allergic airway inflammation, but it is not known if these differ from those detected on the epithelium (Zhang et al., 2007; Patnode et al., 2009). Increases in Siglec-F-Fc binding to airway epithelium were also observed after IL-4 or IL-13 stimulation, but not other cytokines such as TNF-α, in vivo and in vitro (Cho et al., 2010; Kiwamoto et al., 2011a, 2011b). Lungs from St3gal3 null mice, but not St3gal2 null mice, were nearly devoid of Siglec-F-Fc staining (Guo et al., 2011), implicating the St3gal3 sialyltransferase in the generation of Siglec-F ligands. Similar sialidase-sensitive Siglec-F binding molecules were observed in preliminary studies using material derived from mouse primary tracheal epithelial cells (mTECs) and culture supernatants (Cho et al., 2010; Kiwamoto et al., 2011a, 2011b). These results suggest that this Siglec-F-Fc binding molecule is an α2,3-linked sialic acid-containing glycoprotein that increases under allergic inflammatory conditions. Further support for this conclusion comes from early studies reporting that pre-incubation of mouse lung tissue sections or mTEC with a novel anti-6′-sulfo-sLex IgY antiserum blocked Siglec-F-Fc binding (Kiwamoto et al., 2011b, 2012). Although further experimentation is required, this lung epithelial Siglec-F-Fc binding material likely represents a Siglec-F natural tissue ligand that contains the 6′-sulfo-sLex structure.
In contrast, Siglec-8-Fc did not bind human lung epithelium in tissue sections but instead bound human airway glands and was poorly blocked by anti-6′-sulfo-sLex IgY (Kiwamoto et al., 2012). Western blotting using Siglec-8-Fc recognized a ≈500 kDa sialidase sensitive band from supernatants of human bronchial explants (Kiwamoto et al., 2012). Although both Siglec-F-Fc and Siglec-8-Fc detected sialidase-sensitive high molecular weight (≈460–500 kDa) material from both species of lung, a lower molecular weight band (≈225 kDa) was detected only in the mTEC lysate using Siglec-F-Fc (Kiwamoto et al., 2012). Based on these early results from ongoing studies, lung ligands for Siglec-F and Siglec-8 may be different in their location of expression and perhaps in their glycan composition as well. Work is underway to purify and fully biochemically characterize glycoprotein ligands for Siglec-F and Siglec-8 (http://lidpeg.jhmi.edu/). Until these lung-derived candidate ligands are more fully characterized biochemically, it remains unclear whether mouse and human liagnds for Siglec-F and Siglec-8, respectively, are the same or different. Future studies using sialyltransferase and sulfotransferase deficient mice should also help to determine the functional contribution of these enzymes to the generation of natural Siglec-F tissue ligands under normal and inflammatory conditions.
6. Targeting Siglec-F and Siglec-8 via antibodies and glycan ligands
6.1. Anti-eosinophil properties
As introduced above, Siglec-8 expressed on eosinophils has potential as targets for cell-directed therapeutics in a variety of diseases because ligation of Siglec-8 on eosinophils with monoclonal antibodies (mAbs) cause caspase and/or reactive oxygen species (ROS) dependent apoptosis of the cell. This property is amplified under conditions of eosinophil activation such as cytokine priming with IL-5, granulocyte macrophage colony-stimulating factor or IL-33 where ROS dependent mechanisms along with mitochondrial injury are predominantly if not exclusively involved (Nutku et al., 2003, 2005; von Gunten et al., 2007; Nutku-Bilir et al., 2008; Na et al., 2011).
The fact that antibody engagement of Siglec-8 on eosinophils causes their apoptosis suggested that engagement of Siglec-8 with glycan ligands could have similar effects. Indeed, a soluble polymer displaying the glycan 6′-sulfo-sLeX selectively bound to human eosinophils and HEK 293 cells expressing Siglec-8 (Hudson et al., 2009). Polymer binding was inhibited by a polyclonal anti-Siglec-8 antibody. In whole human blood, eosinophils were the only leukocyte subtype to detectably bind polymeric 6′-sulfo-sLeX. IL-5-primed eosinophils underwent apoptosis when incubated with either anti-Siglec-8 mAb or polymeric 6′-sulfo-sLeX, although the glycan polymer was less effective. These data were the first to demonstrate that a soluble, multivalent polymer decorated with a unique glycan, 6′-sulfo-sLeX, selectively binds to human eosinophils and induces their apoptosis. These results provide proof-of-concept that glycan-based approaches can be used to selectively target eosinophils and could be exploited as it relates to glycan nanoparticle targeting.
Several reports have documented that in vivo administration of monoclonal and polyclonal antibodies recognizing Siglec-F reduces blood and tissue eosinophils in vivo, including models of hypereosinophilia and eosinophilic leukemia, and reduces signs and sequelae of eosinophil-mediated lung and intestinal inflammation in murine models (Zimmermann et al., 2008; Song et al., 2009a, 2009b). Furthermore, mice deficient in Siglec-F show exaggerated eosinophilic inflammation and tissue remodeling in asthma models, but there was no significant effect on airways hyperreactivity (Kearley et al., 2007; Zhang et al., 2007; Cho et al., 2010). For example, in a mouse model of gastrointestinal eosinophilia and diarrhea, Siglec-F mAb administration normalized tissue eosinophil levels and weight gain (Song et al., 2009b). In a subsequent paper, this same group demonstrated that mice sensitized to ovalbumin and challenged via the airway repetitively for 1 month while being given anti-Siglec-F not only showed decreased airways eosinophilia, but almost complete normalization of sub-epithelial fibrosis, a reduction in mucus cells and smooth muscle layer thickness, and a significant reduction in airway cells positive for the profibrogenic cytokine transforming growth factor-β (Song et al., 2009a). These data provide encouragement in support of targeting an eosinophil-selective siglec because of the profound effect on eosinophil numbers, tissue injury and disease activity.
