In this randomized open-label trial, tribendimidine was shown to have an efficacy comparable to praziquantel for the treatment of Clonorchis sinensis infection. Patients treated with praziquantel experienced significantly more adverse events than tribendimidine recipients.
Keywords: tribendimidine, praziquantel, Clonorchis sinensis, clonorchiasis, People's Republic of China
Abstract
Background. Clonorchiasis is of considerable public health importance, particularly in the People's Republic of China (PR China), where most of the 15 million individuals infected with Clonorchis sinensis are currently concentrated. Praziquantel is the drug of choice, but tribendimidine might be an alternative.
Methods. We performed a randomized open-label trial in Guangxi, PR China, to assess the efficacy and safety of 400 mg tribendimidine once, 400 mg tribendimidine daily for 3 days, and 75 mg/kg praziquantel in 1 day divided in 3 doses against parasitological-confirmed C. sinensis infections. Cure and egg reduction rates were determined 3 weeks posttreatment using available case analysis. Clinical symptoms were documented at baseline, and adverse events were recorded and graded 3 and 24 hours after each dose.
Results. A total of 74 patients were included in the final analysis. Single-dose tribendimidine achieved a cure rate of 44%, whereas cure rates of 58% and 56% were obtained for tribendimidine administered for 3 days and praziquantel, respectively. High egg reduction rates (97.6%–98.8%) were observed for all treatment regimens. Single-dose tribendimidine was the best-tolerated treatment scheme. Patients treated with praziquantel experienced significantly more adverse events than did tribendimidine recipients (P < .05).
Conclusions. Tribendimidine has an efficacy comparable to praziquantel in the treatment of C. sinensis infection and resulted in fewer adverse events compared to praziquantel. Larger clinical trials are warranted among C. sinensis–infected patients to determine the potential of tribendimidine against clonorchiasis and other helminthiases.
Clinical Trials Registration. Controlled-Trials.com, ISRCTN80829842.
INTRODUCTION
An estimated 15 million people, most of them living in the People's Republic of China (PR China), are infected with the liver fluke Clonorchis sinensis, resulting in a global burden of at least 275 370 disability-adjusted life-years (DALYs) [1]. The consumption of raw freshwater fish is a popular tradition not just in PR China but also in Vietnam and South Korea. Infections occur when raw freshwater fish, harboring infective metacercariae, are consumed. Pathological changes caused by C. sinensis infection are largely confined to the bile duct, liver, and gallbladder. Individuals with heavy infections often suffer from unspecific symptoms, such as fever, fatigue, weakness, nausea, or abdominal pain, but more severe consequences, namely obstructive jaundice and biliary colic, might occur [2]. The most serious complication of C. sinensis infection is cancer of the bile ducts, cholangiocarcinoma [3].
Praziquantel, administered at 3 doses of 25 mg/kg each at 5-hour intervals, is the drug of choice against clonorchiasis [4]. This treatment schedule is highly efficacious, with an egg reduction rate (ERR) of 99% [5]. However, transient adverse events, such as dizziness, sleepiness, headache, and diarrhea, are commonly observed after praziquantel administration [4]. Effective alternative drugs with a good safety profile are not available.
The Chinese anthelmintic drug tribendimidine might be a potential new drug candidate for treating infections with C. sinensis. Tribendimidine was observed to be highly effective in C. sinensis–infected rats [6] and in vitro [7]. In a randomized open-label trial in Lao People's Democratic Republic (Lao PDR), tribendimidine administered as a single oral dose of 200 mg (subjects <14 years of age) or 400 mg (subjects aged ≥14 years) to patients with parasitologically confirmed Opisthorchis viverrini infections, a related liver fluke, was found to be as efficacious as praziquantel (75 mg/kg given in 2 doses) [8]. Clinical trials with tribendimidine have therefore been suggested to determine the efficacy and safety of this drug in patients infected with C. sinensis [4, 9]. Tribendimidine is registered for human use for the treatment of infections with soil-transmitted helminths in PR China. A consortium composed of Chinese, Swiss, and US partners are currently seeking regulatory approval for the use of tribendimidine outside PR China [10], as studies in PR China have demonstrated that the drug is safe and efficacious against hookworm and Ascaris lumbricoides infections [11, 12].
