Abstract
Background
Nonadherence to drug treatment is a major contributor to antihypertensive treatment failure. Mood disorders could impair the patient's desire and ability to follow physician's recommendations. We evaluated the role of symptoms of depression and anxiety on adherence to antihypertensive drug treatment.
Methods
We conducted a longitudinal cohort study in 20–70 years old patients starting antihypertensive drug treatment, without other chronic conditions, and not taking mood-modifying drugs. Severity of symptoms of depression and anxiety were evaluated at enrollment and 3, 6, 9, and 12 months of follow-up, using the Beck depression inventory-II (BDI-II) and the psychological general well-being index (PGWB), respectively. Treatment adherence was measured by pill count. Nonadherence was defined as taking <80% of the prescribed number of pills. Poisson regression was used to model the association of the exposures with adherence.
Results
We enrolled 178 patients (58% male; mean age: 50 years; 508 follow-up visits). The risk of nonadherence was 52.6% in 12 months (95% confidence interval (CI): 46.1, 59.1). After adjusting for other risk factors, individuals with at least mild depression (BDI-II ≥14) and those with at least mild anxiety (PGWB anxiety score <22) were 2.48 (95% CI: 1.47, 4.18) and 1.59 (95% CI: 0.99, 2.56) times more likely to become nonadherent in the following 3 months, respectively.
Conclusions
Patients with at least mild anxiety and depression symptoms are at increased risk of becoming nonadherent to antihypertensive medication. Screening for depression and anxiety symptoms could be used to identify high-risk patients. Further evidence is needed to elucidate whether interventions targeting these conditions improve adherence.
Keywords: anxiety, blood pressure, cohort studies, depression, hypertension, pateint non-adherence
In spite of considerable advances in the treatment of hypertension, only 69% of known hypertensive patients receiving pharmacologic treatment in the United States have their blood pressure controlled.1 Hypertension control rates in other developed countries are even lower, ranging from 20 to 50%.2 Failure to control hypertension is largely due to nonadherence to prescribed treatments. In fact, adherence during the first year of treatment is about 50%3,4 and 50% of patients with refractory hypertension are in fact nonadherent.5 Nonadherence lessens the efficacy of antihypertensive drugs in preventing stroke, coronary heart disease, and chronic kidney disease.6 Moreover, increasing antihypertensive drug use by 20% would result in a fourfold return on investment, mostly attributable to a reduction in hospitalization rates.7
Although multiple risk factors have been proposed, only regimen complexity,8,9 and drug-related side effects9 have been shown to be clearly associated with prospective adherence. Owing to its high incidence and large health impact, it is imperative to identify factors associated to nonadherence, particularly those that are amenable to change. Depression and anxiety could impair cognitive focus, energy, and motivation and might affect the desire and ability of the patient to follow treatment recommendations.10 Indeed, cross-sectional studies have shown higher nonadherence in hypertensive patients with increased severity of depression symptoms,11,12 but these studies are particularly prone to selection, information, and reverse causality bias.13
The aim of our study was to assess the impact of symptoms of depression and anxiety on adherence to antihypertensive medication in a cohort of newly treated hypertensive patients.
Methods
We conducted a longitudinal cohort study among patients 20–70 years old with essential hypertension who had been taking medication for up to 1 week. Hypertension was diagnosed as a systolic/diastolic blood pressure ≥140/90 mm Hg, based on the mean of ≥2 measures taken on separate office visits, before the start of treatment. Pregnant women, patients with self-reported history of cancer, diabetes, rheumatoid arthritis, psychiatric disease requiring drug treatment, coronary heart disease, congestive heart failure, chronic kidney disease, and hepatitis, and those taking mood-modifying medications (paroxetine, sertraline, citalopram, escitalopram, buspirone, zolpidem, trazodone, bupropion, gabapentin, amitriptyline, atomoxetine, carbamazepine, clonazepam, temazepam, diazepam, alprazolam, and mirtazapine) were not eligible. Patients were recruited from clinics at the Department of Family Medicine, University of Wisconsin at Madison, and the Wisconsin Research and Education Network. They were paid $20 per study visit as compensation for their participation. This study was approved by the University of Wisconsin at Madison institutional review board.
Data on type of antihypertensive medications, doses per day, and treatment changes were carefully collected at enrollment and at follow-up visits at 3, 6, 9, and 12 months after the start of treatment. Treatment adherence was defined using pill count data obtained during follow-up visits. Additional baseline data included age, gender, race, education, occupational status, comorbidities, copayment of medications, and the number of physician visits, hospital admissions, and medication use in the year preceding the start of antihypertensive therapy.
