Abstract
No-carrier-added [18F]fluoroarenes were synthesized through the radiofluorination of diaryl sulfoxides with [18F]fluoride ion. Diaryl sulfoxides bearing a para electron-withdrawing substituent readily gave the corresponding 4-[18F]fluoroarenes in high RCYs. This process broadens the scope for preparing novel 18F-labeling synthons and PET radiotracers.
With the growth of positron emission tomography (PET)1 as a powerful modality for biomedical research has emerged a corresponding need for the development of more efficient methods for producing the required positron-emitting radiotracers. Many of these radiotracers are required to be labeled rapidly with fluorine-18, which has a short half-life of 109.7 min. [18F]Fluoride ion has become the preferred source of this radionuclide because it can be prepared in very high activities and in high no-carrier-added (NCA) specific activities (several Ci per µmol of carrier) from a moderate energy cyclotron with the 18O(p,n)18F reaction on [18O]water.2 For many radiotracers, labeling at an aryl position provides for requisite metabolic stability and especially resistance to undesirable defluorination.
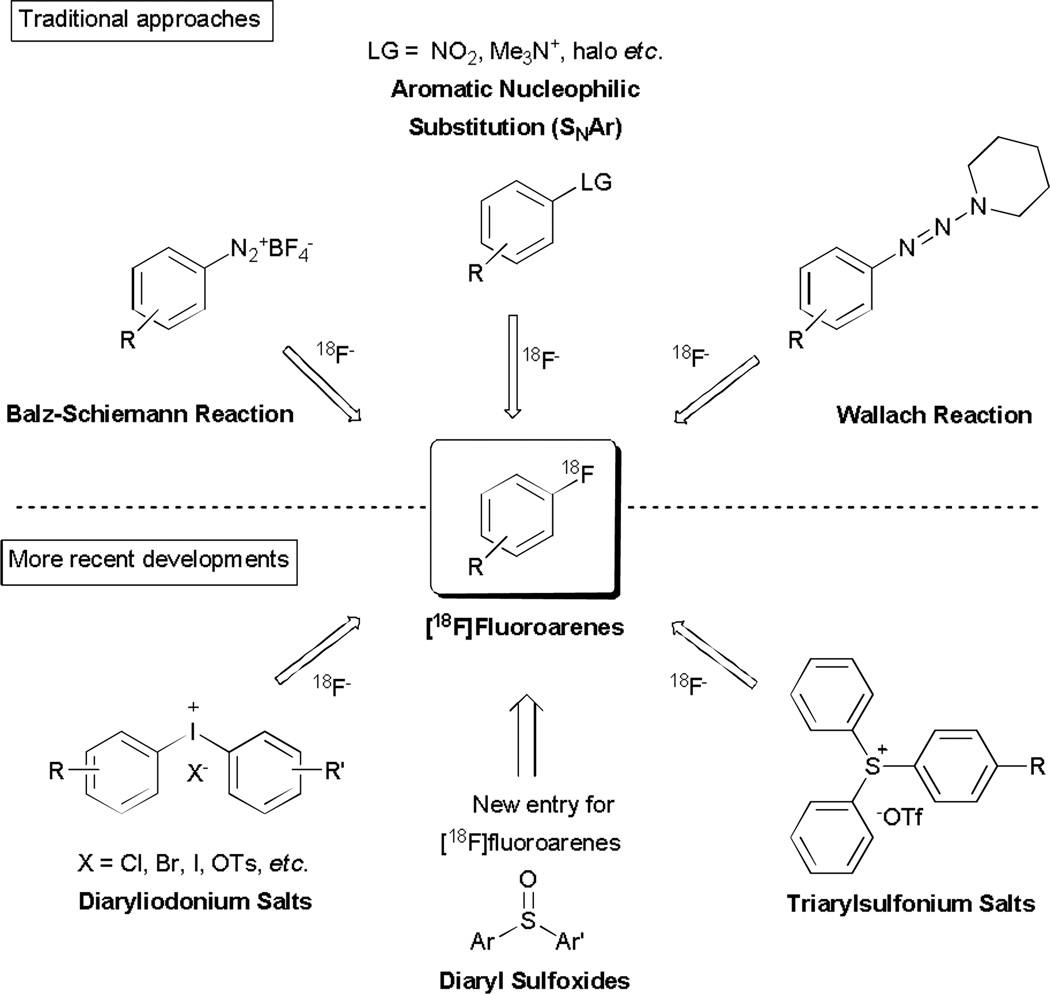
Several methods have been used to produce [18F]fluoroarenes from [18F]fluoride ion (Fig. 1).3 These include the Balz-Schiemann reaction, the Wallach reaction, classical aromatic nucleophilic substitution reactions (SNAr), and the fluorination of hypervalent compounds, such as diaryliodonium and triarylsulfonium salts. The Balz–Schiemann reaction has the disadvantage of low radiochemical yield (RCY; theoretical maximum ~ 25%) and unavoidable dilution of specific radioactivity from tetrafluoroborate anion in the precursor. The Wallach reaction may deliver high specific radioactivity