Cure rates for nongonococcal urethritis (NGU), were approximately 80% and there was no significant difference between azithromycin and doxycycline for clinical or microbiologic cure. Chlamydia trachomatis, Ureaplasma urealyticum biovar 2, and idiopathic NGU remained relatively sensitive to standard therapies, but Mycoplasma genitalium was not.
Keywords: urethritis, treatment, Mycoplasma genitalium, Chlamydia trachomatis, randomized trial
Abstract
Background. Azithromycin or doxycycline is recommended for nongonococcal urethritis (NGU); recent evidence suggests their efficacy has declined. We compared azithromycin and doxycycline in men with NGU, hypothesizing that azithromycin was more effective than doxycycline.
Methods. From January 2007 to July 2011, English-speaking males ≥16 years, attending a sexually transmitted diseases clinic in Seattle, Washington, with NGU (visible urethral discharge or ≥5 polymorphonuclear leukocytes per high-power field [PMNs/HPF]) were eligible for this double-blind, parallel-group superiority trial. Participants received active azithromycin (1 g) + placebo doxycycline or active doxycycline (100 mg twice daily for 7 days) + placebo azithromycin. Urine was tested for Chlamydia trachomatis (CT), Mycoplasma genitalium (MG), Ureaplasma urealyticum biovar 2 (UU-2), and Trichomonas vaginalis (TV) using nucleic acid amplification tests. Clinical cure (<5 PMNs/HPF with or without urethral symptoms and absence of discharge) and microbiologic cure (negative tests for CT, MG, and/or UU-2) were determined after 3 weeks.
Results. Of 606 men, 304 were randomized to azithromycin and 302 to doxycycline; CT, MG, TV, and UU-2 were detected in 24%, 13%, 2%, and 23%, respectively. In modified intent-to-treat analyses, 172 of 216 (80%; 95% confidence interval [CI], 74%–85%) receiving azithromycin and 157 of 206 (76%; 95% CI, 70%–82%) receiving doxycycline experienced clinical cure (P = .40). In pathogen-specific analyses, clinical cure did not differ by arm, nor did microbiologic cure differ for CT (86% vs 90%, P = .56), MG (40% vs 30%, P = .41), or UU-2 (75% vs 70%, P = .50). No unexpected adverse events occurred.
Conclusions. Clinical and microbiologic cure rates for NGU were somewhat low and there was no significant difference between azithromycin and doxycycline. Mycoplasma genitalium treatment failure was extremely common.
Clinical Trials Registration. NCT00358462.
Nongonococcal urethritis (NGU) is common among men attending urologic [1] and sexually transmitted disease (STD) clinics. Chlamydia trachomatis was linked to NGU in the mid-1960s [2], accounts for approximately 25% of cases, and is considered the primary etiologic agent. More recently, Mycoplasma genitalium has been identified in 10%–25% of NGU cases [3], and studies differentiating Ureaplasma urealyticum biovar 2 from Ureaplasma parvum suggest that U. urealyticum biovar 2 causes NGU in select cases (16%–26%) [4–6]. Although NGU rarely results in serious sequelae in men, persistent or recurrent urethritis is common [7], and many female sex partners are at risk for complications [8]. Effective treatment of NGU, therefore, is a key component of male and female reproductive health.
The Centers for Disease Control and Prevention (CDC) treatment guidelines recommend azithromycin or doxycycline for NGU [9]. In the mid-1990s a randomized trial found the 2 treatments to be comparably effective against NGU caused by C. trachomatis and undifferentiated Ureaplasma species [10], and single-dose azithromycin became the preferred therapy in many settings. It has also been consistently more effective than doxycycline against M. genitalium [11]. However, a recent multicenter trial found that azithromycin was significantly less effective than doxycycline in eradicating C. trachomatis [12], and reports of azithromycin resistance in M. genitalium have been increasing [13].