The aim of this trial was to study and compare the efficacy and safety of tribendimidine, given as a single-dose or 3-day regimen, the latter already tested for the treatment of Trichuris trichiura infection [13], with the standard treatment of praziquantel in C. sinensis–infected individuals.
PATIENTS AND METHODS
Ethics Statement
The study was approved by the ethics committees in Basel, Switzerland (EKBB; reference nos. 209/09 and 375/11), the Liverpool School of Tropical Medicine (reference no. 12.02RS), and the National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention (reference no. 2012–02).
Drugs
Tribendimidine (200 mg enteric-coated tablets) was purchased from Shandong Xinhua Pharmaceutical Co, Ltd (Zibo, PR China). Praziquantel (200 mg film-coated tablets) was the product of Nanjing Pharmaceutical Factory Co, Ltd (Nanjing, PR China).
Study Area and Participants
The study was conducted from June to July 2012 in a C. sinensis–endemic village (Hezhuang) located under the township of Gantang, Binyang county, Guangxi Zhuang Autonomous Region, PR China. Most villagers belong to the Zhuang ethnic group and are farmers. Living standards are relatively low as compared to the more developed regions of the country but literacy rate is high, with all villagers having received at least primary-school education.
Eligible individuals were adults of both sexes, aged ≥18 years. During the clinical examination by physicians, villagers who had no major systemic, acute, or chronic illnesses, no known or reported psychiatric or neurological disorders, or hypersensitivity to tribendimidine or praziquantel, and were not pregnant, were invited to join the study. Individuals were excluded from trial participation if they had used any anthelmintic within the past month or were attending other clinical trials during the same period. Only participants with written informed consent, who provided 2 stool samples at baseline, and were willing to provide another 2 stool samples 3 weeks posttreatment were finally included in the study.
Study Flow, Sample Size, and Randomization
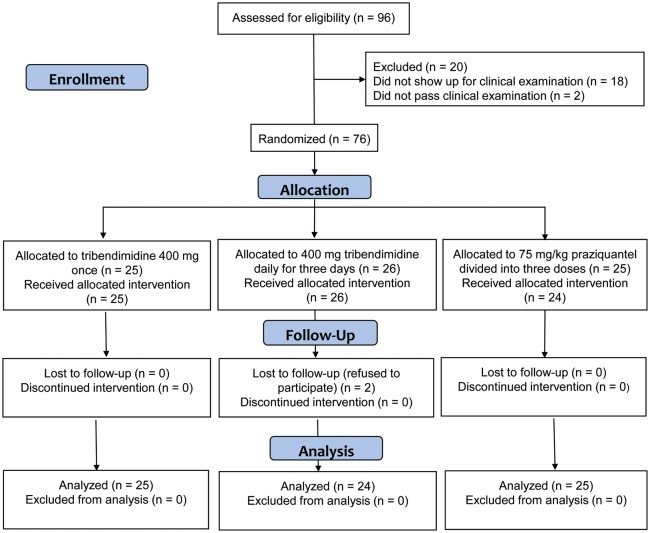
Our study was a randomized, open-label trial aiming to recruit 20–25 patients per treatment arm [14], similar to our earlier study focusing on O. viverrini [8]. Villagers were invited to provide a fresh morning stool sample in a container labeled with a unique identifier. Stool samples were transferred to the laboratory of Binyang Center for Disease Control and Prevention (CDC) and examined for the presence of C. sinensis eggs. Patients with a parasitologically confirmed C. sinensis infection were asked to provide another stool sample over the next 5 days. Written informed consent was obtained from C. sinensis–infected patients, and only consenting individuals were examined clinically, which included a physical examination and the measurement of height, weight, axillary temperature, blood pressure, and pulse. Study participants were randomly allocated, using a computer-generated randomization code prepared by an independent statistician, into 3 treatment arms: (i) tribendimidine 400 mg once, (ii) tribendimidine 400 mg daily for 3 days, and (iii) praziquantel 75 mg/kg divided into 3 doses administered within a day (Figure 1). All patients were treated on the same day. Three and 24 hours after each dosing, each patient was asked open-ended questions for the presence and severity of adverse events. In a next step, the physicians asked for specific adverse events. Adverse events were graded (ie, mild, moderate, severe, and life threatening) [15], recorded in the case report forms (CRFs), and remedial measures were taken if necessary. For each patient, the same physician performed the clinical examination and collected adverse events throughout all follow-ups.