Although the exact signaling mechanisms involved in Siglec-8 and Siglec-F induced eosinophil death are not yet delineated, ongoing work is employing mouse strains deficient in key intracellular molecules to test the roles of phosphatases (e.g., using SHP-1 null mice) and ROS (e.g., using p47 NADPH oxidase deficient mice) in Siglec-F signal transduction using eosinophils grown from bone marrow precursors from such mice. Data generated so far surprisingly suggest neither SHP-1 nor ROS is required for Siglec-F-induced apoptosis, and indeed unlike what is seen with human eosinophils activated via Siglec-8, mouse eosinophils activated via Siglec-F do not produce any detectable ROS (unpublished observations). Recent acquisition of inhibitors of molecules that may be downstream of Siglec-8/-F signaling should allow the comparison of effects in mouse versus human eosinophils, and so far, ERK phosphorylation has been implicated in the IL-5-enhanced apoptosis seen with Siglec-8 and human eosinophils (Kano et al., 2012).
6.2. Anti-mast cell properties
In a separate series of experiments, the hypothesis that activation via Siglec-8 will affect the survival and/or secretory functions of mast cells was tested. Studies using human mast cells generated from CD34+ precursors showed that Siglec-8 engagement failed to induce apoptosis (Yokoi et al., 2008). However, pre-incubation with Siglec-8 mAbs significantly inhibited FcεRI-dependent histamine and PGD2 release from purified mast cells, shifting the dose response curves to stimulation with anti-FcεRI antibody by 1–2 logs. Also significantly inhibited was mast cell-dependent contraction of human bronchial smooth muscle rings in vitro. Further studies with mast cell and Siglec-8 transfectants revealed that preincubation with Siglec-8 mAbs inhibited FcεRI-dependent Ca++ flux and release of β-hexosaminidase that required the presence of the membrane-proximal ITIM tyrosine residue. Thus, although mast cells seem to behave differently than eosinophils following Siglec-8 engagement with respect to apoptosis, both cells display inhibitory responses that fit with a favorable clinical effect profile when considered as treatment for mast cell and eosinophil-related disorders. Unfortunately, Siglec-F is not expressed on mouse mast cells, so its function on these cells cannot easily be determined.
7. Prospects for therapeutic applications of targeting siglecs, including Siglec-8
The fact that siglecs exhibit highly restricted expression in selected hematopoetic cells and oligodendrocytes has drawn attention to their potential as targets for development of cell directed therapies (von Gunten & Bochner, 2008; O’Reilly & Paulson, 2009; Farid et al., 2012). Indeed, CD22 (Siglec-2) and CD33 (Siglec-3) were originally discovered as markers of B cell lymphomas and acute myeloid leukemias, respectively, and soon became targets for the development of immunotherapies to treat these diseases (Ball, 1988; Kreitman & Pastan, 2006). Because many siglecs are endocytic receptors (Shan & Press, 1995; Crocker et al., 2007), immunotoxins have been adopted as an attractive clinical strategy since binding of the antibody to CD22 and CD33 results in endocytosis of the complex, delivering the toxin into the cell. Immunotoxins targeting both CD22 and CD33 continue to be actively pursued in clinical trials for lymphomas and leukemias expressing these siglecs (Kreitman & Pastan, 2006; Tu et al., 2011; Jurcic, 2012; Kreitman et al., 2012; Ogura et al., 2012; Pollard et al., 2012; Walter et al., 2012). B cell depletion strategies using CD22 antibodies are also being pursued for treatment of a number of autoimmune diseases (Dunussi-Joannopoulos et al., 2005; Leonard & Goldenberg, 2007; Fiorina et al., 2008). In a quite different application, several groups have used anti-siglec antibodies for delivery of antigens to antigen-presenting cells. In particular, antigens conjugated to anti-sialoadhesin (Siglec-1) and anti-Siglec-H are efficiently delivered to macrophages and plasmacytoid DCs, respectively, resulting in modulation of antigen-specific immune responses (Backer et al., 2010; Delputte et al., 2011; Loschko et al., 2011).
In recent years, glycan ligands have begun to provide an alternative to antibodies for targeting siglec-expressing cells. This has been stimulated as a result of advances in the generation of siglec ligands with high affinity and selectivity coupled with multivalent platforms to display them (Collins et al., 2006; Zaccai et al., 2007; Blixt et al., 2008; Magesh et al., 2011; Mesch et al., 2012). As observed with antibodies, ligation of Siglec-8 with the polymeric ligand induces apoptosis of eosinophils (Hudson et al., 2009), suggesting that a suitable ligand-bearing nanoparticle might be used to suppress eosinophil mediated inflammation. Efforts to develop siglec decorated nanoparticle platforms that can deliver therapeutic cargo to targeted cells are also bearing fruit (O’Reilly et al., 2008; O’Reilly & Paulson, 2009; Chen et al., 2010, 2012). For example, CD22 ligand decorated liposomal nanoparticles loaded with the chemotherapeutic doxorubicin have recently been shown to prolong survival in a mouse model of human B cell lymphoma (Chen et al., 2010). This is an attractive platform since siglec ligands can be covalently attached to lipids, allowing simple incorporation into liposomes, which are a pharmaceutically proven nanoparticle formulation.
Many of these targeting applications rely on the fact that the siglecs are endocytic receptors that carry the anti-siglec antibody or siglec-ligand conjugate into the cell (Crocker et al., 2007; Crocker & Redelinghuys, 2008). All siglecs studied to date have been found to be endocytic receptors, including sialoadhesin, CD22, CD33, MAG, Siglec-5, Siglec-6, Siglec-7, Siglec-9, Siglec-F, and Siglec-H (Lock et al., 2004; Biedermann et al., 2007; Scott et al., 2008; Winterstein et al., 2008; O’Reilly & Paulson, 2009; Delputte et al., 2011). While CD22 is endocytosed by a clathrin-dependent mechanism and constitutively recycles between the cell surface and endosomes, Siglec-F is endocytosed by a non-clathrin dependent mechanism and traffics directly to lysosomes (Tateno et al., 2007; O’Reilly et al., 2011). Whether this occurs with Siglec-8 is currently under investigation.
