Abstract
BACKGROUND
Assessment of the kidney parenchyma (“kidney”) and urinary bladder (“bladder”) cancer burden among American Indians and Alaska Natives (AI/AN) has been limited. Using a database with improved classification for AI/AN, the authors described patterns of these 2 cancers among AI/AN and non-Hispanic whites (NHW) in the United States.
METHODS
Cases diagnosed during 1999 to 2004 were identified through National Program of Cancer Registries and the Surveillance, Epidemiology and End Results program and linked to the Indian Health Service (IHS) registration records. Age-adjusted incidence rates, rate ratios (RR), annual percent change, and stage at diagnosis were stratified by IHS Contract Health Service Delivery Area (CHSDA) counties to adjust for misclassification.
RESULTS
Kidney cancer incidence among AI/AN in CHSDA counties exceeded that among NHW (RR, 1.51; 95% confidence interval [CI], 1.42-1.61), and was highest among AI/AN in the Northern Plains, Southern Plains, Alaska, and Southwest. Average annual increases were highest among AI/AN (5.9%) and NHW (5.9%) males aged 20 to 49 years, although statistically significant only among NHW. Conversely, bladder cancer incidence was significantly lower among AI/AN than NHW (RR, 0.40; 95% CI, 0.37-0.44). For both sites, AI/AN were significantly less likely to be diagnosed at an earlier stage than NHW.
CONCLUSIONS
AI/AN have about 50% greater risk of kidney cancer and half the risk of bladder cancer than NHW. Although reasons for these enigmatic patterns are not known, sustained primary prevention efforts through tobacco cessation and obesity prevention are warranted.
Keywords: cancer, incidence, American Indian, Alaska Native, misclassification, NPCR, SEER, United States, health disparity
Cancers of the kidney and urinary bladder (“bladder”) are not currently a focus of cancer control and prevention efforts, in part because of the lack of evidence-based screening methods. Previous reports indicate racial/ethnic differences in the incidence of these cancers, including higher incidence and mortality rates of kidney cancer among American Indians and Alaska Natives (AI/AN).1-3
Analyses of cancer patterns among AI/AN have been limited by considerable race misclassification of AI/AN cases in many central cancer registries.4-7 The aim of this study is to provide a comprehensive description of kidney and bladder cancer in AI/AN populations using techniques to lessen the effects of race misclassification. The database used in this analysis provides the most accurate and complete racial/ethnic classification of AI/AN diagnosed with cancer in the United States to date.
MATERIALS AND METHODS
Cancer Case Ascertainment
Newly diagnosed cancers of the bladder and kidney parenchyma (“kidney”) diagnosed during 1999 to 2004 were identified in US population-based cancer registries participating in the National Program of Cancer Registries (NPCR) of the Centers for Disease Control and Prevention (CDC) and the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute (NCI). More detailed methodology can be reviewed elsewhere in this Supplement.8
Analysis of cancers of the kidney included all invasive cancers. Primary site and histology were coded according to the International Classification of Diseases for Oncology (ICD-O) edition in use at the time of diagnosis and converted to the Third Edition (ICD-O-3).9 Kidney cancer analyses focused on cancers of the kidney parenchyma (ICD-O-3 site code C64.9) and renal cell carcinoma overall, according to the Mainz classification(ICD-O-3 morphology codes 8310, 8312, 8317, 8318, and 8260).10 Surveillance reports on kidney and other cancers frequently group the kidney parenchyma and renal pelvis into a composite rate; to facilitate comparisons with other reports, data for kidney and renal pelvis combined (ICD-O-3 site code C65.9) are provided online at http://www.cdc.gov/npcr.
Analysis of cancers of the urinary bladder (ICD-O-3 site code C67.9) included patients with both invasive and in situ cancers.11 We examined data for all histologic types combined, as well as for transitional cell cancers only (ICD-O-3 morphology codes 8120, 8130, and 8050).
Classification of Race and Ethnicity
The database was created by linking cancer registry data to the Indian Health Service (IHS) patient registration database. The IHS registration database consists of persons from federally recognized tribes in the US eligible for IHS services. Individuals whose race or ethnicity was previously classified in participating cancer registries as white, other, or unknown, and then identified as AI/AN by linkage with the IHS data, were reclassified as AI/AN. Probabilistic data record linkages were conducted using CDC’s software LinkPlus. Cases were also classified by Hispanic origin using the North American Association of Central Cancer Registries Hispanic Identification Algorithm.12 All AI/AN were included in AI/AN rates irrespective of Hispanic origin.
Geographic Regions
Geographic regions used for this study were consistent with those used in previous reports.3,13 Contract Health Service Delivery Area (CHSDA) counties are designated by the IHS and consist of 624 counties that, in general, include or are adjacent to federally recognized tribal lands,8 and include approximately 56% of the AI/AN population. CHSDA counties have been used previously2,3 for rate calculations, as there is evidence that less race misclassification occurs in these counties.14 In this study, we calculated incidence for CHSDA counties and for all counties combined. IHS regions and CHSDA counties included in the analysis are shown in Figure 1.
FIGURE 1.
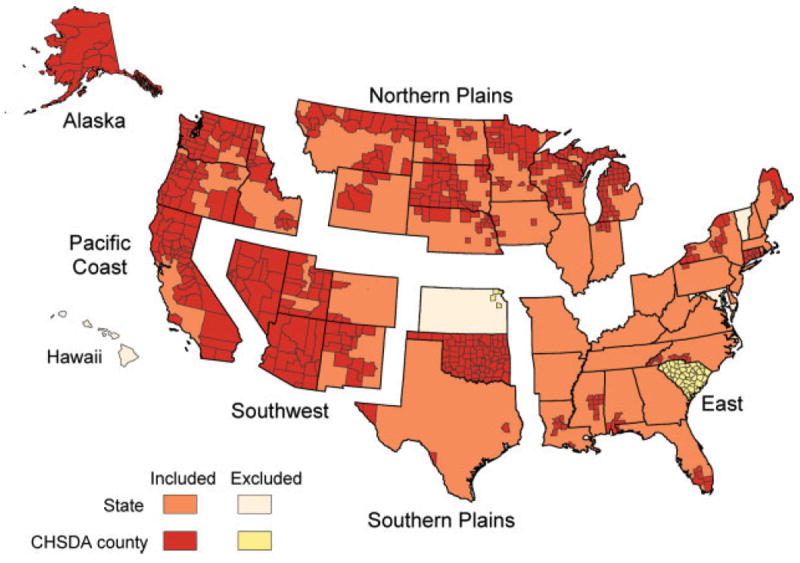
States and Contract Health Service Delivery Area (CHSDA) counties by Indian Health Service Region are illustrated.
Statistical Analysis
Incidence, annual percent change, and rate ratios
Incidence rates were calculated for cancers of the kidney and bladder for 6 geographic regions by sex and by age group, using NCI’s SEER*Stat software (version 6.3.6).15 SEER*Stat uses bridged single-race population estimates as denominator data, developed jointly by the US Census Bureau and CDC, and further modified by NCI.8,16 AI/AN incidence rates were adjusted for age using the direct method with the 2000 US standard population.17 Age-adjusted incidence rates were calculated for 2 age groups: 20 years to 49 years, and 50 years and older. Rate ratios (RR) were calculated to compare incidence rates between AI/AN and NHW. Ninety-five percent confidence intervals (CI) were calculated in SEER*Stat using methods described elsewhere.8 The annual percent change in incidence rates was calculated using weighted linear regression techniques and 2-year moving averages.15 Correlations between regional incidence rates in CHSDA counties and self-reported tobacco use prevalence among AI/AN in CHSDA counties18 were calculated using the Pearson correlation coefficient.
Stage at diagnosis
SEER Summary Stage 2000, age-adjusted to the 2000 US standard population, was used to describe the distribution of stage at diagnosis.8 The statistical significance of differences between AI/AN and NHW stage distribution were determined using the chi-square test. Because of inconsistencies between SEER Summary Stage 2000 and SEER Summary Stage 1977, we report stage data from 2001 to 2003 only.19
RESULTS
Cancer Incidence
Among AI/AN only, incidence rates in CHSDA counties were higher than in all counties combined (CHSDA + non-CHSDA). Consistent with other studies, this difference suggests that misclassification of AI/AN race is greater in the non-CHSDA counties (Tables 1 and 2). Therefore, our presentation of results will focus on CHSDA counties.