Figure 1.
Trial flow diagram demonstrating patient compliance in a randomized open-label trial assessing the efficacy and safety of tribendimidine against C. sinensis in Guangxi, PR China, from June to July 2012.
Laboratory Procedure
From each stool sample, triplicate Kato-Katz thick smears, using 41.7 mg templates, were prepared [16] and quantitatively examined for C. sinensis eggs under a microscope by experienced technicians, who were blinded to treatment allocation. The number of A. lumbricoides, T. trichiura, hookworm, and other helminth eggs were recorded separately. For quality control, the 3 slides were independently read by different technicians, and the results compared. Slides were reread if inconsistencies were detected.
Outcome Measures and Statistics
Statistical analyses were performed with Stata version 10.0 (StataCorp, College Station, Texas). Available case analysis was employed and thus, only those patients who had primary outcome data were used for analysis. Cure rate (CR) was defined as the proportion of C. sinensis egg–positive patients at baseline, who became egg-negative 3 weeks after treatment. CRs represented as odds ratio (OR) between different treatment arms and calculated from logistic regressions were also determined. Fecal egg counts, as expressed as eggs per gram of stool (EPG), were calculated by multiplying the sum of the egg counts from the 6 Kato-Katz thick smears by a factor of 4. Infection intensity of C. sinensis was categorized as light (1–999 EPG), moderate (1000–9999 EPG), and heavy (≥10 000 EPG) according to Yu and colleagues [17]. ERRs for each treatment group were determined based on the geometric mean (GM) fecal egg counts before and 3 weeks after treatment. The 95% confidence intervals (CIs) of ERR were calculated with bootstrap resampling. The average dose (mg/kg) of tribendimidine was also determined and analyzed with a logistic regression to assess whether the dose of treatment had an influence on the CR. Other test statistics included χ2 test, Fisher exact test, and analysis of variance, as appropriate.
RESULTS
Adherence and Baseline Characteristics
From a recruitment pool of 96 individuals assessed for eligibility, 20 patients were excluded because they did not participate at the clinical examination (n = 18) or presented with chronic or severe illnesses (n = 2). In total, 76 participants were randomized to the 3 treatment arms. One patient discontinued praziquantel treatment after 2 doses due to adverse events and 2 patients were lost to follow-up, giving an overall compliance with treatment and follow-up of 96% (73/76).
Stool samples of the patient who discontinued praziquantel treatment were collected at the follow-up, and hence this patient was included in the available case analysis. Demographic and laboratory baseline characteristics of the 74 patients are summarized in Table 1. Fifty-seven participants (77.0%) were male and 17 were female. Only 2 women were randomized to the praziquantel treatment group. The mean age in the 3 treatment arms ranged from 49.9 to 51.2 years. The height ranged from 156 to 162 cm. Most of the patients (n = 41) harbored a moderate C. sinensis infection (1000–9999 EPG). Fecal egg counts ranged from 124 to 31 244 EPG with a GM of 2816 to 4051 EPG according to treatment arms. Overall, 12, 2, and 1 patients were concurrently infected with hookworm, T. trichiura, and A. lumbricoides, respectively. Given the small number of soil-transmitted helminth infections, these were not considered further.