The fact that CD22 is a recycling receptor has consequences for targeted delivery of agents to B cells. Glycan ligands are released in the acidic endosome, allowing the free CD22 to recycle to the cell surface, as illustrated in Fig. 3, which depicts targeting of anti-nitrophenol (NP) IgM to B cells via a hetero-bifunctional ligand comprising NP conjugated to a CD22 ligand (O’Reilly et al., 2008). In contrast, anti-CD22 antibody (clone HIB22) is not released in the endosomes and the CD22-antibody complex recycles between cell surface and endosomes (O’Reilly et al., 2011). As a result, the agent targeted via glycan ligands accumulates in the cell (e.g. the anti-NP-IgM), while the anti-CD22 antibody is not released, and instead equilibrates between the cell surface and endosomal compartments with no net accumulation over time (O’Reilly & Paulson, 2009; Chen et al., 2010). Such considerations will have increasing importance as siglecs, including Siglec-8, are evaluated as receptors for cell-based targeted therapeutics, and suggest that more work should be done to assess the endocytic mechanisms of siglecs.
Fig. 3.
CD22 ligand decorated cargo is released in endosomes and accumulates in the cell, while anti-CD22 antibody recycles to the cell surface. Illustrated is the fate of an anti-NP-IgM (‘the cargo’) targeted to CD22 on B cells using a heterobifunctional ligand comprising the antigen, NP, coupled to a high affinity ligand of CD22. Following ligand driven assembly of the anti-NP IgM:CD22 complex on the surface of the cell, endocytosis results in release of the anti-NP IgM in the acidic endosomal compartments. CD22 then goes back to the cell surface to capture another load of cargo, resulting in the accumulation of the glycan-based cargo (anti-NP IgM) inside the cell. In contrast, an anti-CD22 antibody (clone HIB22) is not released from CD22 in the endosomes, resulting in the complex of CD22 and the anti-CD22 antibody recycles between the cell surface and the endosomes with no accumulation of the antibody. By permission of Mary O’Reilly.
In considering what diseases might be particularly amenable to a therapeutic agent like one targeting Siglec-8 that induces eosinophil apoptosis and inhibits mast cell degranulation, several disorders deserve consideration, not the least of which is asthma. Of potential relevance to a Siglec-8-based therapeutic is that genetic linkage studies of asthma and related phenotypes have previously implicated chromosome 19q13.33–q13.41, where most siglec genes are located (Ober et al., 1998; Venanzi et al., 2001). Further encouragement in support of an important role of Siglec-8 in asthma comes from a study of the genetic association between sequence variants in Siglec-8 and the diagnosis of asthma, where a significant association with asthma was observed for various single nucleotide polymorphisms among African American, Japanese and Brazilian populations (Gao et al., 2010). Whether targeting multiple cell types via Siglec-8 will turn out to be more efficacious in asthma than targeting just the eosinophil with anti-IL-5 antibodies like mepolizumab or reslizumab, the eosinophil and basophil with anti-IL-5 receptor antibodies like benralizumab or the mast cell and basophil with omalizumab remains to be seen (Bochner & Gleich, 2010; Cook & Bochner, 2010).
Beyond asthma, there are other disorders, many of which are orphan diseases, in which eosinophils and/or mast cells are believed to play an important role. Examples include chronic rhinosinusitis with nasal polyposis, eosinophilic gastrointestinal disorders (eosinophilic esophagitis, gastritis and colitis), anaphylaxis, chronic idiopathic urticaria, eosinophilic cellulitis and fasciitis, chronic eosinophilic pneumonia, Churg-Strauss syndrome, hypereosinophilic syndromes, and hematologic malignancies involving eosinophils and mast cells, the latter including systemic mastocytosis. While glycan-based therapeutic strategies with ligands that bind to both Siglec-8 and Siglec-F may be fruitfully tested in mice (at least for eosinophil targeting given the shared activity of the 6′-sulfo-sLex ligand), transgenic models in which Siglec-8 is selectively expressed in eosinophil and/or mast cell compartments, or models where human stem cells are transplanted into immunocompromised mice resulting in maturation and engraftment of human mast cells (Takagi et al., 2012) would be more beneficial for testing mAb-based agents. This could then be followed by testing of anti-eosinophil and mast cell activity in chimpanzees, the only other non-human species to express Siglec-8 (Angata et al., 2004; Cao et al., 2009). Thus, it is fair to say that the regulatory path to the clinic is not straightforward, yet far from insurmountable.
8. Summary and conclusions
Siglec-8, selectively expressed on human eosinophils, mast cells and weakly on basophils, is an intriguing choice for the development of therapies, such as mAbs and glycan-targeting agents including liposomes, for a variety of disorders including asthma, chronic rhinosinusitis, chronic urticaria, hypereosinophilic syndromes, mast cell and eosinophil malignancies and eosinophilic gastrointestinal disorders where excessive, pathologic involvement of eosinophils and mast cells is occurring. Such targeting, depending on the strategies used, could either leverage the known effects of Siglec-8 engagement, namely induction of eosinophil apoptosis and inhibition of mast cell mediator release, or instead could result in depletion of these cells via delivery of toxic payloads via internalization of Siglec-8-binding glycan-coated liposomes or the use of Siglec-8 mAbs that are humanized to maximize antibody-dependent cell-mediated cytotoxicity. Whether engagement of Siglec-8 by naturally occurring lung cell-derived glycan ligands occurs in vivo remains to be determined, but data suggests that such materials may indeed exist in the airways. Regardless, the unique patterns of Siglec-8 expression and mechanisms of inhibition represent a promising and unique opportunity for its exploitation as a novel therapeutic target.
Acknowledgments
The authors thank Jacqueline Schaffer for Figs. 1 and 2 and Mary O’Reilly for Fig. 3. This work was supported by grants AI72265, AI50143 and HL107151 from the National Institutes of Health. Dr. Bochner also received support as a Cosner Scholar in Translational Research from Johns Hopkins University.