but often gives very low radiochemical yields on complex substrates, and like the Balz-Schiemann reaction is now seldom used. Classical SNAr is the most widely established method for preparing [18F]fluoroarenes as PET radiotracers with high specific radioactivity. High radiochemical yields can be achieved in homoarenes with nitro or trimethylamino substituents as the leaving group, provided that the aryl ring is activated with an electron-withdrawing group in ortho or para position.4 This method can also be used to prepare 18F-labeled heteroarenes, such as 2-[18F]fluoropyridines and 4-[18F]fluoropyridines.5 Moreover, the radiofluorination of diaryliodonium salts has now been shown to be effective to label homoarenes and pyridines in any desirable position, irrespective of needs for electron-withdrawing groups.6,7 This method is now finding applications for preparing otherwise difficult to access radiotracers.8 Recently, hypervalent triarylsulfonium salts have also been found useful for preparing [18F]fluoroarenes.9
Fig. 1.
Various radiosynthetic approaches to [18F]fluoroarenes.
As a part of our ongoing interest in the use of hypervalent precursors for radiofluorination, we were attracted to investigate the radiofluorination of diaryl sulfoxides. Here, we show that many diaryl sulfoxides readily react with NCA [18F]fluoride ion to give [18F]fluoroarenes in high RCY.
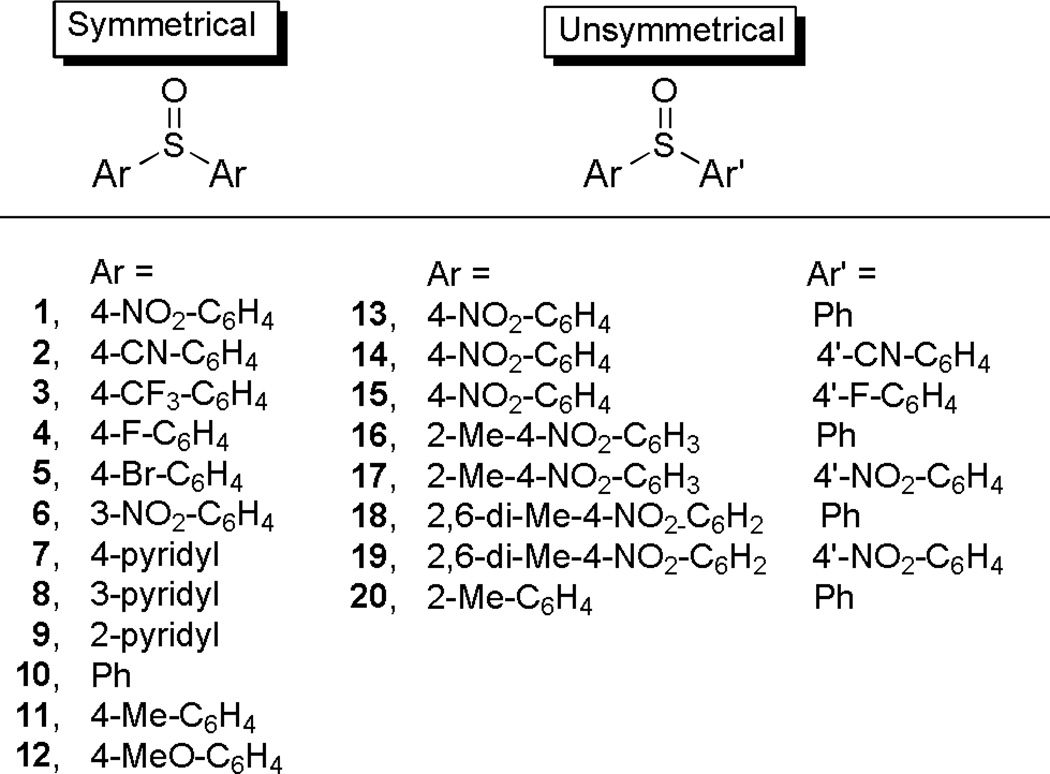
Substituted diaryl sulfoxides (Fig 2) were purchased (10 and 11) or readily synthesized, either through the oxidation of the corresponding diaryl sulfides with m-chloroperbenzoic acid (1–3, 6–9 and 13–20) or by treatment of an appropriate arene with thionyl chloride (4,5,12), and they were obtained in moderate to good yields;10 see ESI† for full synthesis details.