We conducted a double-blind randomized trial testing the efficacy of azithromycin versus doxycycline against NGU, with a focus on M. genitalium and U. urealyticum biovar 2. We hypothesized that azithromycin would be more effective than doxycycline.
METHODS
Design
This was a single-center, double-blind, parallel-group superiority trial. Men with NGU were enrolled by a single study clinician (M.S.L.) and assigned to the intervention or control group using 1:1 randomization.
Setting and Participants
English-speaking males aged ≥16 years, attending the Public Health–Seattle & King County STD clinic in Seattle, Washington, possessing valid contact information were eligible. Men with visible urethral discharge on examination or ≥5 polymorphonuclear leukocytes per high-power field (PMNs/HPF) on a Gram-stained slide of urethral exudates were included. Men who had received antibiotics in the past month or had allergies to study drugs were excluded.
Men with urethral symptoms or signs were referred to the study clinician who assessed eligibility and obtained informed consent. Participants completed a brief computer-assisted self-interview and underwent a routine STD exam during which 2 urethral swab samples were obtained. The first was used for Gram staining and quantitation of PMNs if not done before referral; the second was stored for future testing. Men also provided 25 mL of first-void urine for nucleic acid amplification tests (NAATs) and culture. After examination, men were given a treatment packet and a symptom/coital log to collect daily information on completion of therapy, symptoms, medication side effects, and sexual activity, and were scheduled to return in 3 weeks.
Randomization and Intervention
Randomization and blinding were managed by the Harborview Medical Center Investigational Drug Service (HMC IDS), using Excel to generate the random sequence in blocks of 10. HMC IDS prepared sequentially numbered treatment packets in sealed opaque envelopes; treatment arm was assigned when the clinician gave the patient the next numbered packet in the sequence. All patients, clinicians, and study staff were blinded to treatment assignment until the end of the trial.
The azithromycin group received 1 g of active azithromycin (either 500-mg tablets × 2 or 250-mg tablets × 4), plus 14 placebo doxycycline capsules (100-mg capsules twice daily for 7 days) identical in appearance to the active doxycycline. The doxycycline group received placebo azithromycin tablets identical in appearance to the active drug, plus 14 active doxycycline capsules. Azithromycin tablets (active and placebo) were administered under clinician observation. Patients were instructed to take 1 doxycycline capsule (active or placebo) the evening of their enrollment visit, and 1 each morning and each evening until they were completed.
Follow-up
At the 3-week follow-up visit (allowable window = 2–5 weeks), men underwent examination and specimen collection, turned in their symptom/coital log, and completed a second computed-assisted self-interview. Men with M. genitalium or Ureaplasma species at enrollment and recurrent/persistent NGU or a repeat positive test received the alternate regimen (eg, active azithromycin plus placebo doxycycline if they first received active doxycycline and vice versa) and were scheduled for another visit 6 weeks after enrollment (data not shown). Others with recurrent/persistent NGU were unblinded and treated according to clinic standard of care with no further study visits.
Protocol Modifications
All changes to the protocol occurred early in the trial, after institutional review board approval and prior to study unblinding. Initially, the primary outcome was microbiologic cure of M. genitalium and only M. genitalium–positive men were followed. Enrollment began on 1 January 2007. On 4 May 2007 we began asking all men to return for a 3-week follow-up visit and added testing for Ureaplasma species. Eligibility criteria were modified to include human immunodeficiency virus (HIV)–positive individuals (2 May 2008) and explicitly exclude men taking medications contraindicated for the study drugs (2 September 2008). Beginning 10 September 2010, men with M. genitalium who required therapy at follow-up were offered moxifloxacin (400 mg/day × 7 days) rather than the alternate regimen, given low M. genitalium cure rates revealed at interim analyses. To facilitate comparisons with other trials, in modified intent-to-treat (mITT) analyses we revised the inclusion criteria to “self-reported urethral symptoms (dysuria, discharge, itching, tingling) or clinical signs of visible urethral discharge plus ≥5 PMNs/HPF.”