Severity of depression and anxiety symptoms was evaluated at baseline and at follow-up visits. We used the Beck depression inventory-II (BDI-II),14 a proven reliable and valid tool,15 to assess depression symptoms severity. Following current guidelines, participants were classified based on their BDI-II scores as having minimal or no depression (0–13); mild depression (14–19); moderate depression (20–28); and severe depression (29–63).14 In addition, the level of anxiety was measured with the anxiety subscale of the psychological general well-being index (PGWB),16 and patients were classified by quartiles of the anxiety scores as having minimal (22–25), mild (19–21), moderate (16–18), or severe anxiety (1–15). Those with a score <22 were considered as having at least mild anxiety. These classifications were used for the analysis, but patients in these groups should not be considered clinically depressed or having anxiety disorder based solely on these scores.
The physical symptoms distress index,17 the sexual symptoms distress index,18 and the sleep dysfunction scale18 were used to evaluate symptoms related to antihypertensive medications, sexual function, and sleep problems, respectively. These data forms, as well as those on depression and anxiety symptoms, were completed in private by the participants, and then put in sealed envelopes until data entry. Seated blood pressure was measured three times at each visit, following standard recommendations,19 with a validated automatic device (Omron HEM705CP; Omron Healthcare, Vernon Hills, IL).20 The average of the last two measures was used in the analysis.
We estimated the percent treatment adherence as the proportion of pills taken out of the total prescribed between two consecutive visits (about 3 months apart). Nonadherence was defined conventionally as missing more than 20% of the prescribed doses between consecutive visits.21 We calculated the incidence rate of nonadherence between consecutive visits (cases/person–time), converted the rate to cumulative risk using the exponential approach, calculated the survival free of nonadherence in each period (1 minus the risk), multiplied the survival in each period to obtain the 12-month cumulative survival, and then calculated the 12-month cumulative risk as 1 minus the cumulative survival.22
The effects of the exposures of interest were estimated using Poisson and linear regression, with nonadherence and percent adherence as the outcome variable, respectively. Each period between visits was considered as a separate observation and exposures, outcomes, and confounders were reclassified accordingly. Standard errors were adjusted for clustering at the individual level, to account for the use of repeated observations in the same participant. Results from mixed regression models with a random intercept (not reported) were similar to those from the Poisson and linear regression. Age and gender were forced in all models, and the following variables were retained only if they significantly predicted adherence or confounded the effect of the exposures by more than 10%:23 number of antihypertensive pills per day, marital status, race, education, employment status, average family income, house tenancy, health insurance, physician visits and hospitalizations in the last year, medication copayment, use of medications for chronic diseases, self-reported health status, self-reported physical, sexual, and sleep symptoms distress, and current smoking and alcohol intake. We also fitted a first-order autoregressive model to estimate the effect on adherence of changes in the exposure between consecutive visits within the same individual.24 These “within-individual effects” are more amenable to causal inference than population-average effects, which are a combination of both between-individual (cross-sectional) and within-individual (longitudinal) effects.
Results
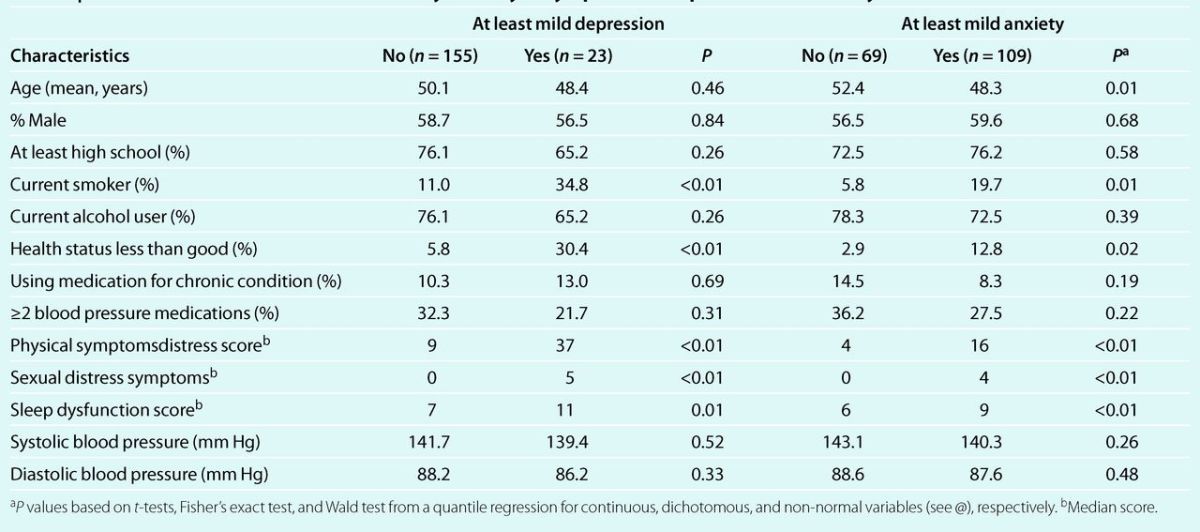
We recruited 214 patients 23–69 years old. A total of 178 with at least 1 follow-up visit are included in this analysis. They contributed 508 out of a possible maximum of 594 follow-up visits and an average follow-up time of 271.2 days (range 68–425 days). Most patients (66%) were 40–60 years old (mean age 49.9 years), 42% (n = 74) were women, 88.8% were white, and 6.2% were African American. Compared to patients with minimal symptoms those with at least mild depression and those with at least mild anxiety were more likely to be smokers, to report less than good health, and to have physical, sexual, and sleep distress symptoms. However, both groups were similar regarding gender, education, alcohol use, smoking, use of medication for chronic conditions, number of antihypertensive medications, and blood pressure level (Table 1).