Fig. 2.
Diaryl sulfoxide precursors used in this study.
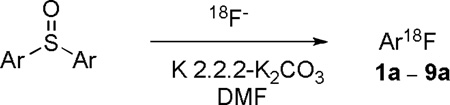
A commercial microfluidic apparatus (Nanotek; Advion, Ithaca, NY) was used as an expedient platform for testing the amenability of the available series of diaryl sulfoxides to radiofluorination. Detailed descriptions of this apparatus and of its operation may be found in our previous publication.7 Dry NCA no-carrier-added [18F]fluoride ion-kryptofix 2.2.2 complex (18F−-K 2.2.2- K+) and the test diaryl sulfoxide were separately dissolved in DMF. Each solution was infused into the fused silica micro-reactor (internal volume: 31 µL) of the apparatus at a fixed equal rate (4−10 µL/min) and at a set temperature. The resulting reactor effluent was quenched with aqueous MeCN and analyzed directly with reverse phase HPLC equipped with a radioactivity detector. Reaction conditions were varied to search for optimal RCYs (see ESI†, Fig. S1, for radiochromatograms from the radiofluorination of 3 between 100 and 200 °C). Because adsorption of [18F]fluoride ion onto reaction vessel walls is commonly encountered in radiofluorination reactions,11 the recoveries of radioactivity from the apparatus were also measured; these were found to be 75 ± 11% (mean ± SD, n = 16).
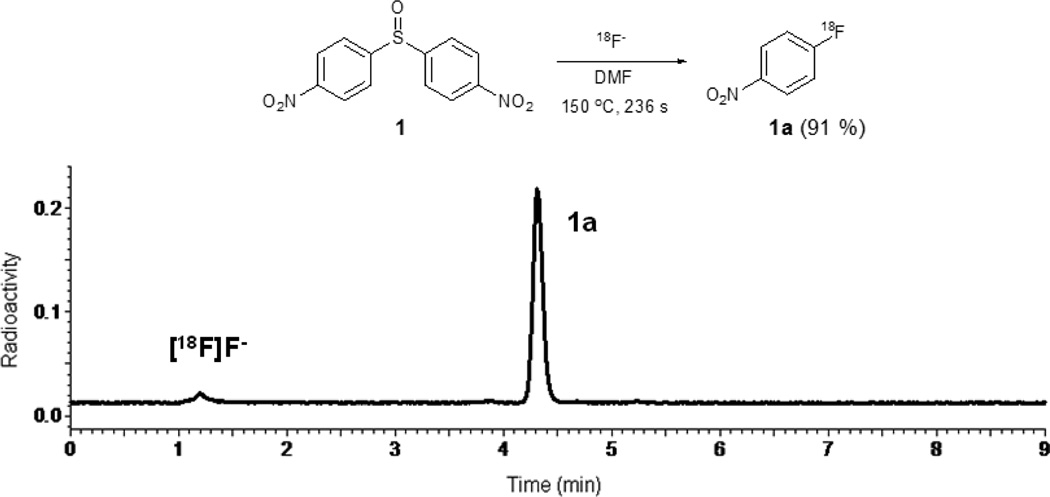
[18F]Fluoroarenes were rapidly obtained from these reactions. In the radiofluorination of symmetrical diaryl sulfoxides, where both aryl rings carried a p-electron withdrawing group (Table 1, entries 1−5), p-substituted [18F]fluorobenzenes were produced in moderate to excellent RCYs, except in the case of the substrate having weakly electron-withdrawing p-bromo substituents (entry 5). A very high RCY of 4-[18F]nitrofluorobenzene was obtained from 1 under quite mild reaction conditions (entry 1). Under these conditions there was no chromatographic evidence of substitution of the nitro group by [18F]fluoride ion (Fig. 3). This result is quite remarkable given the well-known strong nucleofugacity of the nitro group in SNAr reactions, including those with [18F]fluoride ion.4 Similarly, high RCYs were obtained from symmetrical substrates bearing other electron-withdrawing substituents, such as p-cyano or p-trifluoromethyl (entries 2 and 3). The substrate bearing p-fluoro substituents gave only a low yield of [18F]p-difluorobenzene (entry 4), and that bearing m-nitro groups gave almost no yield of 3-[18F]fluoronitrobenzene (entry 6).