Outcomes
The primary outcome was clinical cure of NGU after 3 weeks, defined as <5 PMNs/HPF (with or without urethral symptoms) and absence of urethral discharge. We evaluated 7 secondary outcomes: clinical and microbiologic cure after 3 weeks among men with C. trachomatis, M. genitalium, and U. urealyticum biovar 2, and clinical cure for idiopathic NGU (negative for all pathogens). Ureaplasma parvum–positive men were considered to have idiopathic disease. Microbiologic cure was defined as a negative NAAT for the baseline infecting pathogen.
All tests were performed on urine. We used the APTIMA transcription-mediated amplification assay for C. trachomatis and Neisseria gonorrhoeae and analyte-specific reagents on the same platform for Trichomonas vaginalis (GenProbe, Inc, San Diego, California). Mycoplasma genitalium was assessed by in-house polymerase chain reaction (PCR) [14]; Ureaplasma species were detected in broth urine culture followed by species-specific PCR [6, 15].
Statistical Methods
The sample size was estimated for the original primary outcome of microbiologic cure of M. genitalium. Assuming α = .05 and failure rates of 8% for azithromycin and 33% for doxycycline [16, 17], a total of 45 M. genitalium–positive men per arm would provide 85% power to detect a difference of 25 percentage points. We assumed 12% prevalence of M. genitalium and 20% loss to follow-up, requiring 900 men with NGU.
One interim assessment was performed after half of the target sample of M. genitalium–positive men (n = 45) had been enrolled and completed all 3 scheduled visits (data accrued 2 January 2007–30 October 2009). Using the O'Brien-Fleming stopping rule [18], the level of significance was 0.0052. Upon review, the data safety and monitoring board noted that interim differences in treatment were too small to observe a significant difference and expressed concern over low cure rates for M. genitalium. They advised 1 additional year of recruitment to further investigate apparent high rates of antimicrobial resistance.
An intent-to-treat (ITT) analysis included all randomized men. An mITT analysis excluded men who did not meet the revised mITT inclusion criteria and/or did not return for follow-up. We used Pearson χ2 test (2-sided P value) to identify statistically significant differences in treatment outcomes, calculating exact binomial confidence intervals.
Assuming that men whose symptoms persisted would be more likely to return, participants who were lost to follow-up were considered clinically cured in ITT analyses. Men who returned earlier than 2 weeks before their scheduled visit were considered to have clinical treatment failure (n = 3, all with persistent signs or symptoms) and were retested. Men who attended their follow-up visit >5 weeks past the scheduled date were considered lost to follow-up. Secondary analyses evaluating clinical and microbiologic cure for each bacterium and clinical cure for idiopathic NGU employed these same methods. To examine the impact of our assumptions about losses to follow-up, in sensitivity analyses we considered men lost to follow-up as to have clinical and microbiologic treatment failure rather than cure.
We used multivariable log binomial regression with robust standard errors to adjust the prevalence ratio of clinical and microbiologic cure (azithromycin relative to doxycycline) for unprotected sex between visits. Unprotected sex was defined as sex between visits with any partner with whom condoms were not “always” used. We used Stata software, version 11.2, for all analyses (StataCorp, College Station, Texas).
All study procedures and analyses were approved by the University of Washington Human Subjects Division. The Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health (funder) reviewed the clinical protocol and subsequent amendments and conducted clinical site monitoring.