TABLE 1.
Kidney Parenchyma Cancer Incidence by Indian Health Service Region for American Indians/Alaska Nativesa and Non-Hispanic Whites, United States, 1999 to 2004
| IHS Region | Sex | CHSDA Counties
|
All Counties
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AI/AN Countb | AI/AN Ratec | 95% CI for AI/AN Rate | NHW Rate | Rate Ratioc (AI/AN:NHW) | 95% CI for Rate Ratio | AI/AN Countb | AI/AN Ratec | 95% CI for AI/AN Rate | NHW Ratec | Rate Ratioc (AI/AN:NHW) | 95% CI for Rate Ratio | ||
| Northern Plains | Both sexes | 203 | 23.7 | 20.4-27.5 | 12.1 | 1.96d | 1.68-2.28 | 251 | 17.0 | 14.8-19.4 | 13.0 | 1.31d | 1.14-1.50 |
| Male | 117 | 28.6 | 23.3-34.8 | 16.1 | 1.77d | 1.44-2.16 | 145 | 21.1 | 17.4-25.3 | 17.4 | 1.21 | 1.00-1.45 | |
| Female | 86 | 19.2 | 15.1-23.9 | 8.6 | 2.22d | 1.75-2.78 | 106 | 13.4 | 10.8-16.4 | 9.3 | 1.44d | 1.16-1.76 | |
| Alaskae | Both sexes | 78 | 19.6 | 15.3-24.7 | 12.7 | 1.54d | 1.16-2.03 | 78 | 19.6 | 15.3-24.7 | 12.7 | 1.54d | 1.16-2.03 |
| Male | 52 | 28.6 | 20.7-38.3 | 18.0 | 1.59d | 1.10-2.25 | 52 | 28.6 | 20.7-38.3 | 18.0 | 1.59d | 1.10-2.25 | |
| Female | 26 | 12.0 | 7.7-17.6 | 7.9 | 1.51 | 0.92-2.40 | 26 | 12.0 | 7.7-17.6 | 7.9 | 1.51 | 0.92-2.40 | |
| Southern Plains | Both sexes | 297 | 21.3 | 18.9-23.9 | 12.4 | 1.72d | 1.52-1.94 | 324 | 16.7 | 14.9-18.7 | 13.8 | 1.21d | 1.08-1.36 |
| Male | 163 | 24.7 | 20.9-28.9 | 17.1 | 1.45d | 1.22-1.71 | 177 | 19.0 | 16.2-22.2 | 18.8 | 1.01 | 0.86-1.18 | |
| Female | 134 | 17.9 | 15.0-21.2 | 8.6 | 2.09d | 1.73-2.51 | 147 | 14.4 | 12.1-16.900 | 9.6 | 1.50d | 1.26-1.77 | |
| Pacific Coast | Both sexes | 169 | 11.7 | 9.9-13.8 | 11.3 | 1.04 | 0.87-1.22 | 201 | 7.7 | 6.6-8.9 | 11.1 | 0.69d | 0.59-0.80 |
| Male | 97 | 14.6 | 11.5-18.2 | 15.6 | 0.94 | 0.74-1.17 | 119 | 9.8 | 8.0-12.0 | 15.4 | 0.64d | 0.52-0.78 | |
| Female | 72 | 9.4 | 7.2-11.9 | 7.7 | 1.22 | 0.94-1.56 | 82 | 5.9 | 4.6-7.4 | 7.5 | 0.79d | 0.62-0.99 | |
| East | Both sexes | 46 | 12.9 | 9.3-17.3 | 12.6 | 1.02 | 0.74-1.37 | 163 | 6.3 | 5.3-7.4 | 12.9 | 0.49d | 0.41-0.57 |
| Male | 20 | 12.3 | 7.1-19.6 | 17.6 | 0.70 | 0.41-1.12 | 87 | 6.8 | 5.3-8.5 | 17.7 | 0.38d | 0.30-0.48 | |
| Female | 26 | 13.3 | 8.6-19.6 | 8.5 | 1.56d | 1.01-2.30 | 76 | 5.7 | 4.4-7.1 | 9.0 | 0.63d | 0.49-0.79 | |
| Southwest | Both sexes | 341 | 17.9 | 15.9-19.9 | 11.1 | 1.60d | 1.43-1.80 | 352 | 16.3 | 14.6-18.2 | 10.9 | 1.50d | 1.34-1.67 |
| Male | 206 | 25.0 | 21.5-28.9 | 15.0 | 1.67d | 1.43-1.93 | 216 | 23.2 | 20.0-26.7 | 14.8 | 1.57d | 1.35-1.81 | |
| Female | 135 | 12.4 | 10.3-14.7 | 7.8 | 1.59d | 1.32-1.91 | 136 | 11.1 | 9.2-13.2 | 7.6 | 1.45d | 1.21-1.73 | |
| Total | Both sexes | 1,134 | 17.9 | 16.8-19.0 | 11.8 | 1.51d | 1.42-1.61 | 1,369 | 12.3 | 11.6-13.0 | 12.6 | 0.98 | 0.92-1.03 |
| Male | 655 | 22.7 | 20.8-24.6 | 16.2 | 1.40d | 1.29-1.52 | 796 | 15.6 | 14.4-16.8 | 17.2 | 0.91d | 0.84-0.98 | |
| Female | 479 | 13.9 | 12.7-15.2 | 8.1 | 1.71d | 1.55-1.88 | 573 | 9.6 | 8.8-10.4 | 8.8 | 1.09 | 1.00-1.19 | |
| Total renal cell | Both sexes | 992 | 15.6 | 14.6-16.6 | 10.0 | 1.56d | 1.46-1.67 | 1,199 | 10.7 | 10.1-11.4 | 10.6 | 1.01 | 0.95-1.07 |
| Male | 574 | 19.7 | 18.0-21.5 | 13.7 | 1.44d | 1.31-1.57 | 695 | 13.5 | 12.4-14.6 | 14.6 | 0.92 | 0.85-1.00 | |
| Female | 418 | 12.1 | 11.0-13.4 | 6.8 | 1.79d | 1.62-1.98 | 504 | 8.4 | 7.7-9.2 | 7.3 | 1.15d | 1.04-1.25 | |
Source: Cancer registries in the National Program of Cancer Registries (NPCR) of the Centers for Disease Control and Prevention and/or the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute.
CHSDA indicates Contract Health Service Delivery Areas; IHS, Indian Health Service; AI/AN, American Indians/Alaska Natives; NHW, non-Hispanic whites; CI, confidence interval.
AI/AN race is reported by NPCR and SEER registries or through linkage with the IHS patient registration database. AI/AN persons of Hispanic origin are included.
Rates are per 100,000 persons and are age-adjusted to the 2000 US standard population (19 age groups, Census P25-1130).
Rate ratios are calculated in SEER*Stat prior to rounding of rates and may not equal rate ratios calculated from rates presented in the table.
Rate ratio is statistically significant (P <.05).
Rates and rate ratios for Alaska in the CHSDA counties section are the same as those in the All Counties section because all counties in Alaska are CHSDA counties.
Years of data and registries used: 1999 to 2004 (41 states and the District of Columbia): Alaska,* Alabama,* Arkansas, Arizona,* California,* Colorado,* Connecticut,* the District of Columbia, Delaware, Florida,* Georgia, Hawaii, Iowa,* Idaho,* Illinois, Indiana,* Kentucky, Louisiana,* Massachusetts,* Maine,* Michigan,* Minnesota,* Missouri, Montana,* North Carolina,* Nebraska,* New Hampshire, New Jersey, New Mexico,* Nevada,* New York,* Ohio, Oklahoma,* Oregon,* Pennsylvania,* Rhode Island,* Texas,* Utah,* Washington,* Wisconsin,* West Virginia, and Wyoming*; 1999 and 2002 to 2004: North Dakota*; 2001 to 2004: South Dakota*; 2003 to 2004: Mississippi* and Virginia; 2004: Tennessee. *States with at least one county designated as CHSDA.
Percentage regional coverage of AI/AN in CHSDA counties compared with AI/AN in all counties: Alaska 5 100%; East 5 13.1%; Northern Plains 5 59.0%; Southern Plains 5 64.1%; Pacific Coast 5 55.6%; Southwest 5 87.5%.