Table 1.
RCYs of NCA [18F]fluoroarenes from the radiofluorination of symmetrical diaryl sulfoxides in DMF.
 | ||||
|---|---|---|---|---|
| Entry | Substratea | Conditionsb |
RCYc of Ar18F (%) |
|
| Temp (°C) |
Timed (s) |
|||
| 1 | 1 | 150 | 236 | 1a, 91 (76) |
| 2 | 2 | 200 | 236 | 2a, 82 (66) |
| 3 | 3 | 200 | 251 | 3a, 66 (59) |
| 4 | 4 | 160 | 236 | 4a, 21 (11) |
| 5 | 5 | 200 | 188 | 5a, 7 (5) |
| 6 | 6 | 200 | 188 | 6a, 2 (1) |
| 7 | 7 | 180 | 188 | 7a, 94 (72) |
| 8 | 8 | 200 | 236 | 8a, 4 (3) |
| 9 | 9 | 200 | 188 | 9a, 68 (44) |
| 10 | 10 | 200 | 236 | 10a, 0 |
| 11 | 11 | 200 | 236 | 11a, 0 |
| 12 | 12 | 200 | 236 | 12a, 0 |
5 mM in DMF.
Conditions giving the highest RCYs among 8−12 different runs, except for entries 10−12 where the most extreme of the tested conditions are given.
RCYs are those determined with HPLC. RCYs given in parentheses are with correction for radioactivity adsorption in the apparatus.
Estimated residence time in the micro-reactor.
Fig. 3.
Radio-HPLC chromatogram of the reaction mixture from the radiofluorination of 4,4’-dinitrodiphenyl sulfoxide.
2- and 4-[18F]fluoropyridines were obtained in high RCYs from the respective dipyridinyl sulfoxides (Table 1, entries 7 and 9). However, only a low RCY of 3-[18F]fluoropyridine was obtained from 3,3’-dipyridinyl sulfoxide (entry 8). No radiofluorinated arenes were obtained in the absence of a substituent (entry 10), nor when methyl or methoxy substituents were in p-position (entries 11 and 12). Altogether, these results indicate that the electronic features needed in the diaryl sulfoxides for radiofluorination to proceed efficiently resemble those required for classical SNAr reactions.
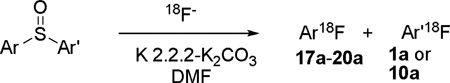
Unsymmetrical diaryl sulfoxides also gave [18F]fluoroarenes. When one aryl ring carried a p-nitro substituent and the other no substituent, 4-[18F]fluoronitrobenzene was obtained in high RCY with no accompanying [18F]fluorobenzene (Table 2, entry 1). When one aryl ring carried a p-nitro group and the other a different electron-withdrawing substituent, formation of 4-[18F]fluoronitrobenzene was dominant (entries 2 and 3). When a single ring was phenyl and the other had one or two o-methyl substituents in addition to a p-nitro group, as in substrates 16 and 18 respectively, the incorporation of fluorine-18 into the substituted ring was again impressively high (entries 4 and 6). Futhermore, the presence of these o-methyl groups had little influence on product selectivity when both rings carried p-nitro groups, as in substrates 17 and 19 (entries 5 and 7). Therefore, these reactions do not display an obvious ‘ortho effect’ as encountered in the radiofluorinations of diaryliodonium salts.7 Generally, for unsymmetrical diaryl sulfoxides bearing at least one p-nitro substituent, the aggregate incorporation of fluorine-18 into nitrofluorobenzenes was very high, at 61 to 93% (entries 1−7). Again, in the absence of a p-electron-withdrawing group on one of the aryl rings, no radiofluorination occurred in the 80 to 200 ° C temperature range (entry 8).