Table 1.
Demographic and Laboratory Baseline Characteristics of Clonorchis sinensis–Infected Patients in Guangxi, PR China, From June to July 2012 (N = 74)
| Treatment Group |
|||
|---|---|---|---|
| Parameter | Tribendimidine 400 mg Once (n = 25) | Tribendimidine 400 mg Once Daily for 3 d (n = 24) | Praziquantel 75 mg/kg Divided in 3 Doses (n = 25) |
| Males/females (no.) | 19/6 | 15/9 | 23/2 |
| Mean (SD) age, years | 51.2 (8.8) | 49.9 (10.1) | 50.5 (10.0) |
| Mean (SD) weight, kg | 61.9 (10.8) | 58.3 (7.6) | 60.4 (12.3) |
| Mean (SD) height, cm | 157 (6) | 156 (8) | 162 (8) |
| C. sinensis infection intensity | |||
| Overall GM fecal egg counts, EPG | 3387 | 2816 | 4051 |
| Fecal egg counts (range), EPG | (124–29 624) | (184–18 772) | (212–31 244) |
| No. of light infections (1–999 EPG) | 5 | 4 | 4 |
| No. of moderate infections (1000–9999 EPG) | 11 | 17 | 13 |
| No. of heavy infections (≥10 000 EPG) | 9 | 3 | 8 |
Abbreviations: EPG, eggs per gram of stool; GM, geometric mean; SD, standard deviation.
Efficacy Against C. sinensis
Single-dose tribendimidine (400 mg) achieved a CR of 44%, while CRs of 58% and 56% were obtained for 400 mg tribendimidine administered for 3 days and praziquantel, respectively (Table 2). All patients harboring a light C. sinensis infection (1–999 EPG) were cured regardless of the treatment administered. In all 3 treatment arms, CRs of 54%–59% were observed against moderate (1000–9999 EPG) infections. Praziquantel was able to cure 3 of 8 patients with a heavy C. sinensis infection (≥10 000 EPG), while tribendimidine failed to cure such heavy infections regardless of dose. High ERRs (97.6%–98.8%) were observed for all treatments. Tribendimidine administered once daily for 3 days had 10% higher odds of curing a C. sinensis infection than praziquantel. On the other hand, single-dose tribendimidine had 38% and 44% lower odds of curing C. sinensis infection than praziquantel and the 3-day tribendimidine regimen, respectively. However, no significant differences were observed for the treatment outcomes in the praziquantel treatment arm vs tribendimidine single-dose or given for 3 days, and between both tribendimidine regimens.
Table 2.
Effect of Tribendimidine Single and Triple Dosages and Praziquantel in Patients Infected With Clonorchis sinensis in Guangxi, PR China, From June to July 2012 (N = 74)
| Treatment Group |
|||
|---|---|---|---|
| Parameter | Tribendimidine 400 mg Once (n = 25) | Tribendimidine 400 mg Once Daily for 3 d (n = 24) | Praziquantel 75 mg/kg Divided in 3 Doses (n = 25) |
| No. of patients cured (%) | 11 (44) | 14 (58) | 14 (56) |
| No. of patients cured of light infection (%) | 5 (100) | 4 (100) | 4 (100) |
| No. of patients cured of moderate infection (%) | 6 (54) | 10 (59) | 7 (54) |
| No. of patients cured of heavy infection (%) | 0 | 0 | 3 (38) |
| C. sinensis infection intensity | |||
| Overall GM fecal egg counts, EPG | 80.0 | 35.1 | 50.0 |
| Fecal egg counts (range), EPG | (0–3636) | (0–1296) | (0–1876) |
| Egg reduction rate (%) (95% CI) | 97.6 (93.0–98.6) | 98.8 (96.9–99.6) | 98.8 (97.0–99.6) |
Abbreviations: CI, confidence interval; EPG, eggs per gram of stool; GM, geometric mean.