TABLE 2.
Urinary Bladder Cancerf Incidence by Indian Health Service Region for American Indians/Alaska Natives and Non-Hispanic Whites, United States, 1999 to 2004
| IHS Region | Sex | CHSDA Counties
|
All Counties
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AI/AN Count | AI/AN Rateb,c | 95% CI for AI/AN Rate | NHW Rate | Rate Ratioc (AI/AN:NHW) | 95% CI for Rate Ratio | AI/AN Count | AI/AN Rateb,c | 95% CI for AI/AN Rate | NHW Rate | Rate Ratioc (AI/AN:NHW) | 95% CI for Rate Ratio | ||
| Northern Plains | Both sexes | 94 | 14.8 | 11.7-18.4 | 22.9 | 0.65d | 0.51-0.80 | 128 | 11.7 | 9.6-14.1 | 23.3 | 0.50d | 0.41-0.60 |
| Male | 70 | 26.8 | 20.1-34.8 | 39.4 | 0.68d | 0.51-0.88 | 93 | 20.3 | 15.8-25.6 | 41.2 | 0.49d | 0.38-0.62 | |
| Female | 24 | 6.4 | 4.0-9.6 | 10.4 | 0.62d | 0.39-0.93 | 35 | 5.6 | 3.8-7.8 | 10.4 | 0.54d | 0.37-0.75 | |
| Alaskae | Both sexes | 45 | 13.4 | 9.6-18.1 | 26.9 | 0.50d | 0.35-0.69 | 45 | 13.4 | 9.6-18.1 | 26.9 | 0.50d | 0.35-0.69 |
| Male | 34 | 23.0 | 15.1-33.1 | 47.3 | 0.49d | 0.31-0.71 | 34 | 23.0 | 15.1-33.1 | 47.3 | 0.49d | 0.31-0.71 | |
| Female | 11 | 6.4 | 3.1-11.3 | 8.6 | 0.74 | 0.34-1.40 | 11 | 6.4 | 3.1-11.3 | 8.6 | 0.74d | 0.34-1.40 | |
| Southern Plains | Both sexes | 184 | 14.6 | 12.6-17.0 | 19.7 | 0.74d | 0.64-0.86 | 196 | 11.6 | 10.0-13.4 | 19.7 | 0.59d | 0.50-0.68 |
| Male | 132 | 25.0 | 20.6-29.9 | 35.0 | 0.71d | 0.59-0.86 | 141 | 19.5 | 16.2-23.2 | 35.2 | 0.55d | 0.46-0.66 | |
| Female | 52 | 7.3 | 5.4-9.5 | 8.5 | 0.85 | 0.63-1.13 | 55 | 5.8 | 4.4-7.6 | 8.3 | 0.70d | 0.52-0.91 | |
| Pacific Coast | Both sexes | 103 | 8.6 | 6.9-10.6 | 24.0 | 0.36d | 0.29-0.44 | 133 | 6.2 | 5.2-7.5 | 23.8 | 0.26d | 0.22-0.31 |
| Male | 75 | 14.1 | 10.8-18.0 | 42.0 | 0.34d | 0.26-0.43 | 96 | 10.1 | 8.0-12.6 | 41.8 | 0.24d | 0.19-0.30 | |
| Female | 28 | 4.3 | 2.8-6.2 | 10.1 | 0.42d | 0.27-0.62 | 37 | 3.2 | 2.2-4.4 | 10.1 | 0.31d | 0.22-0.44 | |
| East | Both sexes | 38 | 11.9 | 8.3-16.5 | 25.8 | 0.46d | 0.32-0.64 | 131 | 6.3 | 5.2-7.5 | 24.6 | 0.26d | 0.21-0.31 |
| Male | 30 | 22.8 | 14.6-33.4 | 44.7 | 0.51d | 0.33-0.75 | 98 | 10.9 | 8.6-13.5 | 43.0 | 0.25d | 0.20-0.31 | |
| Female | 8 | 4.4 | 1.9-8.7 | 12.0 | 0.37d | 0.15-0.73 | 33 | 3.0 | 2.0-4.2 | 11.1 | 0.27d | 0.18-0.38 | |
| Southwest | Both sexes | 54 | 3.2 | 2.4-4.2 | 23.8 | 0.13d | 0.10-0.18 | 60 | 3.2 | 2.4-4.2 | 22.6 | 0.14d | 0.11-0.19 |
| Male | 40 | 5.7 | 4.0-7.8 | 40.7 | 0.14d | 0.10-0.19 | 44 | 5.6 | 4.0-7.6 | 38.8 | 0.14d | 0.10-0.20 | |
| Female | 14 | 1.4 | 0.7-2.3 | 10.1 | 0.14d | 0.07-0.23 | 16 | 1.5 | 0.8-2.4 | 9.7 | 0.15d | 0.09-0.25 | |
| Total | Both sexes | 518 | 9.6 | 8.8-10.5 | 23.9 | 0.40d | 0.37-0.44 | 693 | 7.6 | 7.0-8.2 | 23.7 | 0.32d | 0.29-0.34 |
| Male | 381 | 16.5 | 14.8-18.4 | 41.5 | 0.40d | 0.36-0.44 | 506 | 12.7 | 11.5-14.0 | 41.7 | 0.31d | 0.28-0.34 | |
| Female | 137 | 4.5 | 3.8-5.3 | 10.5 | 0.43d | 0.36-0.51 | 187 | 3.7 | 3.2-4.3 | 10.6 | 0.35d | 0.30-0.41 | |
| Total transitional cell | Both sexes | 452 | 8.3 | 7.6-9.2 | 22.2 | 0.38d | 0.34-0.41 | 615 | 6.7 | 6.1-7.2 | 22.1 | 0.30d | 0.28-0.33 |
| Male | 344 | 14.8 | 13.2-16.6 | 38.7 | 0.38d | 0.34-0.43 | 464 | 11.6 | 10.5-12.8 | 39.0 | 0.30d | 0.27-0.33 | |
| Female | 108 | 3.5 | 2.9-4.3 | 9.5 | 0.37d | 0.30-0.45 | 151 | 3.0 | 2.5-3.5 | 9.6 | 0.31d | 0.26-0.36 | |
Source: Cancer registries in the National Program of Cancer Registries (NPCR) of the Centers for Disease Control and Prevention and/or the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute; see Table 1 for states included.
CHSDA indicates Contract Health Service Delivery Areas; IHS, Indian Health Service; AI/AN, American Indians/Alaska Natives; CI, confidence interval; NHW, non-Hispanic whites.
AI/AN race is reported by NPCR and SEER registries or through linkage with the IHS patient registration database. AI/AN persons of Hispanic origin are included.
Rates are per 100,000 persons and are age-adjusted to the 2000 US standard population (19 age groups, Census P25-1130).
Rate ratios are calculated in SEER*Stat prior to rounding of rates and may not equal RR calculated from rates presented in the table.
Rate ratio is statistically significant (P <.05).
Rates and rate ratios for Alaska in the CHSDA counties section are the same as those in the All Counties section because all counties in Alaska are CHSDA counties.
In situ and invasive cases are included.
For cancer of the kidney parenchyma, incidence rates in CHSDA counties for both sexes combined ranged from 11.7 (Pacific Coast) to 23.7 (Northern Plains) among AI/AN and from 11.1 (Southwest) to 12.7 (East) among NHW (Table 1). Overall, incidence rates were nearly 1.5× higher among AI/AN compared with NHW, and rate ratios comparing AI/AN with NHW were significantly elevated in all regions except the Pacific Coast and East. Rate ratios were somewhat higher among AI/AN females (RR, 1.71; 95% CI, 1.55-1.88) than AI/AN males (RR, 1.40; 95% CI, 1.29-1.52), with highest rate ratios (~2.5-fold higher) among AI/AN females in the Northern and Southern Plains. For all regions combined, these results were similar for renal cell histologic subtypes.
In CHSDA counties, urinary bladder (“bladder”) cancer incidence rates for transitional cell cancers and all bladder cancers combined were significantly lower among AI/AN (9.6) than among NHW (23.9) (Table 2). This pattern was similar in all regions. Incidence rates varied approximately 4-fold among AI/AN, from 3.2 in the Southwest to 14.8 in the Northern Plains. Rate ratios ranged from 0.13 (95% CI, 0.10-0.18) in the Southwest to 0.74 (95% CI, 0.64-0.86) in the Southern Plains.