Table 2.
RCYs of [18F]fluoroarenes from the NCA radiofluorination of unsymmetrical diaryl sulfoxides in DMF.
 | |||||
|---|---|---|---|---|---|
| Entry | Substratea | Conditionsb |
RCY (%)c |
||
| Temp (°C) |
Time (s)d |
Ar18F | Ar’18F | ||
| 1 | 13 | 130 | 224 | 1a, 64 (56) | 10a 0 (0) |
| 2 | 14 | 120 | 236 | 1a, 93 (89) | 2a, <1 (<1) |
| 3 | 15 | 150 | 314 | 1a, 76 (68) | 4a, 0 (0) |
| 4 | 16 | 200 | 236 | 16a, 73 (55) | 10a 0 (0) |
| 5 | 17 | 140 | 314 | 17a, 46 (32) | 1a, 43 (25) |
| 6 | 18 | 200 | 236 | 18a, 61 (43) | 10a 0 (0) |
| 7 | 19 | 140 | 236 | 19a, 36 (20) | 1a, 45 (35) |
| 8 | 20 | 200 | 236 | 20a 0 (0) | 10a 0 (0) |
5 mM in DMF.
Conditions giving highest RCYs among 8−12 different runs, except for entry 8 where the most extreme tested condition is given.
RCYs are those determined with HPLC. RCYs given in parentheses are with correction for radioactivity adsorption in the apparatus.
Estimated residence time in the micro-reactor.
In view of these encouraging results in the microfluidic apparatus, we tested whether they might transfer to more conventional batch reactions. In almost all cases, comparable RCYs of the expected [18F]fluoroarenes were obtained either in DMF or, for higher temperature, in DMSO (ESI, Table S1).
The mechanistic basis of these potentially useful reactions is unclear. Reactions of diaryl sulfoxides with anionic nucleophiles have received scant attention, but we do note previous studies of their reactions with organolithium and Grignard reagents that provide evidence for these reactions to proceed through nucleophilic attack at sulphur resulting in ligand exchange.12 Similar processes might be at work in these radiofluorination reactions, and could for example explain retention of the p-nitro group.
In summary, we report a new radiosynthetic method for producing NCA [18F]fluoroarenes based on the reactions of diaryl sulfoxides bearing p-electron-withdrawing groups with [18F]fluoride ion. These reactions are relatively mild, rapid and efficient and should prove useful in PET radiotracer synthesis, especially where it is desirable to retain a potentially nucleofugic aryl nitro group. The reported TSPO 18 kDa radiotracer, [18F]PK 14105 ([18F](R)-N-sec-butyl-1-(2-fluoro-5-nitrophenyl)-N-methylisoquinoline-3-carboxamide), provides a specific example where this new labeling method might prove useful.13 Moreover, these reactions may also provide alternative access to potentially useful labeling synthons, such as 4-[18F]cyanofluorobenzene.
Supplementary Material
Acknowledgments
This work was funded by the Intramural Research Program of the National Institutes of Health (NIMH). We are grateful to the NIH clinical PET center for supplying fluorine-18.