The actual dose of tribendimidine administered ranged from 4.9 to 9.1 mg/kg for the single-dose and 16.9–29.6 mg/kg for the 3-day regimen. No significant association between CRs and dose was observed.
Adverse Events
No life-threatening adverse events were observed following treatment. One patient treated with praziquantel experienced severe vomiting and discontinued treatment (the patient did not take the third dose). Most adverse event episodes were mild (n = 121), with 9 moderate or heavy adverse events either at 3 hours (n = 2) or 24 hours (n = 7). Table 3 summarizes the number of patients experiencing adverse events, stratified by treatment arm at the different examination time points, while Supplementary Table 1 presents the number of adverse events reported at different time points after treatment.
Table 3.
Number of Patients With Adverse Events Among the 3 Treatment Arms, as Assessed at Different Time Points, in a Randomized Open-Label Trial Assessing the Efficacy and Safety of Tribendimidine Against Clonorchis sinensis in Guangxi, PR China, From June to July 2012a
| Treatment Group |
|||
|---|---|---|---|
| Time Point | Tribendimidine 400 mg Once | Tribendimidine 400 mg Once Daily for 3 d | Praziquantel 75 mg/kg Divided in 3 Doses |
| Related symptoms before treatment | 3 (12) | 1 (4) | 4 (16) |
| No. of patients with adverse events (%) | |||
| 3 hours after first treatment | 5 (20)b | 9 (38) | 12 (48) |
| 24 hours after first treatment | 7 (28) | 10 (42) | NA |
| 3 hours after second treatment | NA | 2 (8)c | 16 (64) |
| 24 hours after second treatment | NA | 4 (16) | NA |
| 3 hours after third treatment | NA | 3 (12.5) | NAd |
| 24 hours after third treatment | NA | 1 (4)c | 17 (71) |
| Overall number of patients experiencing adverse events at any time point | 11 (44)b | 16 (67)b | 23 (92) |
Abbreviation: NA, not applicable.
a P values were calculated using χ2 or Fisher exact test, as appropriate.
b Significantly different from praziquantel group (P < .05).
c Significantly different from praziquantel group (P < .001).
d The third treatment of praziquantel was administered in the evening and it was logistically impossible for the patients to report back 3 hours after treatment. Thus, adverse events were only recorded 24 hours after treatment.
Single-dose tribendimidine was the best-tolerated treatment scheme. Eleven patients treated with a single dose of tribendimidine complained of adverse events, with dizziness/faintness the predominant adverse event reported by 5 patients. However, this symptom was already stated by 3 patients before treatment. Adverse events were reported among 16 patients who were given tribendimidine daily for 3 days, dizziness/faintness being the most commonly reported symptom.
Patients treated with praziquantel experienced significantly more adverse events than patients given tribendimidine (P < .05). Dizziness/faintness was significantly more often reported by patients treated with praziquantel than in patients treated with tribendimidine (P < .05). Praziquantel-treated patients also experienced abdominal pain, vomiting, and nausea. Adverse events labeled as “others” that were commonly reported by praziquantel-treated and tribendimidine-treated patients were bloatedness and dry mouth, respectively.
DISCUSSION
Clonorchiasis is a public health problem in PR China, yet only a single treatment—praziquantel—is currently available [18]. Repurposing existing medicines cuts drug development costs and might provide alternative treatments in a timely manner [19, 20]. We studied tribendimidine, a Chinese broad-spectrum anthelmintic drug marketed for the treatment of soil-transmitted helminthiases, against infections with the liver fluke C. sinensis in a randomized open-label trial.