Age-Specific Incidence Rates
Kidney cancer incidence among AI/AN aged 20 years to 49 years were highest in the Northern (8.6) and Southern Plains (8.4), ranging roughly 2-fold regionally (Table 3). Regional patterns in incidence rates among AI/AN aged 50 years and older were similar to the younger age group, although rates in Alaska were also significantly higher. Over the time period of the study (1999-2004), incidence rates among AI/AN and NHW males in both age groups increased. Average annual percent increases were slightly higher among males aged 20 years to 49 years, compared with males aged 50 years and older, among both AI/ AN (5.9% per year vs 3.2% per year) and NHW (5.9% vs 3.0%) males, respectively, although the increases were statistically significant only among NHW. There was not a significant trend over time among AI/AN females of either age group. Among NHW females aged 50 years and older, the increase was statistically significant (3.5% per year). Among AI/AN aged 20 years to 49 years, bladder cancer incidence rates were highest in the Northern Plains (3.3), Southern Plains (2.4), and East (1.8) (Table 4). Among NHW aged 20 years to 49 years, rates were highest in Alaska (3.4) and the East (3.4). Among individuals aged 50 years and older, the highest rates occurred among NHW in Alaska (91.8). Incidence rates were highest among AI/AN in the Southern Plains (49.2), Northern Plains (48.2), and Alaska (46.1). Rate ratios among individuals aged 50 years and older were all significantly below 1, indicating significantly lower incidence rates among AI/AN in all regions.
TABLE 3.
Kidney Parenchyma Cancer Incidence Rates and Rate Ratios, by Age and Indian Health Service Region for American Indians/Alaska Natives and Non-Hispanic Whites, CHSDA Counties, United States, 1999 to 2004
| IHS Region | Sex | 20-49 Years
|
50+ Years
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AI/AN
|
NHW
|
AI/AN:NHW
|
AI/AN
|
NHW
|
AI/AN:NHW
|
||||||||||||
| Count | Ratea | 95% CI | Count | Ratea | 95% CI | RRb | 95% CI | Count | Ratea | 95% CI | Count | Ratea | 95% CI | RRb | 95% CI | ||
| Northern Plains | Both sexes | 45 | 8.6 | 6.3-11.5 | 814 | 4.0 | 3.7-4.3 | 2.14b | 1.55-2.88 | 154 | 71.6 | 60.1-84.8 | 5,227 | 36.6 | 35.6-37.6 | 1.96b | 1.64-2.32 |
| Male | 25 | 9.9 | 6.4-14.6 | 517 | 5.1 | 4.6-5.5 | 1.96b | 1.26-2.92 | 90 | 87.2 | 68.8-109.5 | 3,186 | 49.6 | 47.9-51.3 | 1.76b | 1.38-2.22 | |
| Female | 20 | 7.3 | 4.5-11.3 | 297 | 3.0 | 2.6-3.3 | 2.48b | 1.49-3.89 | 64 | 57.0 | 43.4-73.7 | 2,041 | 25.7 | 24.6-26.9 | 2.22b | 1.68-2.88 | |
| Alaska | Both sexes | 11 | 4.3 | 2.2-7.7 | 51 | 3.6 | 2.7-4.8 | 1.20 | 0.56-2.33 | 66 | 63.8 | 48.8-82.1 | 202 | 39.7 | 34.0-46.2 | 1.61b | 1.18-2.17 |
| Male | 8 | 6.4 | 2.8-12.6 | 30 | 4.0 | 2.7-5.8 | 1.59 | 0.63-3.54 | 43 | 92.6 | 65.0-128.5 | 144 | 57.9 | 47.3-70.1 | 1.60b | 1.07-2.36 | |
| Female | ~ | 2.3 | 0.5-6.8 | 21 | 3.2 | 2.0-4.9 | 0.74 | 0.14-2.45 | 23 | 39.7 | 24.9-60.3 | 58 | 23.4 | 17.5-30.7 | 1.69 | 0.98-2.84 | |
| Southern Plains | Both sexes | 61 | 8.4 | 6.4-10.8 | 270 | 3.8 | 3.3-4.2 | 2.23b | 1.66-2.95 | 234 | 63.6 | 55.5-72.5 | 2,151 | 38.2 | 36.6-39.8 | 1.67b | 1.45-1.91 |
| Male | 34 | 9.7 | 6.7-13.5 | 183 | 5.1 | 4.4-5.9 | 1.89b | 1.27-2.74 | 128 | 73.8 | 61.2-88.4 | 1,334 | 53.3 | 50.4-56.2 | 1.39b | 1.14-1.67 | |
| Female | 27 | 7.2 | 4.7-10.4 | 87 | 2.4 | 1.9-3.0 | 2.95b | 1.84-4.58 | 106 | 53.2 | 43.5-64.5 | 817 | 26.1 | 24.3-27.9 | 2.04b | 1.65-2.51 | |
| Pacific Coast | Both sexes | 44 | 4.7 | 3.4-6.3 | 1,353 | 3.6 | 3.4-3.8 | 1.29 | 0.93-1.74 | 119 | 34.2 | 27.9-41.4 | 9,050 | 34.4 | 33.7-35.1 | 0.99 | 0.81-1.21 |
| Male | 27 | 5.8 | 3.8-8.5 | 870 | 4.6 | 4.3-5.0 | 1.25 | 0.82-1.83 | 68 | 43.0 | 32.4-56.0 | 5,768 | 48.3 | 47.0-49.5 | 0.89 | 0.67-1.16 | |
| Female | 17 | 3.6 | 2.1-5.7 | 483 | 2.6 | 2.4-2.9 | 1.37 | 0.79-2.21 | 51 | 27.1 | 19.9-36.1 | 3,282 | 22.9 | 22.1-23.7 | 1.19 | 0.87-1.58 | |
| East | Both sexes | 9 | 4.2 | 1.9-8.0 | 980 | 4.1 | 3.9-4.4 | 1.03 | 0.47-1.94 | 37 | 39.8 | 27.6-55.7 | 7,204 | 38.5 | 37.6-39.4 | 1.04 | 0.72-1.45 |
| Male | ~ | 3.8 | 1.0-9.5 | 625 | 5.3 | 4.8-5.7 | 0.72 | 0.20-1.82 | 16 | 38.6 | 20.9-65.9 | 4,495 | 54.7 | 53.1-56.3 | 0.71 | 0.38-1.21 | |
| Female | ~ | 4.7 | 1.5-10.8 | 355 | 3.0 | 2.7-3.3 | 1.55 | 0.50-3.63 | 21 | 40.9 | 25.1-63.2 | 2,709 | 25.5 | 24.5-26.5 | 1.60 | 0.98-2.48 | |
| Southwest | Both sexes | 67 | 5.8 | 4.5-7.4 | 533 | 3.5 | 3.2-3.9 | 1.64b | 1.25-2.11 | 268 | 55.0 | 48.4-62.3 | 4,195 | 34.1 | 33.1-35.2 | 1.61b | 1.41-1.83 |
| Male | 38 | 6.8 | 4.8-9.4 | 323 | 4.3 | 3.8-4.7 | 1.61b | 1.11-2.25 | 165 | 79.4 | 67.2-93.1 | 2,675 | 47.0 | 45.2-48.8 | 1.69b | 1.42-1.99 | |
| Female | 29 | 4.8 | 3.2-6.9 | 210 | 2.8 | 2.4-3.2 | 1.72b | 1.12-2.53 | 103 | 36.7 | 29.8-44.7 | 1,520 | 23.2 | 22.0-24.4 | 1.58b | 1.28-1.94 | |
| Total | Both sexes | 237 | 6.2 | 5.4-7.1 | 4,001 | 3.8 | 3.7-3.9 | 1.63b | 1.42-1.86 | 878 | 54.3 | 50.6-58.1 | 28,029 | 36.0 | 35.6-36.5 | 1.51b | 1.40-1.61 |
| Male | 136 | 7.4 | 6.2-8.7 | 2,548 | 4.8 | 4.6-5.0 | 1.52b | 1.27-1.81 | 510 | 69.9 | 63.5-76.7 | 17,602 | 50.2 | 49.4-50.9 | 1.39 | 1.27-1.53 | |
| Female | 101 | 5.1 | 4.2-6.2 | 1,453 | 2.8 | 2.7-2.9 | 1.84b | 1.49-2.25 | 368 | 41.6 | 37.4-46.2 | 10,427 | 24.4 | 23.9-24.8 | 1.71b | 1.53-1.90 | |
| Total Kidney RCC | Both sexes | 218 | 5.7 | 5.0-6.5 | 3,608 | 3.4 | 3.3-3.5 | 1.67b | 1.45-1.91 | 774 | 47.3 | 43.9-50.9 | 23,748 | 30.6 | 30.2-31.0 | 1.55b | 1.43-1.66 |
| Male | 128 | 6.9 | 5.8-8.3 | 2,294 | 4.4 | 4.2-4.5 | 1.60b | 1.32-1.91 | 446 | 60.4 | 54.6-66.7 | 15,058 | 42.7 | 42.0-43.4 | 1.41b | 1.28-1.56 | |
| Female | 90 | 4.6 | 3.7-5.6 | 1,314 | 2.5 | 2.4-2.7 | 1.82b | 1.45-2.25 | 328 | 36.7 | 32.8-41.0 | 8,690 | 20.5 | 20.1-21.0 | 1.79b | 1.59-2.00 | |
|
| |||||||||||||||||
| APC | APC | APC | APC | ||||||||||||||
| Total Kidney APC | Both sexes | 237 | 3.0 | −8.0-15.3 | 4,001 | 5.3c | 3.1-7.6 | 878 | 3.3 | −2.4-9.3 | 28,029 | 3.2c | 1.9-4.5 | ||||
| Male | 136 | 5.9 | −7.6-21.3 | 2,548 | 5.9c | 2.7-9.2 | 510 | 3.2 | −4.8-11.9 | 17,602 | 3.0c | 0.8-5.2 | |||||
| Female | 101 | −0.4 | −14.3-15.8 | 1,453 | 4.4c | 0.1-8.9 | 368 | 3.1 | −4.4-11.2 | 10,427 | 3.5c | 2.2-4.8 | |||||
Source: Cancer registries in the National Program of Cancer Registries of the Centers for Disease Control and Prevention and/or the Surveillance, Epidemiology, and End Results program of the National Cancer Institute; see Table 1 for states included.