RESULTS
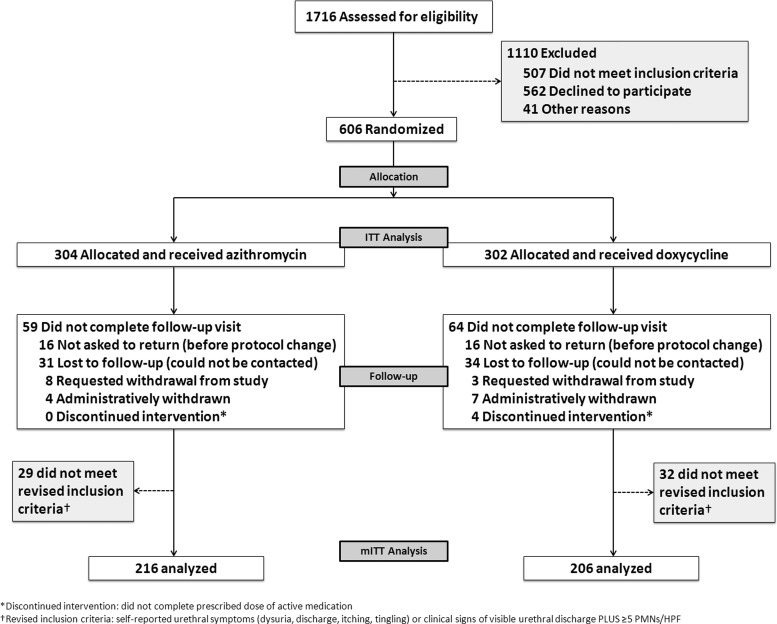
From 1 January 2007 through 31 July 2011 (end of funding period), 1716 men were assessed for eligibility. Of these, 606 met inclusion criteria, agreed to participate, and constituted the ITT population: 304 men were randomized to active azithromycin + placebo doxycycline and 302 were randomized to active doxycycline + placebo azithromycin (Figure 1).
Figure 1.
Flow diagram of enrollment and follow-up in the trial. Abbreviations: ITT, intent to treat; mITT, modified intent to treat; PMNs/HPF, polymorphonuclear leukocytes per high-powered field.
The mITT population consisted of 206 men randomized to doxycycline and 216 men randomized to azithromycin. We excluded 123 men who did not return for follow-up and 61 men who did not meet the revised inclusion criteria (symptoms or visible discharge plus ≥5 PMNs/HPF). Among the 304 men randomized to azithromycin, 245 (81%) returned for follow-up, of whom 29 (12%) did not meet revised inclusion criteria (21 had <5 PMNs, 8 had no discharge or symptoms). Of the 302 men randomized to doxycycline, 238 (79%) returned for follow-up, 32 (13%) of whom did not meet revised inclusion criteria (28 had <5 PMNs, 4 had no discharge or symptoms). All 3-week follow-up visits were completed by 31 August 2011.
Characteristics of the 606 men in ITT analyses were similar by study arm (Table 1). Mean age was 33.7 years and the majority was white (55%). Half (53%) presented with symptoms of urethral discharge, 51% complained of dysuria, and 26% reported other urethral symptoms. Chlamydia trachomatis and U. urealyticum biovar 2 were most commonly detected (23%–24% each), followed by M. genitalium (13%). Fifteen men (2.5%) were HIV-positive.
Table 1.