We found that single-dose tribendimidine (400 mg) achieved a high ERR and moderate CR in patients infected with C. sinensis, an efficacy profile comparable to standard triple-dose praziquantel. Moreover, tribendimidine was better tolerated than triple-dose praziquantel. Indeed, and in line with findings from previous studies [21, 22], praziquantel-treated patients commonly reported dizziness, abdominal pain, nausea, and vomiting. It is interesting to note that dizziness has also been reported previously in praziquantel-treated patients infected with O. viverrini [8] and Hymenolepis nana [23]. To our knowledge, it is not known why praziquantel causes dizziness. More investigation into this issue is warranted, as praziquantel is routinely used in preventive chemotherapy, and dizziness as a common adverse event is not desirable.
Our results confirm recent results obtained with single-dose tribendimidine in patients infected with O. viverrini in Lao PDR. Indeed, in that proof-of-concept study, 19 of 27 O. viverrini–infected-patients were cured (70%) and an ERR of over 99% was achieved. In line with our findings, tribendimidine was better tolerated than praziquantel [8].
Though our study was not designed as an equivalence trial, our findings hint that single-dose tribendimidine is equally efficacious as 3 doses of the drug. Note that in preventive chemotherapy programs, which aim to prevent morbidity due to helminthiases using the available armamentarium of anthelmintics, drugs are administered as single doses to entire at risk-populations without prior diagnosis, often in remote rural areas where access to public services is a formidable challenge [24]. A clonorchicidal drug, which is effective as a single dose, would therefore greatly facilitate preventive chemotherapy programs.
In terms of CR, all 3 treatment regimens had an excellent efficacy against light infections and a reasonable efficacy against moderate infections, but a poor efficacy against heavy C. sinensis infections. The differing susceptibility to praziquantel depending on infection intensity of trematode infections is well known [22, 25], and therefore different doses and treatment schedules have been proposed [22]. For example, praziquantel given at 3 × 25 mg/kg was found to be superior to a single dose of 40 mg/kg, in particular for C. sinensis infection of heavy intensities [22]. It has been suggested that genetic polymorphism might be a reason for the low efficacy of praziquantel against heavy C. sinensis infection. For example, patients with a particular single-nucleotide polymorphism of their cytochrome P450 gene revealed lower CRs than patients without [26]. In addition, genetic diversity of C. sinensis could also play a factor in the success of praziquantel treatment, with strains in Southeast Asia showing lower susceptibility to the drug than strains from East Asia [27, 28].
Because soil-transmitted helminthiases and clonorchiasis often overlap geographically [29], praziquantel and albendazole have been coadministered in areas where trematode (eg, C. sinensis and O. viverrini) and nematode infections (eg, soil-transmitted helminths) coexist [30]. Our findings point to an additional benefit of tribendimidine; because of the broad spectrum of activity of this drug, concurrent infections with soil-transmitted helminths and liver flukes could be targeted in one go. In the present setting, only few C. sinensis–infected patients were coinfected, and hence the efficacy and safety of tribendimidine in patients infected with both soil-transmitted helminths and liver flukes remains to be investigated.
In conclusion, our results suggest that single-dose tribendimidine is comparable to triple-dose praziquantel, both in terms of efficacy and safety, in the treatment of C. sinensis infections. Larger clinical trials, which have been initiated with O. viverrini–infected patients in Lao PDR, should also be planned in C. sinensis–infected patients.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the people of Hezhuang village for their participation. The smooth execution of this study would not have been possible without dedicated efforts from the local staff of Guangxi CDC, Binyang CDC, and Gantang township hospital. Statistical support from Mr Dimitrios Alexios Karagiannis Voules and Mr Benjamin Speich is deeply appreciated. We are grateful to Prof Peter Odermatt and Dr Somphou Sayasone for their continuous support and leading role in the grants funded by DFID/MRC/Wellcome Trust Joint Global Health Trials Scheme.