Footnotes
Electronic Supplementary Information (ESI) available: Experimental details, 1H and 13C NMR spectra of prepared diaryl sulfoxides, and selected radio HPLC radiochromatograms. See DOI: 10.1039/b000000x/
Notes and references
- 1.Phelps ME. Proc. Natl. Acad. Sci. U. S. A. 2000;97:9226–9233. doi: 10.1073/pnas.97.16.9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guillaume M, Luxen A, Nebeling B, Argentini M, Clark JC, Pike VW. Appl. Radiat. Isot. 1992;42:749–762. [Google Scholar]
- 3.(a) Coenen HH. ch. 2. In: Schubiger PA, Lehmann L, Friebe M, editors. PET Chemistry the Driving Force in Molecular Imaging. Heidelberg: Springer; 2007. pp. 15–50. [Google Scholar]; (b) Cai L, Lu S, Pike VW. Eur. J. Org. Chem. 2008:2853–2873. [Google Scholar]
- 4.(a) Angelini G, Speranza M, Wolf AP, Shiue CY, Fowler JS, Watanabe M. J. Label. Compd. Radiopharm. 1989;27:823–833. [Google Scholar]; (b) Haka HS, Kilbourn MR, Watkins GL, Toorongian SA. J. Label. Compd. Radiopharm. 1989;27:823–833. [Google Scholar]
- 5.(a) Dolci L, Dollé F, Jubeau S, Vaufrey F, Crouzel C. J. Labelled Compd. Radiopharm. 1999;42:975–985. [Google Scholar]; (b) Dollé F. Curr. Pharm. Des. 2005;11:3221–3235. doi: 10.2174/138161205774424645. [DOI] [PubMed] [Google Scholar]; (c) Dollé F. ch. 5. In: Schubiger PA, Lehmann L, Friebe M, editors. PET Chemistry the Driving Force in Molecular Imaging. Heidelberg: Springer; 2007. pp. 113–157. [Google Scholar]
- 6.(a) Pike VW, Aigbirhio FI. J. Chem. Soc., Chem. Commun. 1995:2215–2216. [Google Scholar]; (b) Shah A, Pike VW, Widdowson DA. J. Chem. Soc., Perkin Trans. 1. 1998:2043–2046. [Google Scholar]; (c) Ross L, Ermert J, Hocke C, Coenen HH. J. Am. Chem. Soc. 2007;129:8018–8025. doi: 10.1021/ja066850h. [DOI] [PubMed] [Google Scholar]; (d) Chun J-H, Lu S, Pike VW. Eur. J. Org. Chem. 2011:4439–4447. doi: 10.1002/ejoc.201100382. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Chun J-H, Pike VW. Chem. Commun. 2012:9921–9923. doi: 10.1039/c2cc35005j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chun J-H, Lu S, Lee Y-S, Pike VW. J. Org. Chem. 2010;75:3332–3338. doi: 10.1021/jo100361d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Zhang MR, Kumata K, Suzuki K. Tetrahedron Lett. 2007;48:8632–8635. [Google Scholar]; (b) Telu S, Chun J-H, Siméon FG, Lu S, Pike VW. Org. Biomol. Chem. 2011;9:9921–9923. doi: 10.1039/c1ob05555k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mu L, Fischer CR, Holland JP, Becaud J, Schubiger PA, Schibli R, Ametamey SM, Graham K, Stellfeld T, Dinkelborg LM, Lehmann L. Eur. J. Org. Chem. 2012:889–892. [Google Scholar]
- 10.(a) Collins SG, Maguire AR. Sci. Synthesis. 2007;31a:907–948. [Google Scholar]; (b) Paquette LA, Carr RVC. Org. Synth. Coll. Vol VII. 1990:453. [Google Scholar]; (c) Granoth J, Kalir A, Pelah Z. J. Chem. Soc. C. 1969:2424–2425. [Google Scholar]; (d) Olah G, Marinez ER, Prakash GKS. Synlett. 1999:1397–1398. [Google Scholar]; (e) Shockravi A, Rostami E, Zakeri M. Iran J. Chem. Chem. Eng. 2005;24:47–52. [Google Scholar]
- 11.Brodack JW, Kilbourn MR, Welch MJ, Katzenellenbogen JA. Appl. Radiat. Isot. 1986;37:217–221. doi: 10.1016/0883-2889(86)90174-7. [DOI] [PubMed] [Google Scholar]
- 12.(a) Furukawa N, Ogawa S, Matsumura K, Fujihara H. J. Org. Chem. 1991;56:6341–6348. [Google Scholar]; (b) Oae S, Kawai T, Furukawa N, Iwasaki F. J. Chem. Soc., Perkin Trans 2. 1987:405–411. [Google Scholar]; (c) Oae S, Uchida Y. Acc. Chem. Res. 1991;24:202–208. [Google Scholar]
- 13.(a) Pascali C, Luthra SK, Pike VW, Price GW, Ahier RG, Hume SP, Myers R, Manjil L, Cremer JE. Appl. Radiat. Isot. 1990;41:477–482. doi: 10.1016/0883-2889(90)90008-5. [DOI] [PubMed] [Google Scholar]; (b) Price GW, Ahier RG, Hume SP, Myers R, Manjil L, Cremer JE, Luthra SK, Pascali C, Pike VW, Frackowiak R. J. Neurochem. 1990;55:175–185. doi: 10.1111/j.1471-4159.1990.tb08836.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.