CHSDA indicates Contract Health Service Delivery Areas; AI/AN, American Indians/Alaska Natives; NHW, non-Hispanic whites; IHS, Indian Health Service; CI, confidence interval; RR, rate ratio; APC, annual percentage change.
Rates are per 100,000 persons and are age-adjusted to the 2000 US standard population (19 age groups, Census P25-1130).
Rate ratio is statistically significant (P <.05).
APC is statistically significant (P <.05).
Counts less than 6 are suppressed.
TABLE 4.
Urinary Bladder Cancera Incidence Rates and Rate Ratios, by Age and Indian Health Service Region for American Indians/Alaska Natives and Non-Hispanic Whites, CHSDA Counties, United States, 1999 to 2004
| IHS Region | 20-49 Years
|
50+ Years
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AI/AN
|
NHW
|
AI/AN:NHW
|
AI/AN
|
NHW
|
AI/AN:NHW
|
|||||||||||
| Count | Rateb | 95% CI | Count | Rateb | 95% CI | RRc | 95% CI | Count | Rateb | 95% CI | Count | Rateb | 95% CI | RRc | 95% CI | |
| Northern Plains | 17 | 3.3 | 1.9-5.2 | 597 | 2.9 | 2.7-3.2 | 1.13 | 0.65-1.81 | 76 | 48.2 | 37.4-61.1 | 11,265 | 78.1 | 76.7-79.6 | 0.62c | 0.48-0.78 |
| Alaska | ~ | 1.6 | 0.4-4.1 | 48 | 3.4 | 2.5-4.5 | 0.47 | 0.12-1.28 | 41 | 46.1 | 32.5-63.4 | 405 | 91.8 | 82.5-101.8 | 0.50c | 0.35-0.70 |
| Southern Plains | 18 | 2.4 | 1.4-3.8 | 214 | 2.9 | 2.6-3.4 | 0.82 | 0.48-1.33 | 166 | 49.2 | 41.8-57.4 | 3,777 | 66.6 | 64.5-68.8 | 0.74c | 0.63-0.86 |
| Pacific Coast | 7 | 0.8 | 0.3-1.5 | 1,086 | 2.9 | 2.7-3.1 | 0.26c | 0.10-0.53 | 96 | 30.0 | 23.9-37.2 | 21,723 | 82.1 | 81.0-83.2 | 0.37c | 0.29-0.45 |
| East | ~ | 1.8 | 0.5-4.7 | 821 | 3.4 | 3.2-3.7 | 0.54 | 0.15-1.37 | 34 | 40.2 | 27.3-57.0 | 16,941 | 87.7 | 86.4-89.1 | 0.46c | 0.31-0.65 |
| Southwest | ~ | 0.2 | 0.0-0.7 | 436 | 2.9 | 2.6-3.2 | 0.08c | 0.02-0.24 | 51 | 11.2 | 8.2-14.8 | 10,017 | 81.4 | 79.8-83.0 | 0.14c | 0.10-0.18 |
| Total CHSDA | 53 | 1.4 | 1.0-1.8 | 3,202 | 3.0 | 2.9-3.1 | 0.45c | 0.34-0.60 | 464 | 32.6 | 29.6-35.8 | 64,128 | 81.5 | 80.9-82.1 | 0.40c | 0.36-0.44 |
| Total bladder TCC | 44 | 1.1 | 0.8-1.5 | 2,957 | 2.8 | 2.7-2.9 | 0.41c | 0.30-0.55 | 407 | 28.3 | 25.5-31.4 | 59,531 | 75.7 | 75.1-76.3 | 0.37c | 0.34-0.41 |
|
| ||||||||||||||||
| APC | APC | APC | APC | |||||||||||||
| Total bladder APC | 53 | −16.3d | −25.2-−6.3 | 3,202 | −2.9 | −6.9-1.2 | 464 | −1.4 | 0.4 −5.2 | 64,128 | −0.7d | −0.9-−0.4 | ||||
Source: Cancer registries in the National Program of Cancer Registries of the Centers for Disease Control and Prevention and/or the Surveillance, Epidemiology, and End Results program of the National Cancer Institute; see Table 1 for states included.
CHSDA indicates Contract Health Service Delivery Areas; AI/AN, American Indians/Alaska Natives; NHW, non-Hispanic whites; IHS, Indian Health Service; CI, confidence interval; RR, rate ratio; TCC, transitional cell carcinoma; APC, annual percentage change.
In situ and invasive cases are included.
Rates are per 100,000 persons and are age-adjusted to the 2000 US standard population (19 age groups, Census P25-1130).
Rate ratio is statistically significant (P <.05).
APC is statistically significant (P <.05).
Counts less than 6 are suppressed.
Regional Correlation of Incidence With Smoking and Obesity
The correlation between self-reported tobacco use and bladder cancer incidence rates in CHSDA counties was statistically significant (r = 0.9, P = .02) (Table 5). The magnitude of this correlation for kidney cancer incidence rates was not statistically significant and less pronounced (r = 0.5, P = .30), appearing somewhat greater among AI/AN females (r = 0.7, P = .15) than AI/AN males (r = 0.4, P = .49). Correlations between incidence and the prevalence of obesity were not statistically significant (data not shown).
TABLE 5.
Regional Correlation of Cancer Incidence Rates (1999 to 2004) With Self-Reported Tobacco Use (BRFSS 2000 to 2006) Among American Indian and Alaska Natives, CHSDA Counties, United States
| Correlation Coefficient
| ||
|---|---|---|
| Tobacco Use | Urinary Bladdera (P) | Kidney Parenchyma (P) |
| Both sexes | .9 (.02) | .5 (.30) |
| Male | .9 (.01) | .4 (.49) |
| Female | .8 (.07) | .7 (.15) |
Source: Cancer registries in the National Program of Cancer Registries of the Centers for Disease Control (CDC) and Prevention and the Surveillance, Epidemiology, and End Results program of the National Cancer Institute (see Table 1 for states included); CDC’s BRFSS.
BRFSS indicates Behavioral Risk Factor Surveillance System; CHSDA, Contract Health Service Delivery Areas.