Table 1.
Baseline characteristics of the cohort by severity of symptoms of depression and anxiety
At enrollment 95% (n = 169) of all patients were taking one antihypertensive pill, but the pill had two blood pressure- lowering drugs in 9.5% of them (n = 16). Eight additional patients (4.5%) were taking two pills with two different drugs, and one patient was taking two pills with three drugs. The antihypertensive medications most frequently used at baseline were diuretics (44.4%), angiotensin-converting enzyme-inhibitors (32.6%), and calcium channel blockers (14.0%). Only 11% were regular users of medications for other chronic conditions and 3.4% had no health insurance.
The average BDI-II score was 4.9 points (95% confidence interval (CI): 3.9, 6.0). The prevalence of at least mild depression (BDI-II score ≥14) through the study was slightly higher in women than in men: 10.7% (95% CI: 4.2, 17.3) vs. 10.0 (95% CI: 5.0, 15.0) and ranged from 7.6% in visit two to 12.2% at baseline. Overall, 74 episodes of at least mild depression were experienced by 32 subjects during the follow-up. The median PGWB anxiety score was 18.6 points (95% CI: 18.1, 19.2) and was also similar in men and women and throughout the follow-up.
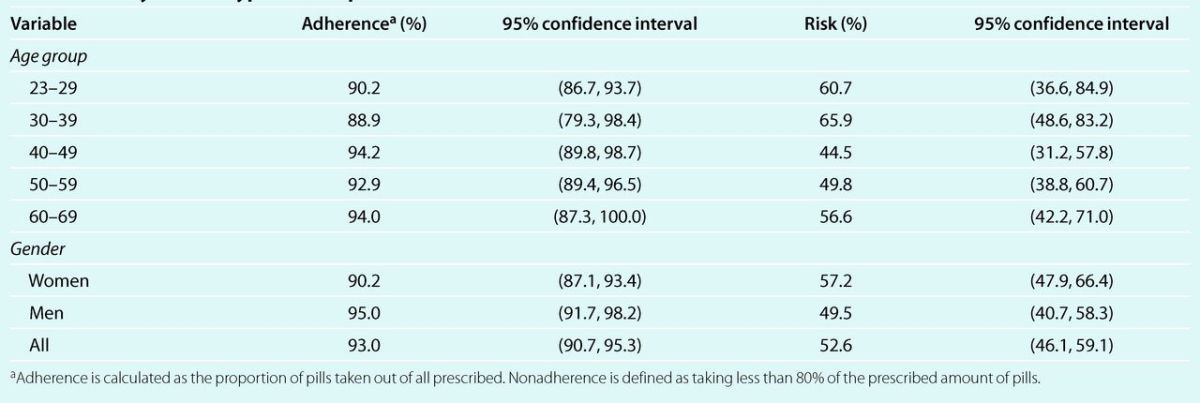
On average patients took 93% of all the prescribed pills, and adherence was at or above 90% in all age groups in both men and women (Table 2). A total of 60 patients were nonadherent in 1 visit; 16 in 2 visits; 2 in 3 visits; and 1 in 4 visits. The total number of instances of nonadherence was 102. Cumulative risk of nonadherence was 23.6% between baseline and the first follow-up visit (about 3 months apart), 16.6% between follow-up visits 1 and 2, 12.1% between follow-up visits 2 and 3, and 15.4% between follow-up visits 3 and 4. The 12-month cumulative risk of nonadherence was 52.6% (95% CI: 46.1, 59.1), and was somehow higher in women than in men. Moreover, the risk of nonadherence was higher in the youngest and oldest, and lowest in the middle-aged group (Table 2).
Table 2.