Abbreviations
- 6′-sulfo-sLex
6′-sulfated sialyl Lewis X (NeuAcα2–3(6S)Galβ1–4 (Fucα1–3)GlcNAc
- CD
cluster of differentiation
- Fc
fragment crystalizable region of IgG
- Ig
immunoglobulin
- IL
interleukin
- ITIM
immunoreceptor tyrosine-based inhibitory motif
- mAb
monoclonal antibody
- mTEC
mouse tracheal epithelial cells
- ROS
reactive oxygen species
- Siglec-8-Fc
a fusion protein containing two extracellular Siglec-8 domains linked to the human Fc (fragment crystalizable) region of IgG
- Siglec-F-Fc
a fusion protein containing two extracellular Siglec-F domains linked to the human Fc (fragment crystalizable) region of IgG
Footnotes
Conflict of Interest statement
Dr. Bochner is a co-inventor on existing and pending Siglec-8-related patents. Dr. Bochner may be entitled to a share of royalties received by the University on the potential sales of such products. Dr. Bochner is also a co-founder of, and owns stock in, Allakos, Inc., which makes him subject to certain restrictions under University policy. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies. Drs. Paulson and Kawasaki are inventors on patent applications managed by The Scripps Research Institute relating to the use of siglec ligands for targeting siglec-bearing cells, and they may be entitled to a share of license revenues in the event that they are realized.
References
- Aizawa H, Plitt J, Bochner BS. Human eosinophils express two siglec-8 splice variants. J Allergy Clin Immunol. 2002;109:176. doi: 10.1067/mai.2002.120550. [DOI] [PubMed] [Google Scholar]
- Aizawa H, Zimmermann N, Carrigan PE, Lee JJ, Rothenberg ME, Bochner BS. Molecular analysis of human Siglec-8 orthologs relevant to mouse eosinophils: identification of mouse orthologs of Siglec-5 (mSiglec-F) and Siglec-10 (mSiglec-G) Genomics. 2003;82:521–530. doi: 10.1016/s0888-7543(03)00171-x. [DOI] [PubMed] [Google Scholar]
- Angata T, Hingorani R, Varki NM, Varki A. Cloning and characterization of a novel mouse Siglec, mSiglec-F: differential evolution of the mouse and human (CD33) Siglec-3-related gene clusters. J Biol Chem. 2001a;276:45128–45136. doi: 10.1074/jbc.M108573200. [DOI] [PubMed] [Google Scholar]
- Angata T, Varki NM, Varki A. A second uniquely human mutation affecting sialic acid biology. J Biol Chem. 2001b;276:40282–40287. doi: 10.1074/jbc.M105926200. [DOI] [PubMed] [Google Scholar]
- Angata T, Margulies EH, Green ED, Varki A. Large-scale sequencing of the CD33-related Siglec gene cluster in five mammalian species reveals rapid evolution by multiple mechanisms. Proc Natl Acad Sci U S A. 2004;101:13251–13256. doi: 10.1073/pnas.0404833101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avril T, Attrill H, Zhang J, Raper A, Crocker PR. Negative regulation of leucocyte functions by CD33-related siglecs. Biochem Soc Trans. 2006;34:1024–1027. doi: 10.1042/BST0341024. [DOI] [PubMed] [Google Scholar]
- Backer R, Schwandt T, Greuter M, Oosting M, Jungerkes F, Tuting T, et al. Effective collaboration between marginal metallophilic macrophages and CD8+ dendritic cells in the generation of cytotoxic T cells. Proc Natl Acad Sci U S A. 2010;107:216–221. doi: 10.1073/pnas.0909541107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball ED. In vitro purging of bone marrow for autologous marrow transplantation in acute myelogenous leukemia using myeloid-specific monoclonal antibodies. Bone Marrow Transplant. 1988;3:387–392. [PubMed] [Google Scholar]
- Biedermann B, Gil D, Bowen DT, Crocker PR. Analysis of the CD33-related siglec family reveals that Siglec-9 is an endocytic receptor expressed on subsets of acute myeloid leukemia cells and absent from normal hematopoietic progenitors. Leuk Res. 2007;31:211–220. doi: 10.1016/j.leukres.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Blasius AL, Cella M, Maldonado J, Takai T, Colonna M. Siglec-H is an IPC-specific receptor that modulates type I IFN secretion through DAP12. Blood. 2006;107:2474–2476. doi: 10.1182/blood-2005-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blixt O, Han S, Liao L, Zeng Y, Hoffmann J, Futakawa S, et al. Sialoside analogue arrays for rapid identification of high affinity siglec ligands. J Am Chem Soc. 2008;130:6680–6681. doi: 10.1021/ja801052g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner BS. Siglec-8 on human eosinophils and mast cells, and Siglec-F on murine eosinophils, are functionally related inhibitory receptors. Clin Exp Allergy. 2009;39:317–324. doi: 10.1111/j.1365-2222.2008.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner BS, Gleich GJ. What targeting eosinophils has taught us about their role in diseases. J Allergy Clin Immunol. 2010;126:16–25. doi: 10.1016/j.jaci.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner BS, Alvarez RA, Mehta P, Bovin NV, Blixt O, White JR, et al. Glycan array screening reveals a candidate ligand for Siglec-8. J Biol Chem. 2005;280:4307–4312. doi: 10.1074/jbc.M412378200. [DOI] [PubMed] [Google Scholar]
- Brinkman-Van der Linden EC, Hurtado-Ziola N, Hayakawa T, Wiggleton L, Benirschke K, Varki A, et al. Human-specific expression of Siglec-6 in the placenta. Glycobiology. 2007;17:922–931. doi: 10.1093/glycob/cwm065. [DOI] [PubMed] [Google Scholar]
- Campanero-Rhodes MA, Childs RA, Kiso M, Komba S, Le Narvor C, Warren J, et al. Carbohydrate microarrays reveal sulphation as a modulator of siglec binding. Biochem Biophys Res Commun. 2006;344:1141–1146. doi: 10.1016/j.bbrc.2006.03.223. [DOI] [PubMed] [Google Scholar]