In situ and invasive cases are included.
Stage at Diagnosis
AI/AN were significantly less likely to have been diagnosed with earlier stages of cancer (in situ and localized stage), including both bladder (76.3% vs 82.8%, P =.002) and kidney (51.9% vs 58.7%, P =.001) cancer (Table 6). In addition, a higher proportion of AI/AN were diagnosed with late-stage kidney cancer compared with NHW (P =.026).
TABLE 6.
Urinary Bladdera and Kidney Parenchyma Cancer Incidence Rates and Percentage Distributions by Stageb and Indian Health Service Region for American Indians/Alaska Natives and Non-Hispanic Whites, CHSDA Counties, United States, 2001 to 2003
| Localized | Race | Regional
|
Distant
|
Unstaged
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Count | Ratec | 95% CI | % of Casesd | Count | Ratec | 95% CI | % of Casesd | Count | Ratec CI | 95% CI | % of Casesd | Count | Ratec | 95% CI | % of Casesd | ||
| Urinary bladder | AI/AN | 200 | 7.1 | 6.1-8.2 | 76.3 | 23 | 0.8 | 0.5-1.3 | 8.6 | 12 | 0.4 | 0.2-0.8 | 4.3 | 23 | 0.9 | 0.6-1.4 | 9.7 |
| NHW | 27,929 | 19.7 | 19.4-19.9 | 82.8 | 2,611 | 1.8 | 1.8-1.9 | 7.6 | 1,063 | 0.7 | 0.7-0.8 | 2.9 | 2,200 | 1.5 | 1.5-1.6 | 6.3 | |
| P | .002 | .691 | .123 | .364 | |||||||||||||
| Kidney parenchyma | AI/AN | 310 | 9.4 | 8.4-10.6 | 51.9 | 83 | 2.5 | 1.9-3.1 | 13.8 | 130 | 4.0 | 3.3-4.8 | 22.1 | 62 | 2.1 | 1.6-2.8 | 11.6 |
| NHW | 9,665 | 7.1 | 6.9-7.2 | 58.7 | 2,597 | 1.9 | 1.8-1.9 | 15.7 | 3,015 | 2.2 | 2.1-2.2 | 18.2 | 1,471 | 1.0 | 1.0-1.1 | 8.3 | |
| P | .001 | .548 | .026 | ||||||||||||||
Source: Cancer registries in the National Program of Cancer Registries of the Centers for Disease Control and Prevention and/or the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute; see Table 1 for states included.
CHSDA indicates Contract Health Service Delivery Areas; CI, confidence interval; AI/AN, American Indians/Alaska Natives; NHW, non-Hispanic whites.
In situ urinary bladder cases were included.
SEER Summary Stage 2000 was used.
Rates are per 100,000 persons and are age-adjusted to the 2000 US standard population (19 age groups, Census P25-1130).
Percentage stage distribution is age-adjusted to the 2000 US standard population; percentageages may not add to 100% due to rounding.
DISCUSSION
Summary
Compared with NHW, this study reports that kidney parenchyma cancer incidence is approximately 1.5-fold higher among AI/AN overall, and as great as 2.5-fold higher among females in some regions. Temporal increases in kidney cancer incidence rates appear to be comparable among AI/AN and NHW males, although statistically significant only among NHW males. Although bladder cancer incidence is significantly lower among AI/AN compared with NHW, there is substantial regional variation among AI/AN alone, which correlates crudely with tobacco use. For both cancer sites, AI/AN are less likely to be diagnosed with early-stage disease.
Kidney cancer
Histologically, most kidney cancers (>80%) are renal cell carcinomas (RCC), and therefore it is not surprising that differences between AI/AN and NHW were similar for RCC compared with all cancers occurring in the kidney.1 In most regions of the United States, kidney cancer incidence rates were significantly higher among AI/AN compared with NHW, and this relationship was apparent in both younger and older adult age groups. Smoking, obesity, and hypertension together are estimated to account for approximately 50% to 60% of kidney cancers.1,20 Obesity alone is attributed to approximately 25% of kidney cancers.21 AI/AN are more likely to be overweight and/or obese than NHW.13,18,22-25 However, body mass index among AI/AN does not vary as considerably as smoking by region and did not explain well the regional differences observed among AI/AN in this study.18 Other factors may be responsible for the 2-fold regional variation in incidence among AI/AN. There is some evidence that the use of diuretics may increase the risk of kidney cancer in both hypertensive and normotensive individuals.21,26-28 According to the Strong Heart Study of American Indians in the United States, the prevalence of the use of diuretics to treat hypertension among AI/AN may vary as much as 11% to 30% by study region.29 However, the extent to which the use of diuretics may contribute to regional variation in kidney cancer incidence is not known.
A corollary condition to obesity is diabetes. Type 2 diabetes mellitus (“diabetes”) has been inconsistently associated with an increased risk of RCC, and it is possible that diabetes contributes to the etiology of this cancer through increased risk of kidney failure among diabetics. Studies among kidney dialysis patients report an increased risk of kidney cancer.30,31 Kidney failure leading to dialysis is more prevalent among AI/AN with diabetes and may be an important kidney cancer risk factor among AI/AN.32,33 In addition, more recent research has focused on the importance of in utero exposures that may impact kidney development and function.34 Two studies report that children of mothers who were diabetic during pregnancy are more likely to have reduced diabetes control (ie, higher HbA1c) and a higher incidence of kidney disease.35,36 Further research is needed to elucidate the implications of these early life exposures on later development of cancer.
Several other risk factors have been proposed, including infections, diet, occupational exposures, and family history.1,20,37 Approximately 4% of kidney cancers diagnosed in the US are estimated to be familial,38 and those diagnosed at earlier ages may be more likely to have a genetic component,39 although known familial kidney cancer syndromes have not been described among AI/AN.38,40 Population-based case-control studies suggest that multiple genetic and environmental triggers are likely responsible.1
Recent increases in kidney cancer incidence rates in the United States and abroad over the past few decades suggest that nongenetic factors are driving kidney cancer trends.41,42 Among AI/AN, cancer of the kidney is now the fourth most frequently diagnosed cancer.1,2 The average annual increases in kidney cancer incidence among AI/AN and NHW over age 50 years in this study (3.0% to 3.5%) are comparable to studies from earlier time periods reporting increases of 1% to 3% annually.1,41,43 The 5.9% average annual increase among younger adult NHW and AI/AN males (aged 20 to 49 years) is of concern because of the magnitude of the annual increase and the morbidity of this cancer. However, this study involves a relatively brief time period (6 years), which may or may not be indicative of longer-term trends.
Kidney cancer incidence is generally 2-fold higher among males than females.2 However, we report that AI/AN females in some regions have incidence rates closer to those of AI/AN males. Certain exposures, including tobacco use and pregnancy, can increase oxidative stress on the kidney, which may be a common pathway increasing cancer risk.44 At least 2 large cohort studies report an increased risk of renal cell cancer among females with higher parity, compared with nulliparous females.45,46 This suggests that AI/AN females with higher parity may also be at increased risk of kidney cancer.