Average adherence and 12-month cumulative risk of nonadherence to antihypertensive medication by age and gender in a cohort of newly treated hypertensive patients
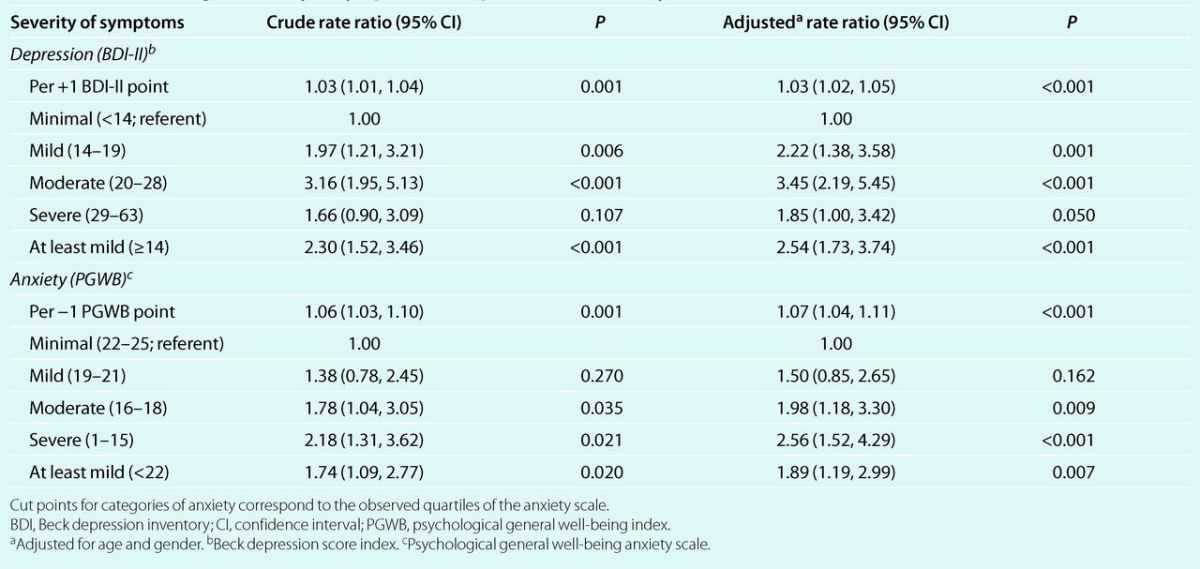
Crude and age–gender adjusted analyses showed that the incidence of nonadherence increased 1.03 times (P < 0.001) for each increase of 1 point in the BDI-II score (Table 3). Also, incident nonadherence was more than two times higher in patients who had at least mild depression, as compared to those with minimal depression (rate ratio: 2.54; P < 0.001). On the other hand, for each decrease of 1 point in the PGWB anxiety score, the age- and gender-adjusted incidence of nonadherence increased 7% (P < 0.001; Table 3). Moreover, the incidence of nonadherence increased progressively and significantly in patients with mild, moderate, and severe anxiety, as compared to those with minimal anxiety. In fact, in patients with at least mild anxiety, the risk of nonadherence increased 1.89 times (P = 0.01).
Table 3.
Crude and age- and gender-adjusted rate ratios (95% confidence interval) for incident nonadherence to antihypertensive medication, according to severity of symptoms of depression and anxiety
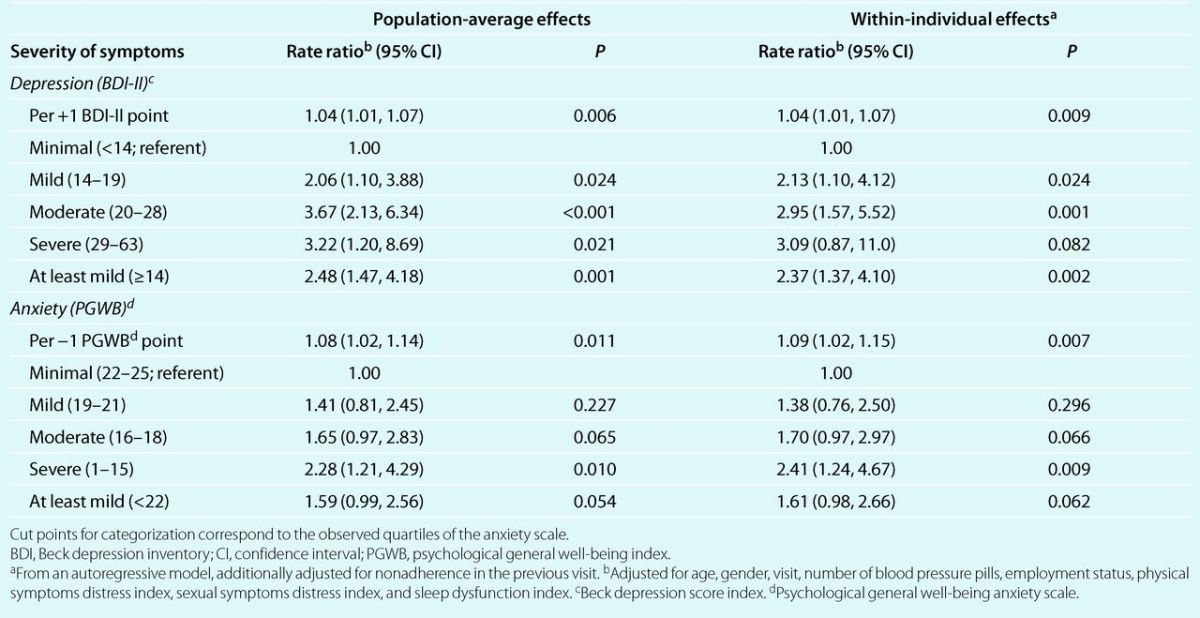
Associations adjusted for additional risk factors were consistent with those from the age- and gender-adjusted analysis (Table 4). Specifically, patients with mild depression were twice and those with moderate and severe depression were three times more likely to become nonadherent than patients with minimal depression. Overall, patients with at least mild depression were 2.48 times more likely to become nonadherent (95% CI: 1.47, 4.18; P < 0.01). Significantly higher incidence of nonadherence was also observed in patients with anxiety after adjustment for other risk factors. Indeed, the risk of nonadherence increased progressively with higher levels of anxiety, up to being 2.28 times higher in patients with severe than in those with minimal anxiety (P = 0.01). For patients with at least mild anxiety, the incidence of nonadherence increased 1.59 times (P = 0.05). The within-individual effects of both depression and anxiety were strikingly similar to the population-average (marginal) effects (Table 4). For instance, if an individual experienced a change from a BDI-II score <14 to a score ≥14 his/her risk of becoming nonadherent in the following 3 months increased by a factor of 2.37, a figure very close to the population-average effect of 2.48.