Financial support. This work was supported by DFID/MRC/Wellcome Trust Joint Global Health Trials Scheme. J. K. is financially supported by the Swiss National Science Foundation (SNSF; project nos. PPOOA-114941 and PPOOP3_135170). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential conflicts of interests. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Fürst T, Keiser J, Utzinger J. Global burden of human food-borne trematodiasis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:210–221. doi: 10.1016/S1473-3099(11)70294-8. [DOI] [PubMed] [Google Scholar]
- 2.Fürst T, Sayasone S, Odermatt P, Keiser J, Utzinger J. Manifestation, diagnosis, and management of foodborne trematodiasis. BMJ. 2012;344:e4093. doi: 10.1136/bmj.e4093. [DOI] [PubMed] [Google Scholar]
- 3.Hong ST, Fang Y. Clonorchis sinensis and clonorchiasis, an update. Parasitol Int. 2012;61:17–24. doi: 10.1016/j.parint.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Choi MH, Park SK, Li Z, et al. Effect of control strategies on prevalence, incidence and re-infection of clonorchiasis in endemic areas of China. PLoS Negl Trop Dis. 2010;4:e601. doi: 10.1371/journal.pntd.0000601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rim HJ. The current pathobiology and chemotherapy of clonorchiasis. Korean J Parasitol. 1986;24:1–141. doi: 10.3347/kjp.1986.24.suppl.1. [DOI] [PubMed] [Google Scholar]
- 6.Keiser J, Xiao SH, Chollet J, Tanner M, Utzinger J. Evaluation of the in vivo activity of tribendimidine against Schistosoma mansoni, Fasciola hepatica, Clonorchis sinensis, and Opisthorchis viverrini. Antimicrob Agents Chemother. 2007;51:1096–8. doi: 10.1128/AAC.01366-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao SH, Xue J, Xu LL, Zheng Q, Qiang HQ, Zhang YN. The in vitro and in vivo effect of tribendimidine and its metabolites against Clonorchis sinensis. Parasitol Res. 2009;105:1497–507. doi: 10.1007/s00436-009-1579-6. [DOI] [PubMed] [Google Scholar]
- 8.Soukhathammavong P, Odermatt P, Sayasone S, et al. Efficacy and safety of mefloquine, artesunate, mefloquine-artesunate, tribendimidine, and praziquantel in patients with Opisthorchis viverrini: a randomised, exploratory, open-label, phase 2 trial. Lancet Infect Dis. 2011;11:110–8. doi: 10.1016/S1473-3099(10)70250-4. [DOI] [PubMed] [Google Scholar]
- 9.Keiser J, Utzinger J. The drugs we have and the drugs we need against major helminth infections. Adv Parasitol. 2010;73:197–230. doi: 10.1016/S0065-308X(10)73008-6. [DOI] [PubMed] [Google Scholar]
- 10.Olliaro P, Seiler J, Kuesel A, et al. Potential drug development candidates for human soil-transmitted helminthiases. PLoS Negl Trop Dis. 2011;5:e1138. doi: 10.1371/journal.pntd.0001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao SH, Wu ZX, Zhang JH, et al. Clinical observation on 899 children infected with intestinal nematodes and treated with tribendimidine enteric coated tablets. Chin J Parasitol Parasit Dis. 2007;25:372–5. [in Chinese] [PubMed] [Google Scholar]
- 12.Zhang JH, Xiao SH, Wu ZX, et al. Tribendimidine enteric coated tablet in treatment of 1,292 cases with intestinal nematode infection—a phase IV clinical trial. Chin J Parasitol Parasit Dis. 2008;26:6–9. [in Chinese] [PubMed] [Google Scholar]
- 13.Wu ZX, Fang YY, Liu YS. Effect of a novel drug—enteric coated tribendimidine in the treatment of intestinal nematode infections. Chin J Parasitol Parasit Dis. 2006;24:23–6. [in Chinese] [PubMed] [Google Scholar]
- 14.Julious S. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat. 2005;4:287–91. [Google Scholar]