Bladder cancer
Previous studies report lower bladder cancer incidence and mortality among AI/AN than in other populations.2,3,47 In the US, smoking is the major risk factor for cancers of the bladder, and it may account for approximately 70% of new bladder cancer cases among males and 20% to 30% among females.20,48 In our study, among both males and females, approximately 90% of the regional variation in bladder cancer among AI/AN was correlated with tobacco use. The Behavioral Risk Factor Surveillance System for years 2000 to 2006 reports higher smoking prevalence among AI/AN in the Northern Plains (40.1%), Alaska (39.4%), and the East (36.3%), regions that also have higher bladder cancer incidence among AI/AN.18 Consistent with this smoking pattern, Inuit populations (Alaska, Canada, and Greenland) and American Indians of the Northern Plains also have a high incidence of lung cancer that exceeds rates among NHW by 30% to 60%.3,49,50 Given the higher incidence of lung cancer and higher prevalence of smoking among AI/AN, the comparative deficit of bladder cancer among AI/AN is somewhat enigmatic. Further complicating this picture are other studies that suggest that diabetes, again more prevalent among AI/AN, may increase the risk of bladder cancer.51,52 These contrasting patterns suggest the need to understand intervening factors that may affect the risk of bladder cancer. Several case-control studies have identified an elevated risk of bladder cancer among certain occupations, including those in the rubber, chemical, leather, metal, printing, painting, textile, and truck driving industries. Occupational exposures account for approximately 5% to 25% of bladder cancer cases among males and 8% to 11% among females, although the proportion of AI/AN with exposure to occupational bladder carcinogens is not known.48
Other possible risks for bladder cancer include family history, exposure to cyclophosphamide, ionizing radiation, arsenic, disinfection by-products contained in chlorinated drinking water, and decreased fluid intake.20 Individuals with a first primary bladder cancer are at approximately 4-fold increased risk of a secondary bladder cancer.37 Finally, there may be molecular studies suggest important genes related to bladder cancer susceptibility, although the prevalence of at-risk or protective genetic polymorphisms among AI/AN populations is not known.53
Strengths and Limitations
Strengths
This is the first study to identify broad regional patterns of urologic cancers among the AI/AN population on a national scale. Misclassification of AI/AN race and ethnicity was reduced by linkage to the IHS patient registration records and limiting the analysis to CHSDA counties. Other statistics, such as survival and stage at diagnosis, were similar in CHSDA and non-CHSDA areas (data not presented here), suggesting that the CHSDA areas provide a reasonable representation of cancer cases, while at the same time reducing misclassification of AI/AN race and ethnicity.
Limitations
It is possible that regions with fewer reservation land-based tribes, such as the East and Pacific Coast, may have had greater misclassification of AI/AN race and ethnicity than when compared with regions such as the Northern Plains and Southwest, which would result in lower than actual incidence rates among AI/AN. For example, we did not observe an excess of kidney cancer in these regions, whereas we did observe an excess among AI/AN in all other regions. Although AI/AN patients must return to a CHSDA to become eligible for contract care services (eg, for cancer treatment) through the Indian Health Service, it is likely that a significant proportion do not. Efforts to improve the classification of race and ethnicity in these areas will still be important.
Our study could not account for increased medical surveillance in detecting cancer that may result in a higher rate of case identification among some individuals. For example, the assessment of certain types of cancer—particularly multiple low-grade bladder cancers—may be lower among AI/AN populations that reside in rural regions further from medical care. In addition, SEER summary staging combines in situ and local-stage bladder cancer for the staging systems used during the time period of this study. It is possible that there are more subtle differences in in situ versus invasive cancer stages that could not be analyzed by this study.
Conclusions
Our study highlights major differences in bladder and kidney cancer incidence rates between AI/AN and NHW populations in the United States. AI/AN have significantly higher kidney cancer incidence, with recent increases evident for both AI/AN and NHW. Despite the finding that bladder cancer incidence among AI/AN is about half that of NHW, regional incidence rates among AI/AN alone correlate crudely with tobacco use. These results, combined with the higher prevalence of late-stage bladder and kidney cancers among AI/AN, emphasize the need for sustained efforts to prevent smoking and reduce obesity.
Footnotes
This supplement was sponsored by Cooperative Agreement Number U50 DP424071-04 from the Centers for Disease Control and Prevention, Division of Cancer Prevention and Control.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
This article is a US Government work and, as such, is in the public domain in the United States of America.
References
- 1.Moore LE, Wilson RT, Campleman SL. Lifestyle factors, exposures, genetic susceptibility and renal cell cancer risk: a review. Cancer Invest. 2005;23:240–255. doi: 10.1081/cnv-200055962. [DOI] [PubMed] [Google Scholar]
- 2.Espey DK, Wu XC, Swan J, et al. Annual report to the nation on the status of cancer, 1975-2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007;110:2119–2152. doi: 10.1002/cncr.23044. [DOI] [PubMed] [Google Scholar]
- 3.Espey D, Paisano R, Cobb N. Regional patterns and trends in cancer mortality among American Indians and Alaska Natives, 1990-2001. Cancer. 2005;103:1045–1053. doi: 10.1002/cncr.20876. [DOI] [PubMed] [Google Scholar]
- 4.Frost F, Taylor V, Fries E. Racial misclassification of Native Americans in a surveillance, epidemiology, and end results cancer registry. J Natl Cancer Inst. 1992;84:957–962. doi: 10.1093/jnci/84.12.957. [DOI] [PubMed] [Google Scholar]
- 5.Sugarman JR, Holliday M, Ross A, Castorina J, Hui Y. Improving American Indian Cancer Data in the Washington State Cancer Registry using linkages with the Indian Health Service and tribal records. Cancer. 1996;78(7 suppl):1564–1568. [PubMed] [Google Scholar]
- 6.Partin MR, Rith-Najarian SJ, Slater JS, Korn JE, Cobb N, Soler JT. Improving cancer incidence estimates for American Indians in Minnesota. Am J Public Health. 1999;89:1673–1677. doi: 10.2105/ajph.89.11.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foote M, Matloub J, Strickland R, Stephenson L, Vaughan-Batten H. Improving cancer incidence estimates for American Indians in Wisconsin. WMJ. 2007;106:196–204. [PubMed] [Google Scholar]
- 8.Espey DK, Wiggins CL, Jim MA, Miller BA, Johnson CJ, Becker TM. Methods for improving cancer surveillance data in American Indian and Alaska Native Populations. Cancer. 2008;113(5 suppl):1120–1130. doi: 10.1002/cncr.23724. [DOI] [PubMed] [Google Scholar]
- 9.Fritz A, Percy C, Jack A. International classification of diseases of oncology. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 10.Diaz JI, Mora LB, Hakam A. The Mainz classification of renal cell tumors. Cancer Control. 1999;6:571–579. doi: 10.1177/107327489900600603. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. International classification of diseases for oncology. 3. Geneva, Switzerland: WHO; 2000. [Google Scholar]
- 12.North American Association of Central Cancer Registries, Logistics Requirements Working Group. NAACCR guideline for enhancing Hispanic/Latino identification: Revised NAACCR Hispanic/Latino identification algorithm [NHIA v2] Springfield, IL: North American Association of Central Cancer Registries; 2005. [Google Scholar]
- 13.Denny CH, Holtzman D, Cobb N. Surveillance for health behaviors of American Indians and Alaska Natives. Findings from the Behavioral Risk Factor Surveillance System, 1997-2000. MMWR Surveill Summ. 2003;52:1–13. [PubMed] [Google Scholar]
- 14.Jim MA, Espey DK, Wiggins CL, Cobb N, Wingo PA. Racial misclassification of American Indians residing near IHS facilities. Poster P-47, Final Program and Abstracts; Presented at the North American Association of Central Cancer Registries Conference; Regina, Saskatchewan, Canada. June 10-16; 2006. [Google Scholar]
- 15.National Cancer Institute, Surveillance Research Program, Cancer Statistics Branch. [July 12, 2008];SEER*Stat software. 2007 Available at: http://www.seer.cancer.gov/seerstat.