Table 4.
Population-average and within-individual multivariate adjusted rate ratios for incident nonadherence to antihypertensive medication, according to severity of symptoms of depression and anxiety.
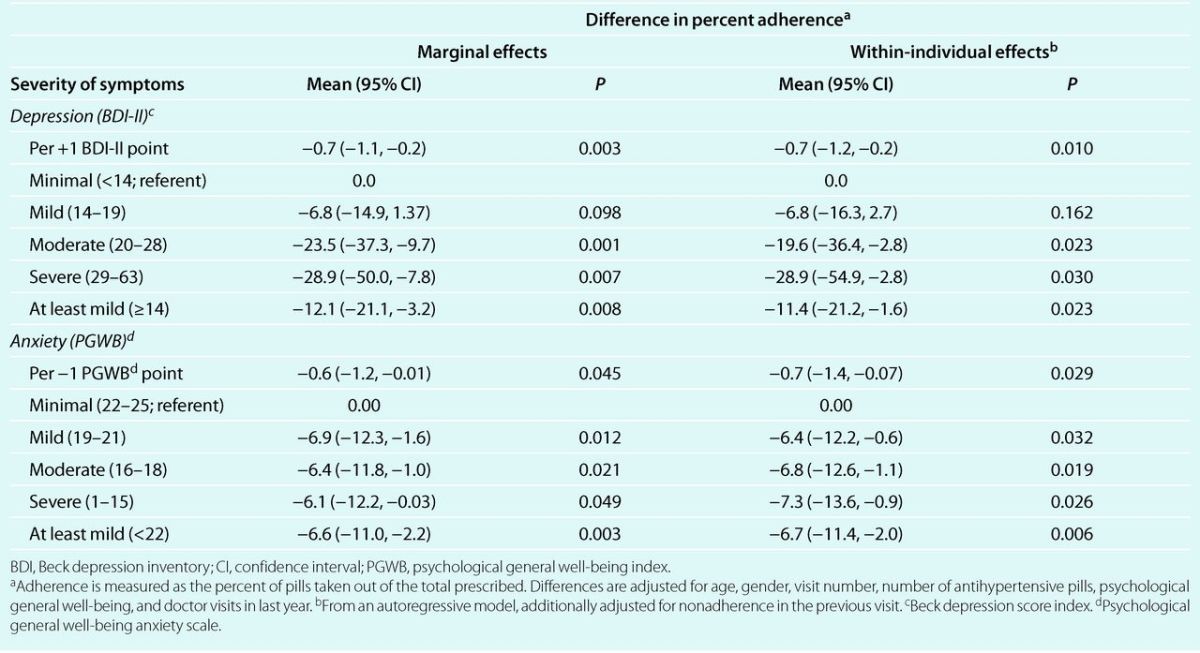
Depression and anxiety symptoms were also significant predictors of percent adherence (Table 5). After adjustment for other risk factors, adherence in the next 3 months decreased by 6.8, 23.5, and 28.9 percent-points in patients with mild, moderate, and severe depression, as compared to those with minimal depression, and the trend was statistically significant (P < 0.01). Also, adherence decreased 12.1 percent-points in those with at least mild depression (P = 0.01). On the other hand, adherence decreased significantly in patients with any level of anxiety, as compared to minimal anxiety, but a trend with increasing anxiety was not statistically significant (P = 0.09, from autoregressive model). Nevertheless, patients with at least mild anxiety experienced a prospective decrease of 6.6 percent points in adherence, as compared to those with minimal anxiety (P < 0.01). Within-individual effects followed closely the population-average effects (Table 5).
Table 5.