- Cao H, de Bono B, Belov K, Wong ES, Trowsdale J, Barrow AD. Comparative genomics indicates the mammalian CD33rSiglec locus evolved by an ancient large-scale inverse duplication and suggests all Siglecs share a common ancestral region. Immunogenetics. 2009;61:401–417. doi: 10.1007/s00251-009-0372-0. [DOI] [PubMed] [Google Scholar]
- Castro M, Mathur S, Hargreave F, Boulet LP, Xie F, Young J, et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011;184:1125–1132. doi: 10.1164/rccm.201103-0396OC. [DOI] [PubMed] [Google Scholar]
- Chen WC, Completo GC, Sigal DS, Crocker PR, Saven A, Paulson JC. In vivo targeting of B-cell lymphoma with glycan ligands of CD22. Blood. 2010;115:4778–4786. doi: 10.1182/blood-2009-12-257386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WC, Sigal DS, Saven A, Paulson JC. Targeting B lymphoma with nanoparticles bearing glycan ligands of CD22. Leuk Lymphoma. 2012;53:208–210. doi: 10.3109/10428194.2011.604755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JY, Song DJ, Pham A, Rosenthal P, Miller M, Dayan S, et al. Chronic OVA allergen challenged Siglec-F deficient mice have increased mucus, remodeling, and epithelial Siglec-F ligands which are up-regulated by IL-4 and IL-13. Respir Res. 2010;11:154. doi: 10.1186/1465-9921-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins BE, Blixt O, Han S, Duong B, Li H, Nathan JK, et al. High-affinity ligand probes of CD22 overcome the threshold set by cis ligands to allow for binding, endocytosis, and killing of B cells. J Immunol. 2006;177:2994–3003. doi: 10.4049/jimmunol.177.5.2994. [DOI] [PubMed] [Google Scholar]
- Cook ML, Bochner BS. Update on biological therapeutics for asthma. World Allergy Organ J. 2010;3:188–194. doi: 10.1097/WOX.0b013e3181e5ec5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker PR. Siglecs: sialic-acid-binding immunoglobulin-like lectins in cell-cell interactions and signalling. Curr Opin Struct Biol. 2002;12:609–615. doi: 10.1016/s0959-440x(02)00375-5. [DOI] [PubMed] [Google Scholar]
- Crocker PR. Siglecs in innate immunity. Curr Opin Pharmacol. 2005;5:431–437. doi: 10.1016/j.coph.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Crocker PR, Redelinghuys P. Siglecs as positive and negative regulators of the immune system. Biochem Soc Trans. 2008;36:1467–1471. doi: 10.1042/BST0361467. [DOI] [PubMed] [Google Scholar]
- Crocker PR, Clark EA, Filbin M, Gordon S, Jones Y, Kehrl JH, et al. Siglecs: a family of sialic-acid binding lectins. Glycobiology. 1998;8:v–vi. doi: 10.1093/oxfordjournals.glycob.a018832. [DOI] [PubMed] [Google Scholar]
- Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- Delputte PL, Van Gorp H, Favoreel HW, Hoebeke I, Delrue I, Dewerchin H, et al. Porcine sialoadhesin (CD169/Siglec-1) is an endocytic receptor that allows targeted delivery of toxins and antigens to macrophages. PLoS One. 2011;6:e16827. doi: 10.1371/journal.pone.0016827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunussi-Joannopoulos K, Hancock GE, Kunz A, Hegen M, Zhou XX, Sheppard BJ, et al. B-cell depletion inhibits arthritis in a collagen-induced arthritis (CIA) model, but does not adversely affect humoral responses in a respiratory syncytial virus (RSV) vaccination model. Blood. 2005;106:2235–2243. doi: 10.1182/blood-2004-11-4547. [DOI] [PubMed] [Google Scholar]
- Dyer KD, Moser JM, Czapiga M, Siegel SJ, Percopo CM, Rosenberg HF. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J Immunol. 2008;181:4004–4009. doi: 10.4049/jimmunol.181.6.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farid SS, Mirshafiey A, Razavi A. Siglec-8 and Siglec-F, the new therapeutic targets in asthma. Immunopharmacol Immunotoxicol. 2012 doi: 10.3109/08923973.2011.589453. http://dx.doi.org/10.3109/08923973.2011.589453. [DOI] [PubMed]
- Fiorina P, Vergani A, Dada S, Jurewicz M, Wong M, Law K, et al. Targeting CD22 reprograms B-cells and reverses autoimmune diabetes. Diabetes. 2008;57:3013–3024. doi: 10.2337/db08-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian S, Sonneck K, Czerny M, Hennersdorf F, Hauswirth AW, Buhring HJ, et al. Detection of novel leukocyte differentiation antigens on basophils and mast cells by HLDA8 antibodies. Allergy. 2006;61:1054–1062. doi: 10.1111/j.1398-9995.2006.01171.x. [DOI] [PubMed] [Google Scholar]
- Floyd H, Ni J, Cornish AL, Zeng Z, Liu D, Carter KC, et al. Siglec-8: a novel eosinophil-specific member of the immunoglobulin superfamily. J Biol Chem. 2000;275:861–866. doi: 10.1074/jbc.275.2.861. [DOI] [PubMed] [Google Scholar]
- Foussias G, Yousef GM, Diamandis EP. Molecular characterization of a Siglec-8 variant containing cytoplasmic tyrosine-based motifs, and mapping of the Siglec-8 gene. Biochem Biophys Res Commun. 2000;278:775–781. doi: 10.1006/bbrc.2000.3866. [DOI] [PubMed] [Google Scholar]
- Gao PS, Shimizu K, Grant AV, Rafaels N, Zhou LF, Hudson SA, et al. Polymorphisms in the sialic acid-binding immunoglobulin-like lectin-8 (Siglec-8) gene are associated with susceptibility to asthma. Eur J Hum Genet. 2010;18:713–719. doi: 10.1038/ejhg.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JP, Brummet ME, Myers AC, Na HJ, Rowland E, Schnaar RL, et al. Characterization of expression of glycan ligands for Siglec-F in normal mouse lungs. Am J Respir Cell Mol Biol. 2011;44:238–243. doi: 10.1165/rcmb.2010-0007OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartnell A, Steel J, Turley H, Jones M, Jackson DG, Crocker PR. Characterization of human sialoadhesin, a sialic acid binding receptor expressed by resident and inflammatory macrophage populations. Blood. 2001;97:288–296. doi: 10.1182/blood.v97.1.288. [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Kerr S, Jellusova J, Zhang J, Weisel F, Wellmann U, et al. Siglec-G is a B1 cell-inhibitory receptor that controls expansion and calcium signaling of the B1 cell population. Nat Immunol. 2007;8:695–704. doi: 10.1038/ni1480. [DOI] [PubMed] [Google Scholar]