- 15.Cancer Therapy Evaluation Program. Common Toxicity Criteria, Version 2.0. DCTD, NCI, NIH, DHHS 1998. [Google Scholar]
- 16.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop São Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 17.Yu SH, Kawanaka M, Li XM, Xu LQ, Lan CG, Rui L. Epidemiological investigation on Clonorchis sinensis in human population in an area of South China. Jpn J Infect Dis. 2003;56:168–71. [PubMed] [Google Scholar]
- 18.Lun ZR, Gasser RB, Lai DH, et al. Clonorchiasis: a key foodborne zoonosis in China. Lancet Infect Dis. 2005;5:31–41. doi: 10.1016/S1473-3099(04)01252-6. [DOI] [PubMed] [Google Scholar]
- 19.Dakshanamurthy S, Issa NT, Assefnia S, et al. Predicting new indications for approved drugs using a proteochemometric method. J Med Chem. 2012;55:6832–6848. doi: 10.1021/jm300576q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keiser J, Utzinger J. Antimalarials in the treatment of schistosomiasis. Curr Pharm Des. 2012;18:3531–8. [PubMed] [Google Scholar]
- 21.Rim HJ, Lyu KS, Lee JS, Joo KH. Clinical evaluation of the therapeutic efficacy of praziquantel (Embay 8440) against Clonorchis sinensis infection in man. Ann Trop Med Parasitol. 1981;75:27–33. doi: 10.1080/00034983.1981.11687405. [DOI] [PubMed] [Google Scholar]
- 22.Rim HJ, Lee YM, Lee JS, Joo KH. Therapeutic field trial with praziquantel (Biltricide®) in a rural population infected with Clonorchis sinensis. Korean J Parasitol. 1982;20:1–8. doi: 10.3347/kjp.1982.20.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Rim HJ, Park CY, Lee JS, Joo KH, Lyu KS. Therapeutic effects of praziquantel (Embay 8440) against Hymenolepis nana infection. Korean J Parasitol. 1978;16:82–7. doi: 10.3347/kjp.1978.16.2.82. [DOI] [PubMed] [Google Scholar]
- 24.WHO. Preventive chemotherapy in human helminthiasis: coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. Geneva: World Health Organization; 2006. [Google Scholar]
- 25.Augusto G, Magnussen P, Kristensen TK, Appleton CC, Vennervald BJ. The influence of transmission season on parasitological cure rates and intensity of infection after praziquantel treatment of Schistosoma haematobium–infected schoolchildren in Mozambique. Parasitology. 2009;136:1771–9. doi: 10.1017/S0031182009006210. [DOI] [PubMed] [Google Scholar]
- 26.Kim CH, Lee JK, Chung BS, Li S, Choi MH, Hong ST. Influencing factors for cure of clonorchiasis by praziquantel therapy: infection burden and CYP3A5 gene polymorphism. Korean J Parasitol. 2011;49:45–9. doi: 10.3347/kjp.2011.49.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tatonova YV, Chelomina GN, Besprosvannykh VV. Genetic diversity of nuclear ITS1–5.8S-ITS2 rDNA sequence in Clonorchis sinensis Cobbold, 1875 (Trematoda: Opisthorchidae) from the Russian Far East. Parasitol Int. 2012;61:664–74. doi: 10.1016/j.parint.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Tinga N, De N, Vien HV, et al. Little effect of praziquantel or artemisinin on clonorchiasis in northern Vietnam. A pilot study. Trop Med Int Health. 1999;4:814–8. doi: 10.1046/j.1365-3156.1999.00499.x. [DOI] [PubMed] [Google Scholar]
- 29.Utzinger J, Keiser J. Schistosomiasis and soil-transmitted helminthiasis: common drugs for treatment and control. Expert Opin Pharmacother. 2004;5:263–85. doi: 10.1517/14656566.5.2.263. [DOI] [PubMed] [Google Scholar]
- 30.WHO. Vientiane: World Health Organization; 2011. Report of the WHO expert consultation on foodborne trematode infections and taeniasis/cysticercosis. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.