Population-average and within-individual multivariate adjusted changes in percent adherence to antihypertensive medication, according to severity of symptoms of depression and anxiety
Average treatment adherence during the period preceding a visit was weakly associated with current blood pressure levels. Age- and gender-adjusted systolic and diastolic blood pressure were 1.61 (P = 0.28) and 1.44 (P = 0.18) mm Hg higher, respectively, in patients who were nonadherent to treatment, but the increase was not statistically significant.
Discussion
This study shows that newly treated patients with at least mild depression and anxiety symptoms are at increased risk of becoming nonadherent to antihypertensive medication. Patients with at least mild depression symptoms were 2.5 times and those with at least mild anxiety symptoms were 1.6 times more likely to become nonadherent in the following 3 months than patients with minimal symptoms. To the best of our knowledge, this is the first longitudinal cohort study showing an association between symptoms of these disorders and adherence to treatment.
Our finding of an association between depression symptoms and treatment adherence expands and corroborates those from previous studies. Wang et al.25 found that hypertensive patients ≥65 years old with clinical depression were 50% less likely to fill out at least one prescription for antihypertensive drugs during the following year. Also, Siegel et al. found a 16% increase in nonadherence (medication possession ratio <80%) in the following year in patients with clinical depression (P < 0.001).26 Lower treatment adherence among patients with depression symptoms has also been reported in cross-sectional studies.11,12 Unfortunately, cross-sectional studies of treatment adherence are particularly prone to both selection and information bias,13 limitations that are less likely in longitudinal studies.
As far as we know, there are no previous reports of an association between anxiety symptoms and adherence to antihypertensive medication. Results from a small meta-analysis show that associations with adherence to other medications are mostly uncertain.10
The effects of depression and anxiety symptoms on adherence may be mediated by the patient's perception of his/her capacity to carry out a specific task (self-efficacy expectation).27,28
Patients with low self-efficacy expectation make less efforts in taking medication as prescribed,27 and are also more prone to maximize the severity of obstacles to treatment adherence. This could lead to inefficacious thinking, impaired level of functioning, and low treatment adherence. Also, patients with anxiety may have low coping and thought control self-efficacy and this could enhance avoidance behavior.27 Briefly, perceived self-efficacy seems to have a direct impact on whether patients decide to follow treatment recommendations, whether they enlist the motivation and perseverance needed to succeed, and how well they maintain their treatment related habits.
The relationship between self-efficacy expectation, mood disorders, and treatment adherence appears to occurs in feedback loops. Low self-efficacy expectation seems to be a risk factor for,29,30 as well as a consequence of, symptoms of depression and anxiety.31,32 In turn, depression and anxiety symptoms could lead to10,33 and result from low treatment adherence.31,32 Finally, low adherence decreases self-efficacy expectation,32 which could by itself decrease treatment adherence.27 Therefore, investigating whether successful intervention in one of these factors could improve the other factors and lead to better blood pressure control is warranted.
We found that blood pressure was higher in patients who were poor compliers, but not significantly so. This finding could be explained by high adherence, particularly in the days preceding the study visit (“white-coat adherence”),34,35 and suggests that measurements of office blood pressure may be of little use and that regular home blood pressure monitoring or more direct adherence measurements such as self-report, pill-count, or review of pharmacy records, may be needed to identify patients with poor adherence to medication.
The longitudinal cohort design is a major strength of our study. By evaluating participants every 3 months, we reliably assessed symptoms of depression and anxiety and treatment adherence, and ascertained that severity of current symptoms influenced adherence in the following months. In addition, this design allowed us to measure the relationship between longitudinal changes in symptoms and longitudinal changes in adherence, and to isolate the actual effects of predictor variables (within-subject effects) from their cross-sectional effects (between-subject effects).
Another strength of our study is the use of pill count to assess adherence. Although pill count is known to overestimate adherence,36,37 its performance in identifying non-adherers and adherence patterns over time is similar to that of electronic pill-bottles, a potentially more accurate method.38 Moreover, pill count avoids the social desirability response bias that could occur with self-reported adherence. Using pill count was also important because patients with more severe symptoms of depression could under-report drug use (pessimism bias).39 Lastly, we are confident that confounding bias is an unlikely explanation of our findings because withinsubjects effects, which are free from confounding by measured and unmeasured time-fixed variables, such as gender and genetic make-up, were similar to the population-average effects. Nevertheless, we cannot rule out the possibility of confounding by unmeasured variables that changed with time.
Some limitations qualify our study inferences. Studying a homogeneous group of relatively healthy patients starting antihypertensive medication improved the validity, but compromised the generalizability of our findings. Therefore, we recommend replication in patients with concomitant chronic conditions such as diabetes and coronary heart disease and with ongoing antihypertensive treatment. Also, study procedures such as informed consent and pill count could have improved adherence. However, this increase in adherence should have been similar in all patients, resulting in little bias in the estimate of the rate ratio. Moreover, 14.5% of the follow-up visits were missing, but selection bias seems unlikely because risk factors for nonadherence were not associated with missing visits in a multivariate model (data not shown). A lack of gender difference in depression scores may have resulted from lower participation rate among women with higher depression score. Nevertheless, adjusting by gender likely should have prevented any bias resulting from this type of selective participation.