- Hudson SA, Bovin N, Schnaar RL, Crocker PR, Bochner BS. Eosinophil-selective binding and pro-apoptotic effect in vitro of a synthetic Siglec-8 ligand, polymeric 6′-sulfated sialyl Lewis X. J Pharmacol Exp Ther. 2009;330:608–612. doi: 10.1124/jpet.109.152439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson SA, Herrmann H, Du J, Cox P, Haddad el B, Butler B, et al. Developmental, malignancy-related, and cross-species analysis of eosinophil, mast cell, and basophil siglec-8 expression. J Clin Immunol. 2011;31:1045–1053. doi: 10.1007/s10875-011-9589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara Y, Ikehara SK, Paulson JC. Negative regulation of T cell receptor signaling by Siglec-7 (p70/AIRM) and Siglec-9. J Biol Chem. 2004;279:43117–43125. doi: 10.1074/jbc.M403538200. [DOI] [PubMed] [Google Scholar]
- Jurcic JG. What happened to anti-CD33 therapy for acute myeloid leukemia? Curr Hematol Malig Rep. 2012;7:65–73. doi: 10.1007/s11899-011-0103-0. [DOI] [PubMed] [Google Scholar]
- Kano G, Bochner BS, Zimmermann N. Mechanisms involved in IL-5 enhancement of Siglec-8-induced eosinophil apoptosis: Role for reactive oxygen species (ROS)-enhanced MEK/ERK activation. J Allergy Clin Immunol. 2012;129:AB210. doi: 10.1016/j.jaci.2013.03.024. (abstr.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearley J, Jones C, McMillan SJ, Cromie K, Crocker PR, Lloyd CM. Anti-Siglec-F antibody treatment during allergen-induced airway inflammation reduces eosinophil numbers but has no effect on airway hyperreactivity in vivo. Am J Respir Crit Care Med. 2007;175:A690. (abstr.) [Google Scholar]
- Kikly KK, Bochner BS, Freeman S, Tan KB, Gallagher KT, D’Alessio K, et al. Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells and basophils. J Allergy Clin Immunol. 2000;105:1093–1100. doi: 10.1067/mai.2000.107127. [DOI] [PubMed] [Google Scholar]
- Kimura N, Ohmori K, Miyazaki K, Izawa M, Matsuzaki Y, Yasuda Y, et al. Human B-lymphocytes express alpha2-6-sialylated 6-sulfo-N-acetyllactosamine serving as a preferred ligand for CD22/Siglec-2. J Biol Chem. 2007;282:32200–32207. doi: 10.1074/jbc.M702341200. [DOI] [PubMed] [Google Scholar]
- Kiwamoto T, Brummet ME, Na HJ, Hudson SA, Bochner BS. Characterization of the synthesis of the eosinophil Siglec-F glycan ligand by mouse airway epithelial cells. J Allergy Clin Immunol. 2011a;127:AB204. (abstr.) [Google Scholar]
- Kiwamoto T, Na HJ, Brummet ME, Finn MG, Smith DF, Hong V, et al. Use of Siglec-F-Fc and a novel IgY antibody recognizing 6′-sulfated-sialyl Lewis X to identify endogenous lung ligands for Siglec-F. Glycobiology. 2011b;21:1484. (abstr.) [Google Scholar]
- Kiwamoto T, Na H-J, Brummet ME, Finn MG, Smith DF, Bochner BS. Similarities and differences between lung ligands for mouse Siglec-F and human Siglec-8. J Allergy Clin Immunol. 2012;129:AB49. (abstr.) [Google Scholar]
- Kreitman RJ, Pastan I. Immunotoxins in the treatment of hematologic malignancies. Curr Drug Targets. 2006;7:1301–1311. doi: 10.2174/138945006778559139. [DOI] [PubMed] [Google Scholar]
- Kreitman RJ, Tallman MS, Robak T, Coutre S, Wilson WH, Stetler-Stevenson M, et al. Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J Clin Oncol. 2012;30:1822–1828. doi: 10.1200/JCO.2011.38.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard JP, Goldenberg DM. Preclinical and clinical evaluation of epratuzumab (anti-CD22 IgG) in B-cell malignancies. Oncogene. 2007;26:3704–3713. doi: 10.1038/sj.onc.1210370. [DOI] [PubMed] [Google Scholar]
- Liu SM, Xavier R, Good KL, Chtanova T, Newton R, Sisavanh M, et al. Immune cell transcriptome datasets reveal novel leukocyte subset-specific genes and genes associated with allergic processes. J Allergy Clin Immunol. 2006;118:496–503. doi: 10.1016/j.jaci.2006.04.040. [DOI] [PubMed] [Google Scholar]
- Lock K, Zhang J, Lu J, Lee SH, Crocker PR. Expression of CD33-related siglecs on human mononuclear phagocytes, monocyte-derived dendritic cells and plasmacytoid dendritic cells. Immunobiology. 2004;209:199–207. doi: 10.1016/j.imbio.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Loschko J, Heink S, Hackl D, Dudziak D, Reindl W, Korn T, et al. Antigen targeting to plasmacytoid dendritic cells via Siglec-H inhibits Th cell-dependent autoimmunity. J Immunol. 2011;187:6346–6356. doi: 10.4049/jimmunol.1102307. [DOI] [PubMed] [Google Scholar]
- Magesh S, Ando H, Tsubata T, Ishida H, Kiso M. High-affinity ligands of Siglec receptors and their therapeutic potentials. Curr Med Chem. 2011;18:3537–3550. doi: 10.2174/092986711796642580. [DOI] [PubMed] [Google Scholar]
- McMillan SJ, Crocker PR. CD33-related sialic-acid-binding immunoglobulin-like lectins in health and disease. Carbohydr Res. 2008;343:2050–2056. doi: 10.1016/j.carres.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Mesch S, Lemme K, Wittwer M, Koliwer-Brandl H, Schwardt O, Kelm S, et al. From a library of MAG antagonists to nanomolar CD22 ligands. ChemMedChem. 2012;7:134–143. doi: 10.1002/cmdc.201100407. [DOI] [PubMed] [Google Scholar]