- 16.Ingram DD, Parker JD, Schenker N, et al. United States Census 2000 population with bridged race categories. Vital Health Stat 2. 2003 Sep;:1–55. [PubMed] [Google Scholar]
- 17.Shryock HS, Siegel JS. The methods and materials of demography. New York, NY: Academic Press; 1976. [Google Scholar]
- 18.Steele CB, Cardinez CJ, Richardson LC, Tom-Orme L, Shaw K. Surveillance for health behaviors of American Indians and Alaska Natives—findings from the Behavioral Risk Factor Surveillance System, 2000-2006. Cancer. 2008;113(5 suppl):1131–1141. doi: 10.1002/cncr.23727. [DOI] [PubMed] [Google Scholar]
- 19.Phillips JL. Springfield, IL: North American Association of Central Cancer Registries; 2003. [July 12, 2008]. Summary stage: data effects of the changes in 2000. Available at: www.naaccr.org. [Google Scholar]
- 20.Scelo G, Brennan P. The epidemiology of bladder and kidney cancer. Nat Clin Pract Urol. 2007;4:205–217. doi: 10.1038/ncpuro0760. [DOI] [PubMed] [Google Scholar]
- 21.Chow WH, Devesa SS, Moore LE. Epidemiology of renal cell carcinoma. In: Vogelzang NJ, Scardino PJ, Shipley WU, D FMJ, L WM, editors. Comprehensive textbook of genitourinary oncology. 2. Vol. 110 Philadelphia, PA: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 22.Doshi SR, Jiles R. Health behaviors among American Indian/Alaska Native women, 1998-2000 BRFSS. J Womens Health (Larchmt) 2006;15:919–927. doi: 10.1089/jwh.2006.15.919. [DOI] [PubMed] [Google Scholar]
- 23.Xu J, Eilat-Adar S, Loria CM, et al. Macronutrient intake and glycemic control in a population-based sample of American Indians with diabetes: the Strong Heart Study. Am J Clin Nutr. 2007;86:480–487. doi: 10.1093/ajcn/86.2.480. [DOI] [PubMed] [Google Scholar]
- 24.Quandt SA, Bell RA, Snively BM, et al. Ethnic disparities in glycemic control among rural older adults with type 2 diabetes. Ethn Dis. 2005;15:656–663. [PMC free article] [PubMed] [Google Scholar]
- 25.Story M, Stevens J, Himes J, et al. Obesity in American-Indian children: prevalence, consequences, and prevention. Prev Med. 2003;37(6 pt 2):S3–S12. doi: 10.1016/j.ypmed.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Flaherty KT, Fuchs CS, Colditz GA, et al. A prospective study of body mass index, hypertension, and smoking and the risk of renal cell carcinoma (United States) Cancer Causes Control. 2005;16:1099–1106. doi: 10.1007/s10552-005-0349-8. [DOI] [PubMed] [Google Scholar]
- 27.Setiawan VW, Stram DO, Nomura AM, Kolonel LN, Henderson BE. Risk factors for renal cell cancer: the multiethnic cohort. Am J Epidemiol. 2007;166:932–940. doi: 10.1093/aje/kwm170. [DOI] [PubMed] [Google Scholar]
- 28.Grossman E, Messerli FH, Goldbourt U. Antihypertensive therapy and the risk of malignancies. Eur Heart J. 2001;22:1343–1352. doi: 10.1053/euhj.2001.2729. [DOI] [PubMed] [Google Scholar]
- 29.Hayslett JA, Eichner JE, Yeh JL, et al. Hypertension treatment patterns in American Indians: the strong heart study. Am J Hypertens. 2001;14(9 pt 1):950–956. doi: 10.1016/s0895-7061(01)02146-x. [DOI] [PubMed] [Google Scholar]
- 30.Stewart JH, Buccianti G, Agodoa L, et al. Cancers of the kidney and urinary tract in patients on dialysis for endstage renal disease: analysis of data from the United States, Europe, and Australia and New Zealand. J Am Soc Nephrol. 2003;14:197–207. doi: 10.1097/01.asn.0000039608.81046.81. [DOI] [PubMed] [Google Scholar]
- 31.Kalantar-Zadeh K, Kopple JD. Obesity paradox in patients on maintenance dialysis. Contrib Nephrol. 2006;151:57–69. doi: 10.1159/000095319. [DOI] [PubMed] [Google Scholar]
- 32.Narva AS. The spectrum of kidney disease in American Indians. Kidney Int Suppl. 2003 Feb;:S3–S7. doi: 10.1046/j.1523-1755.63.s83.2.x. [DOI] [PubMed] [Google Scholar]
- 33.Lemley KV. A basis for accelerated progression of diabetic nephropathy in Pima Indians. Kidney Int Suppl. 2003 Feb;:S38–S42. doi: 10.1046/j.1523-1755.63.s83.9.x. [DOI] [PubMed] [Google Scholar]
- 34.Chugh SS, Wallner EI, Kanwar YS. Renal development in high-glucose ambience and diabetic embryopathy. Semin Nephrol. 2003;23:583–592. doi: 10.1053/s0270-9295(03)00136-0. [DOI] [PubMed] [Google Scholar]
- 35.Nelson RG, Morgenstern H, Bennett PH. Intrauterine diabetes exposure and the risk of renal disease in diabetic Pima Indians. Diabetes. 1998;47:1489–1493. doi: 10.2337/diabetes.47.9.1489. [DOI] [PubMed] [Google Scholar]
- 36.Bunt JC, Tataranni PA, Salbe AD. Intrauterine exposure to diabetes is a determinant of hemoglobin Ac and systolic blood pressure in pima Indian children. J Clin Endocrinol Metab. 2005;90:3225–3229. doi: 10.1210/jc.2005-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson RT, Silverman DK, Fraumeni JF, Curtis R. Multiple primaries in cancer survivors National Cancer Institute monograph. Bethesda, MD: National Cancer Institute; 2007. New malignancies following cancer of the bladder, renal pelvis and kidney. [Google Scholar]
- 38.Cohen D, Zhou M. Molecular genetics of familial renal cell carcinoma syndromes. Clin Lab Med. 2005;25:259–277. doi: 10.1016/j.cll.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Pavlovich CP, Schmidt LS, Phillips JL. The genetic basis of renal cell carcinoma. Urol Clin North Am. 2003;30:437–454. vii. doi: 10.1016/s0094-0143(03)00023-5. [DOI] [PubMed] [Google Scholar]
- 40.Linehan WM, Walther MM, Zbar B. The genetic basis ofcancer of the kidney. J Urol. 2003;170(6 pt 1):2163–2172. doi: 10.1097/01.ju.0000096060.92397.ed. [DOI] [PubMed] [Google Scholar]
- 41.Chow WH, Devesa SS, Warren JL, Fraumeni JF., Jr Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628–1631. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 42.Mathew A, Devesa SS, Fraumeni JF, Jr, Chow WH. Global increases in kidney cancer incidence, 1973-1992. Eur J Cancer Prev. 2002;11:171–178. doi: 10.1097/00008469-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 43.Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) program. Oncologist. 2007;12:20–37. doi: 10.1634/theoncologist.12-1-20. [DOI] [PubMed] [Google Scholar]
- 44.Gago-Dominguez M, Castelao JE. Lipid peroxidation and renal cell carcinoma: further supportive evidence and new mechanistic insights. Free Radic Biol Med. 2006;40:721–733. doi: 10.1016/j.freeradbiomed.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 45.Kabat GC, Silvera SA, Miller AB, Rohan TE. A cohort study of reproductive and hormonal factors and renal cell cancer risk in women. Br J Cancer. 2007;96:845–849. doi: 10.1038/sj.bjc.6603629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lambe M, Lindblad P, Wuu J, Remler R, Hsieh CC. Pregnancy and risk of renal cell cancer: a population-based study in Sweden. Br J Cancer. 2002;86:1425–1429. doi: 10.1038/sj.bjc.6600263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelly JJ, Lanier AP, Alberts S, Wiggins CL. Differences in cancer incidence among Indians in Alaska and New Mexico and U.S. Whites, 1993-2002. Cancer Epidemiol Biomarkers Prev. 2006;15:1515–1519. doi: 10.1158/1055-9965.EPI-05-0454. [DOI] [PubMed] [Google Scholar]
- 48.Silverman DT, Devesa SS, Moore LE, Rothman N. Bladder cancer. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer epidemiology and prevention. 3. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 49.Lanier AP, Kelly JJ, Maxwell J, McEvoy T, Homan C. Cancer in Alaska Native people, 1969-2003. Alaska Med. 2006;48:30–59. [PubMed] [Google Scholar]
- 50.Bliss A, Cobb N, Solomon T, Cravatt K, Marshall L, Campbell J. Lung cancer incidence among American Indians and Alaska Natives in the United States, 1999-2004. Cancer. 2008;113(5 suppl):1168–1178. doi: 10.1002/cncr.23738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tripathi A, Folsom AR, Anderson KE. Risk factors for urinary bladder carcinoma in postmenopausal women. The Iowa Women’s Health Study Cancer. 2002;95:2316–2323. doi: 10.1002/cncr.10975. [DOI] [PubMed] [Google Scholar]
- 52.Larsson SC, Orsini N, Brismar K, Wolk A. Diabetes mellitus and risk of bladder cancer: a meta-analysis. Diabetologia. 2006;49:2819–2823. doi: 10.1007/s00125-006-0468-0. [DOI] [PubMed] [Google Scholar]
- 53.Garcia-Closas M, Malats N, Silverman D, et al. NAT2 slow acetylation, GSTM1 null genotype, and risk of bladder cancer: results from the Spanish Bladder Cancer Study and meta-analyses. Lancet. 2005;366:649–659. doi: 10.1016/S0140-6736(05)67137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