In summary, we have found that severity of symptoms of depression and anxiety were associated with lower prospective adherence to antihypertensive medication among patients starting treatment. Although adherence level was not significantly associated with blood pressure in our study, a finding likely explained by the high level of adherence, symptoms of depression and anxiety could result in poor adherence and poor blood pressure control in other groups of patients. In consequence, testing for depression and anxiety symptoms could be useful for identifying patients at high risk of nonadherence. Future research should examine our findings and test whether managing depression and anxiety symptoms or improving self-efficacy expectation may contribute to better treatment adherence and improved blood pressure control.
Acknowledgment
This study was funded by the American Heart Association, Award No. MSN101929 and by the University of Wisconsin Institute for Clinical and Translational Research (NIH Clinical and Translational Science Award, Award No. 1 UL1 RR025011).
Disclosure
The authors declared no conflict of interest.
References
- 1.Egan BM.Zhao Y.Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA 2010;303:2043–2050 [DOI] [PubMed] [Google Scholar]
- 2.Wolf-Maier K.Cooper RS.Kramer H.Banegas JR.Giampaoli S.Joffres MR.Poulter N.Primatesta P.Stegmayr B.Thamm M. Hypertension treatment and control in five European countries, Canada, and the United States. Hypertension 2004;43:10–17 [DOI] [PubMed] [Google Scholar]
- 3.Vrijens B.Vincze G.Kristanto P.Urquhart J.Burnier M. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ 2008;336:1114–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan SG.Yan L. Persistence with hypertension treatment among community-dwelling BC seniors. Can J Clin Pharmacol 2004;11:e267–e273 [PubMed] [Google Scholar]
- 5.Oparil S.Calhoun DA. Managing the patient with hard-to-control hypertension. Am Fam Physician 1998;57:1007–14, 1019–1020 [PubMed] [Google Scholar]
- 6.Mazzaglia G.Ambrosioni E.Alacqua M.Filippi A.Sessa E.Immordino V.Borghi C.Brignoli O.Caputi AP.Cricelli C.Mantovani LG. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation 2009;120:1598–1605 [DOI] [PubMed] [Google Scholar]
- 7.Sokol MC.McGuigan KA.Verbrugge RR.Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care 2005;43:521–530 [DOI] [PubMed] [Google Scholar]
- 8.Dezii CM. A retrospective study of persistence with single-pill combination therapy vs. concurrent two-pill therapy in patients with hypertension. Manag Care 2000;9:2–6 [PubMed] [Google Scholar]
- 9.Bloom BS. Continuation of initial antihypertensive medication after 1 year of therapy. Clin Ther 1998;20:671–681 [DOI] [PubMed] [Google Scholar]
- 10.DiMatteo MR.Lepper HS.Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med 2000;160:2101–2107 [DOI] [PubMed] [Google Scholar]
- 11.Morris AB.Li J.Kroenke K.Bruner-England TE.Young JM.Murray MD. Factors associated with drug adherence and blood pressure control in patients with hypertension. Pharmacotherapy 2006;26:483–492 [DOI] [PubMed] [Google Scholar]
- 12.Wang PS.Bohn RL.Knight E.Glynn RJ.Mogun H.Avorn J. Noncompliance with antihypertensive medications: the impact of depressive symptoms and psychosocial factors. J Gen Intern Med 2002;17:504–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bautista LE. Response to “depression and adherence to antihypertensive therapy”. Am J Hypertens 2008;21:726. [DOI] [PubMed] [Google Scholar]
- 14.Beck AT.Steer RA.Brown GK. Manual for Beck Depression Inventory-II. Psychological Corporation: San Antonio, TX, 1996 [Google Scholar]
- 15.Arnau RC.Meagher MW.Norris MP.Bramson R. Psychometric evaluation of the Beck Depression Inventory-II with primary care medical patients. Health Psychol 2001;20:112–119 [DOI] [PubMed] [Google Scholar]
- 16.Dupuy HJ. Utility of the National Center for Health Statistics General Well-Being Schedule in the Assessment of Self-Representations of Subjective Well-Being and Distress. The national conference on evaluation in alcohol, drug abuse and mental health programs ADAMHA: Washington, DC, 1974 [Google Scholar]