- Munitz A, Levi-Schaffer F. Inhibitory receptors on eosinophils: a direct hit to a possible Achilles heel? J Allergy Clin Immunol. 2007;119:1382–1387. doi: 10.1016/j.jaci.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Na HJ, Hudson SA, Bochner BS. IL-33 enhances Siglec-8 mediated apoptosis of human eosinophils. Cytokine. 2011;57:169–174. doi: 10.1016/j.cyto.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DH, Hurtado-Ziola N, Gagneux P, Varki A. Loss of Siglec expression on T lymphocytes during human evolution. Proc Natl Acad Sci U S A. 2006;103:7765–7770. doi: 10.1073/pnas.0510484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003;101:5014–5020. doi: 10.1182/blood-2002-10-3058. [DOI] [PubMed] [Google Scholar]
- Nutku E, Hudson SA, Bochner BS. Mechanism of Siglec-8-induced human eosinophil apoptosis: role of caspases and mitochondrial injury. Biochem Biophys Res Commun. 2005;336:918–924. doi: 10.1016/j.bbrc.2005.08.202. [DOI] [PubMed] [Google Scholar]
- Nutku-Bilir E, Hudson SA, Bochner BS. Interleukin-5 priming of human eosinophils alters Siglec-8 mediated apoptosis pathways. Am J Respir Cell Mol Biol. 2008;38:121–124. doi: 10.1165/rcmb.2007-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober C, Cox NJ, Abney M, Di Rienzo A, Lander ES, Changyaleket B, et al. Genome-wide search for asthma susceptibility loci in a founder population. Hum Mol Genet. 1998;7:1393–1398. doi: 10.1093/hmg/7.9.1393. [DOI] [PubMed] [Google Scholar]
- Ogura M, Hatake K, Ando K, Tobinai K, Tokushige K, Ono C, et al. Phase I study of anti-CD22 immunoconjugate inotuzumab ozogamicin plus rituximab in relapsed/refractory B-cell non-Hodgkin lymphoma. Cancer Sci. 2012;103:933–938. doi: 10.1111/j.1349-7006.2012.02241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly MK, Paulson JC. Siglecs as targets for therapy in immune-cell-mediated disease. Trends Pharmacol Sci. 2009;30:240–248. doi: 10.1016/j.tips.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly MK, Collins BE, Han S, Liao L, Rillahan C, Kitov PI, et al. Bifunctional CD22 ligands use multimeric immunoglobulins as protein scaffolds in assembly of immune complexes on B cells. J Am Chem Soc. 2008;130:7736–7745. doi: 10.1021/ja802008q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly MK, Tian H, Paulson JC. CD22 is a recycling receptor that can shuttle cargo between the cell surface and endosomal compartments of B cells. J Immunol. 2011;186:1554–1563. doi: 10.4049/jimmunol.1003005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patnode ML, Abraham WM, Crocker PR, Rosen SD. Goblet cells express carbohydrate ligands for Siglec-8 and Siglec-F. Glycobiology. 2009;19:1309. (abstr.) [Google Scholar]
- Paulson JC, Macauley MS, Kawasaki N. Siglecs as sensors of self in innate and adaptive immune responses. Ann N Y Acad Sci. 2012;1253:37–48. doi: 10.1111/j.1749-6632.2011.06362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard JA, Alonzo TA, Loken M, Gerbing RB, Ho PA, Bernstein ID, et al. Correlation of CD33 expression level with disease characteristics and response to gemtuzumab ozogamicin containing chemotherapy in childhood AML. Blood. 2012;119:3705–3711. doi: 10.1182/blood-2011-12-398370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell LD, Varki A. I-type lectins. J Biol Chem. 1995;270:14243–14246. doi: 10.1074/jbc.270.24.14243. [DOI] [PubMed] [Google Scholar]
- Rinaldi S, Brennan KM, Goodyear CS, O’Leary C, Schiavo G, Crocker PR, et al. Analysis of lectin binding to glycolipid complexes using combinatorial glycoarrays. Glycobiology. 2009;19:789–796. doi: 10.1093/glycob/cwp049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salicru AN, Hermann H, Hudson SA, Steinke JW, Borish L, Valent P, et al. Analysis of eosinophil, mast cell, and basophil Siglec-8 expression on human cell lines and hematologic malignancies. J Allergy Clin Immunol. 2012;129:AB118. (abstr.) [Google Scholar]
- Scott CJ, Marouf WM, Quinn DJ, Buick RJ, Orr SJ, Donnelly RF, et al. Immunocolloidal targeting of the endocytotic siglec-7 receptor using peripheral attachment of siglec-7 antibodies to poly(lactide-co-glycolide) nanoparticles. Pharm Res. 2008;25:135–146. doi: 10.1007/s11095-007-9400-7. [DOI] [PubMed] [Google Scholar]
- Shan D, Press OW. Constitutive endocytosis and degradation of CD22 by human B cells. J Immunol. 1995;154:4466–4475. [PubMed] [Google Scholar]
- Shik D, Munitz A. Regulation of allergic inflammatory responses by inhibitory receptors. Clin Exp Allergy. 2010;40:700–709. doi: 10.1111/j.1365-2222.2010.03501.x. [DOI] [PubMed] [Google Scholar]
- Song DJ, Cho JY, Lee SY, Miller M, Rosenthal P, Soroosh P, et al. Anti-Siglec-F antibody reduces allergen-induced eosinophilic inflammation and airway remodeling. J Immunol. 2009a;183:5333–5341. doi: 10.4049/jimmunol.0801421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song DJ, Cho JY, Miller M, Strangman W, Zhang M, Varki A, et al. Anti-Siglec-F antibody inhibits oral egg allergen induced intestinal eosinophilic inflammation in a mouse model. Clin Immunol. 2009b;131:157–169. doi: 10.1016/j.clim.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spergel JM, Rothenberg ME, Collins MH, Furuta GT, Markowitz JE, Fuchs G, III, et al. Reslizumab in children and adolescents with eosinophilic esophagitis: Results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2011;129:456–463. doi: 10.1016/j.jaci.2011.11.044. [DOI] [PubMed] [Google Scholar]