Characteristics of Study Participants in the Intent-to-Treat and Modified Intent-to-Treat Population at Enrollment, by Randomization Arm
| ITT |
mITT |
|||||
|---|---|---|---|---|---|---|
| Randomization Arm |
Total |
Randomization Arm |
Total |
|||
| Characteristica | Azithromycin (n = 304) | Doxycycline (n = 302) | N = 606 | Azithromycin (n = 216) | Doxycycline (n = 206) | N = 422 |
| Age, mean (SD) | 34.1 (9.8) | 33.3 (10.1) | 33.7 (10.0) | 34.6 (9.5) | 33.9 (10.0) | 34.3 (9.8) |
| Race | ||||||
| White | 159 (52.3) | 172 (57.0) | 331 (54.6) | 112 (53.9) | 119 (61.7) | 231 (57.6) |
| Black | 113 (37.2) | 93 (30.8) | 206 (34.0) | 77 (37.0) | 59 (30.6) | 136 (33.9) |
| Otherb | 23 (7.6) | 19 (6.3) | 42 (6.9) | 19 (9.1) | 15 (7.8) | 34 (8) |
| Hispanic/Latino | 9 (3.0) | 14 (4.6) | 23 (3.8) | 6 (2.8) | 9 (4.4) | 15 (3.6) |
| Highest level of education completed | ||||||
| ≤High school/GED | 130 (42.8) | 139 (46.0) | 269 (44.4) | 91 (42.3) | 91 (44.4) | 182 (43.3) |
| >High school/GED | 173 (56.9) | 161 (53.3) | 334 (55.1) | 124 (57.7) | 114 (55.6) | 238 (56.7) |
| Annual income, $ | ||||||
| <10 000 | 106 (34.9) | 104 (34.4) | 210 (34.7) | 73 (34.8) | 69 (34.7) | 142 (34.7) |
| 10 000–29 999 | 104 (34.2) | 96 (31.8) | 200 (33.0) | 75 (35.7) | 67 (33.7) | 142 (34.7) |
| ≥30 000 | 88 (28.9) | 93 (30.8) | 181 (29.9) | 62 (29.5) | 63 (31.6) | 125 (30.6) |
| Sexual history, past 12 mo | ||||||
| Heterosexual | 208 (68.4) | 193 (63.9) | 401 (66.2) | 142 (65.7) | 125 (60.7) | 267 (63.3) |
| Homosexual | 81 (26.6) | 89 (29.5) | 170 (28.1) | 62 (28.7) | 65 (31.6) | 127 (30.1) |
| Bisexual | 14 (4.6) | 18 (6.0) | 32 (5.3) | 12 (5.6) | 15 (7.3) | 27 (6.4) |
| Not sexually active | 1 (0.3) | 2 (0.7) | 3 (0.5) | 0 (0.0) | 1 (0.5) | 1 (0.2) |
| HIV status | ||||||
| Positive | 7 (2.3) | 8 (2.6) | 15 (2.5) | 5 (2.4) | 7 (3.6) | 12 (3.0) |
| Negative | 243 (79.9) | 250 (82.8) | 493 (81.4) | 175 (85.0) | 169 (86.7) | 344 (85.8) |
| Never tested or unknown | 40 (13.2) | 31 (10.3) | 71 (11.7) | 26 (12.6) | 19 (9.7) | 45 (11.2) |
| No. sex partners, past 12 mo, median (IQR) | 4 (2–6) | 3 (2–6) | 3 (2–6) | 3 (2–6) | 3 (2–6) | 3 (2–6) |
| Circumcised | 257 (84.5) | 235 (77.8) | 492 (81.2) | 180 (83.7) | 163 (79.1) | 343 (81.3) |
| Symptoms at enrollment | ||||||
| Urethral discharge | 164 (53.9) | 159 (52.6) | 323 (53.3) | 117 (54.2) | 114 (55.3) | 231 (54.7) |
| Dysuria | 158 (52.0) | 152 (50.3) | 310 (51.2) | 120 (55.6) | 109 (52.9) | 229 (54.3) |
| Other urethral symptomsc | 81 (26.6) | 79 (26.2) | 160 (26.4) | 60 (27.8) | 58 (28.2) | 118 (28.0) |
| Nonurethral symptomsd | 44 (14.5) | 56 (18.5) | 100 (16.5) | 26 (12.0) | 32 (15.5) | 58 (13.7) |
| Infecting pathogen | ||||||
| Mycoplasma genitalium | 45 (14.8) | 35 (11.6) | 80 (13.2) | 38 (17.6) | 27 (13.1) | 65 (15.4) |
| Chlamydia trachomatis | 76 (25.0) | 68 (22.5) | 144 (23.8) | 53 (24.5) | 50 (24.3) | 103 (24.4) |
| Trichomonas vaginalis | 5 (1.6) | 6 (2.0) | 11 (1.8) | 3 (1.4) | 5 (2.4) | 8 (1.9) |
| Ureaplasma urealyticume | 65 (21.4) | 77 (25.5) | 142 (23.4) | 52 (26.0) | 55 (28.1) | 107 (27.0) |
| Ureaplasma parvume | 41 (13.5) | 40 (13.2) | 81 (13.4) | 31 (15.5) | 29 (14.8) | 60 (15.2) |
| Ureaplasma spp unspeciatedf | 14 (4.6) | 8 (2.6) | 22 (3.6) | 13 (6.1) | 8 (3.9) | 21 (5.0) |
| Idiopathicg | 117 (38.5) | 128 (42.4) | 245 (40.4) | 81 (38.8) | 85 (41.9) | 166 (40.3) |