- 17.Anderson RB.Hollenberg NK.Williams GH. Physical Symptoms Distress Index: a sensitive tool to evaluate the impact of pharmacological agents on quality of life. Arch Intern Med 1999;159:693–700 [DOI] [PubMed] [Google Scholar]
- 18.Croog SH.Levine S.Testa MA.Brown B.Bulpitt CJ.Jenkins CD.Klerman GL.Williams GH. The effects of antihypertensive therapy on the quality of life. N Engl J Med 1986;314:1657–1664 [DOI] [PubMed] [Google Scholar]
- 19.Pickering TG.Hall JE.Appel LJ.Falkner BE.Graves J.Hill MN.Jones DW.Kurtz T.Sheps SG.Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005;111:697–716 [DOI] [PubMed] [Google Scholar]
- 20.O'Brien E.Mee F.Atkins N.Thomas M. Evaluation of three devices for self-measurement of blood pressure according to the revised British Hypertension Society Protocol: the Omron HEM-705CP, Philips HP5332, and Nissei DS-175. Blood Press Monit 1996;1:55–61 [PubMed] [Google Scholar]
- 21.Ebrahim S. Detection, adherence and control of hypertension for the prevention of stroke: a systematic review. Health Technol Assess 1998;2:i–iv, 1–78 [PubMed] [Google Scholar]
- 22.Collet D. Some non-parametric procedures. Modelling Survival Data in Medical Research. Chapman & Hall: New York, NY, 1994 [Google Scholar]
- 23.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health 1989;79:340–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Twisk JWR. Applied Longitudinal Data Analysis for Epidemiology: A Practical Guide. Cambridge University Press: Cambridge, UK, 2003 [Google Scholar]
- 25.Wang PS.Avorn J.Brookhart MA.Mogun H.Schneeweiss S.Fischer MA.Glynn RJ. Effects of noncardiovascular comorbidities on antihypertensive use in elderly hypertensives. Hypertension 2005;46:273–279 [DOI] [PubMed] [Google Scholar]
- 26.Siegel D.Lopez J.Meier J. Antihypertensive medication adherence in the Department of Veterans Affairs. Am J Med 2007;120:26–32 [DOI] [PubMed] [Google Scholar]
- 27.Bandura A. Self-efficacy. In Ramachaudran VS. (ed), Encyclopedia of Human Behavior. Academic Press: New York, 1994 [Google Scholar]
- 28.Schoenthaler A.Ogedegbe G.Allegrante JP. Self-efficacy mediates the relationship between depressive symptoms and medication adherence among hypertensive African Americans. Health Educ Behav 2009;36:127–137 [DOI] [PubMed] [Google Scholar]
- 29.Bandura A.Pastorelli C.Barbaranelli C.Caprara GV. Self-efficacy pathways to childhood depression. J Pers Soc Psychol 1999;76:258–269 [DOI] [PubMed] [Google Scholar]
- 30.Muris P. Relationships between self-effcacy and symptoms of anxiety disorders and depression in a normal adolescent sample. Pers Individ Differ 2002;32:337–348 [Google Scholar]
- 31.Wing RR.Phelan S.Tate D. The role of adherence in mediating the relationship between depression and health outcomes. J Psychosom Res 2002;53:877–881 [DOI] [PubMed] [Google Scholar]
- 32.Sacco WP.Wells KJ.Vaughan CA.Friedman A.Perez S.Matthew R. Depression in adults with type 2 diabetes: the role of adherence, body mass index, and self-efficacy. Health Psychol 2005;24:630–634 [DOI] [PubMed] [Google Scholar]
- 33.Stilley CS.Sereika S.Muldoon MF.Ryan CM.Dunbar-Jacob J. Psychological and cognitive function: predictors of adherence with cholesterol lowering treatment. Ann Behav Med 2004;27:117–124 [DOI] [PubMed] [Google Scholar]
- 34.Feinstein AR. On white-coat effects and the electronic monitoring of compliance. Arch Intern Med 1990;150:1377–1378 [PubMed] [Google Scholar]
- 35.Cramer JA.Scheyer RD.Mattson RH. Compliance declines between clinic visits. Arch Intern Med 1990;150:1509–1510 [PubMed] [Google Scholar]
- 36.Hamilton GA. Measuring adherence in a hypertension clinical trial. Eur J Cardiovasc Nurs 2003;2:219–228 [DOI] [PubMed] [Google Scholar]
- 37.Guerrero D.Rudd P.Bryant-Kosling C.Middleton B.Middleton BF [corrected to Middleton B. Antihypertensive medication-taking. Investigation of a simple regimen. Am J Hypertens 1993;6:586–592 [DOI] [PubMed] [Google Scholar]
- 38.Liu H.Golin CE.Miller LG.Hays RD.Beck CK.Sanandaji S.Christian J.Maldonado T.Duran D.Kaplan AH.Wenger NS. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med 2001;134:968–977 [DOI] [PubMed] [Google Scholar]
- 39.Morgado A.Raoux N.Smith M.Allilaire JF.Widlöcher D. Subjective bias in reports of poor work adjustment in depressed patients. Acta Psychiatr Scand 1989;80:541–547 [DOI] [PubMed] [Google Scholar]