- Stevens WW, Kim TS, Pujanauski LM, Hao X, Braciale TJ. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J Immunol Methods. 2007;327:63–74. doi: 10.1016/j.jim.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi H, Fukaya T, Eizumi K, Sato Y, Sato K, Shibazaki A, et al. Plasmacytoid dendritic cells are crucial for the initiation of inflammation and T cell immunity in vivo. Immunity. 2011;35:958–971. doi: 10.1016/j.immuni.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Takagi S, Saito Y, Hijikata A, Tanaka S, Watanabe T, Hasegawa T, et al. Membrane-bound human SCF/KL promotes in vivo human hematopoietic engraftment and myeloid differentiation. Blood. 2012;119:2768–2777. doi: 10.1182/blood-2011-05-353201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateno H, Crocker PR, Paulson JC. Mouse Siglec-F and human Siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6′-sulfo-sialyl Lewis X as a preferred glycan ligand. Glycobiology. 2005;15:1125–1135. doi: 10.1093/glycob/cwi097. [DOI] [PubMed] [Google Scholar]
- Tateno H, Li H, Schur MJ, Bovin N, Crocker PR, Wakarchuk WW, et al. Distinct endocytic mechanisms of CD22 (Siglec-2) and Siglec-F reflect roles in cell signaling and innate immunity. Mol Cell Biol. 2007;27:5699–5710. doi: 10.1128/MCB.00383-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu X, LaVallee T, Lechleider R. CD22 as a target for cancer therapy. J Exp Ther Oncol. 2011;9:241–248. [PubMed] [Google Scholar]
- Varki A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature. 2007;446:1023–1029. doi: 10.1038/nature05816. [DOI] [PubMed] [Google Scholar]
- Varki A. Natural ligands for CD33-related Siglecs? Glycobiology. 2009;19:810–812. doi: 10.1093/glycob/cwp063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. Colloquium paper: uniquely human evolution of sialic acid genetics and biology. Proc Natl Acad Sci U S A. 2010;107(Suppl 2):8939–8946. doi: 10.1073/pnas.0914634107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A, Angata T. Siglecs — the major sub-family of I-type lectins. Glycobiology. 2006;16:1R–27R. doi: 10.1093/glycob/cwj008. [DOI] [PubMed] [Google Scholar]
- Vely F, Vivier E. Conservation of structural features reveals the existence of a large family of inhibitory cell surface receptors and noninhibitory/activatory counterparts. J Immunol. 1997;159:2075–2077. [PubMed] [Google Scholar]
- Venanzi S, Malerba G, Galavotti R, Lauciello MC, Trabetti E, Zanoni G, et al. Linkage to atopy on chromosome 19 in north-eastern Italian families with allergic asthma. Clin Exp Allergy. 2001;31:1220–1224. doi: 10.1046/j.1365-2222.2001.01132.x. [DOI] [PubMed] [Google Scholar]
- von Gunten S, Bochner BS. Basic and clinical immunology of Siglecs. Ann N Y Acad Sci. 2008;1143:61–82. doi: 10.1196/annals.1443.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gunten S, Bochner BS. Expression and function of Siglec-8 in human eosinophils, basophils and mast cells. In: Pawankar R, Holgate S, Rosenwasser LJ, editors. Allergy frontiers: Classification and pathomechanisms. Tokyo: Springer; 2009. pp. 297–313. [Google Scholar]
- von Gunten S, Vogel M, Schaub A, Stadler BM, Miescher S, Crocker PR, et al. Intravenous immunoglobulin preparations contain anti-Siglec-8 autoanti-bodies. J Allergy Clin Immunol. 2007;119:1005–1011. doi: 10.1016/j.jaci.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Walter RB, Appelbaum FR, Estey EH, Bernstein ID. Acute myeloid leukemia stem cells and CD33-targeted immunotherapy. Blood. 2012;119:6198–6208. doi: 10.1182/blood-2011-11-325050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterstein C, Trotter J, Kramer-Albers EM. Distinct endocytic recycling of myelin proteins promotes oligodendroglial membrane remodeling. J Cell Sci. 2008;121:834–842. doi: 10.1242/jcs.022731. [DOI] [PubMed] [Google Scholar]
- Yokoi H, Myers A, Matsumoto K, Crocker PR, Saito H, Bochner BS. Alteration and acquisition of Siglecs during in vitro maturation of CD34+ progenitors into human mast cells. Allergy. 2006;61:769–776. doi: 10.1111/j.1398-9995.2006.01133.x. [DOI] [PubMed] [Google Scholar]
- Yokoi H, Choi OH, Hubbard W, Lee H-S, Canning BJ, Lee HH, et al. Inhibition of FcεRI-dependent mediator release and calcium flux from human mast cells by Siglec-8 engagement. J Allergy Clin Immunol. 2008;121:499–505. doi: 10.1016/j.jaci.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Zaccai NR, May AP, Robinson RC, Burtnick LD, Crocker PR, Brossmer R, et al. Crystallographic and in silico analysis of the sialoside-binding characteristics of the Siglec sialoadhesin. J Mol Biol. 2007;365:1469–1479. doi: 10.1016/j.jmb.2006.10.084. [DOI] [PubMed] [Google Scholar]
- Zhang JQ, Biedermann B, Nitschke L, Crocker PR. The murine inhibitory receptor mSiglec-E is expressed broadly on cells of the innate immune system whereas mSiglec-F is restricted to eosinophils. Eur J Immunol. 2004;34:1175–1184. doi: 10.1002/eji.200324723. [DOI] [PubMed] [Google Scholar]
- Zhang J, Raper A, Sugita N, Hingorani R, Salio M, Palmowski MJ, et al. Characterization of Siglec-H as a novel endocytic receptor expressed on murine plasmacytoid dendritic cell precursors. Blood. 2006;107:3600–3608. doi: 10.1182/blood-2005-09-3842. [DOI] [PubMed] [Google Scholar]
- Zhang M, Angata T, Cho JY, Miller M, Broide DH, Varki A. Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood. 2007;109:4280–4287. doi: 10.1182/blood-2006-08-039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann N, McBride ML, Yamada Y, Hudson SA, Jones C, Cromie KD, et al. Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy. 2008;63:1156–1163. doi: 10.1111/j.1398-9995.2008.01709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]