| No. PMNs/HPF on Gram-stained slide | ||||||
| 0–4 | 24 (7.9) | 31 (10.3) | 55 (9.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5–9 | 72 (23.7) | 79 (26.1) | 151 (24.9) | 48 (22.2) | 60 (29.1) | 108 (25.6) |
| ≥10 | 208 (68.4) | 192 (63.6) | 400 (66.0) | 168 (77.8) | 146 (70.9) | 314 (74.4) |
| History of antibiotic use 1–3 mo ago | ||||||
| None | 255 (83.9) | 264 (87.4) | 519 (85.6) | 177 (81.9) | 182 (88.3) | 359 (85.1) |
| Someh | 29 (9.5) | 16 (5.3) | 45 (7.4) | 26 (12.0) | 12 (5.8) | 38 (6.6) |
| Doesn't know | 15 (4.9) | 13 (4.3) | 28 (4.6) | 13 (6.0) | 8 (3.9)i | 21 (4.9) |
| Sex since last visit (vaginal, oral, and/or anal) | … | … | … | 101 (47.0) | 103 (51.0) | 204 (48.9) |
| If yes, used condom every single time | … | … | … | 23 (24.7) | 29 (30.2) | 52 (27.5) |
| If yes, used condom some of the time | … | … | … | 44 (47.3) | 45 (46.9) | 89 (47.1) |
| If yes, never used a condom | … | … | … | 26 (28.0) | 22 (22.9) | 48 (25.4) |
| Unprotected sex in between visits | … | … | … | 70 (33.8) | 67 (34.4) | 137 (34.1) |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: GED, General Educational Development test; HIV, human immunodeficiency virus; IQR, interquartile range; ITT, intent to treat; mITT, modified intent to treat; PMNs/HPF, polymorphonuclear leukocytes per high-powered field.
a Owing to missing values, not all variables sum to column totals. Denominators for ITT proportions represent all randomized participants (azithromycin, n = 304; doxycycline, n = 302). Denominators for mITT proportions represent those who had data available for that characteristic.
b Other includes Asian (20), Native American or Alaska Native (4), Pacific Islander or Hawaiian Native (4), and multiple (14) for ITT; and Asian (17), Native American or Alaska Native (4), Pacific Islander or Hawaiian Native (3), and multiple (10) for mITT.
c Other includes unspecified urethral symptoms (70), itching (34), irritation (23), tingling (8), soreness (7), stinging/burning (6), and other (12) for ITT; and unspecified urethral symptom (49), itching (26), irritation (17), tingling (7), soreness (4), stinging/burning (5), and other (10) for mITT.
d Nonurethral symptoms include genital lesion/rash (51), nongenital rash (17), oral/pharyngeal (17), testicular (13), and anorectal (2) for ITT; and genital lesion/rash (38), nongenital rash (14), oral/pharyngeal (14), testicular (9), and anorectal (1) for mITT.
e Excludes Ureaplasma + unspeciated from denominator of mITT.
f This represents men who were Ureaplasma-positive by culture at the enrollment visit and who could not be speciated at the enrollment or follow-up visit.
g This represents men who were negative for M. genitalium, C. trachomatis, U. urealyticum, and T. vaginalis at enrollment (U. parvum was not considered a pathogen).
h Some includes macrolide (azithromycin; 16), tetracycline (doxycycline; 9), cephalosporin (cefixime, cefpodoxime, ceftriaxone; 6), penicillin (5), fluoroquinolone (ciprofloxacin; 3), and other antibiotic (6) for ITT, and macrolide (azithromycin; 13), tetracycline (doxycycline; 9), cephalosporin (cefixime, cefpodoxime, ceftriaxone; 6), penicillin (3), fluoroquinolone (ciprofloxacin; 2), and other antibiotic (5) for mITT.
i P = .051. All other comparisons by arm P > .05.
Characteristics of the 422 men in mITT analyses also did not differ by study arm and were similar to the ITT group, although the prevalences of M. genitalium and U. urealyticum biovar 2 were slightly higher in the mITT group.
Clinical Cure
In mITT analyses (primary outcome), 172 of 216 men (80%; 95% confidence interval [CI], 74%–85%) receiving azithromycin experienced clinical cure versus 157 of 206 (76%; 95% CI, 70%–82%) receiving doxycycline (P = .40; Table 2).
Table 2.
Clinical and Microbiologic Cure at Follow-up in the Modified Intent-to-Treat Population, by Infection at Enrollment
| Clinical Cure |
Microbiologic Cure |
|||
|---|---|---|---|---|
| Azithromycin (n = 216) | Doxycycline (n = 206) | Azithromycin (n = 216) | Doxycycline (n = 206) | |
| All participants | 79.6 (73.6–84.8) | 76.2 (69.8–81.9) | … | … |
| Chlamydia trachomatisa | 86.8 (74.7–94.5) | 76.0 (61.8–86.9) | 86.3 (73.7–94.3) | 90.0 (78.2–96.7) |
| Mycoplasma genitaliumb | 63.2 (46.0–78.2) | 48.1 (28.7–68.1) | 39.5 (24.0–56.6) | 29.6 (13.8–50.2) |
| Ureaplasma urealyticumc | 82.7 (69.7–91.8) | 72.7 (59.0–83.9) | 75.0 (61.1–86.0) | 69.1 (55.2–80.9) |
| Idiopathicd | 79.0 (68.5–87.3) | 85.7 (76.6–92.5) | … | … |
Data are presented as % (95% confidence interval).
a Denominator sample size: azithromycin, n = 53 for clinical cure and n = 51 for microbiologic cure; doxycycline, n = 50.
b Denominator sample size: azithromycin, n = 38; doxycycline, n = 27.
c Denominator sample size: azithromycin, n = 52; doxycycline, n = 55.
d Denominator sample size: azithromycin, n = 81; doxycycline, n = 85.
Clinical cure occurred less often among the 98 (23%) men who returned early (2–3 weeks) versus after 3–5 weeks (67% vs 81%, P = .004), and somewhat less often among HIV-positive men (58% vs 79%, P = .15).
In analyses of specific pathogens, there were also no significant differences by arm. Men with M. genitalium at baseline experienced the lowest clinical cure rates (63% for azithromycin vs 48% for doxycycline, P = .23). Of men with clinical treatment failure, 30% had M. genitalium, 26% had U. urealyticum biovar 2, 20% had C. trachomatis, and 31% had idiopathic disease.
Results were similar in ITT analyses. There were no significant differences in clinical cure rates for all-cause NGU (83% receiving azithromycin vs 82% receiving doxycycline, P = .64) or for men with C. trachomatis, M. genitalium, U. urealyticum biovar 2, or idiopathic NGU. In sensitivity analyses, assuming losses to follow-up had experienced clinical failure, clinical cure rates for all-cause NGU were 64% for azithromycin and 61% for doxycycline (P = .41; Table 3).
Table 3.
Sensitivity Analysis of Intent-to-Treat Population: Clinical and Microbiologic Cure at Follow-up by Infection at Enrollment and Assumptions About Losses to Follow-up (N = 606)
| ITT Primary Analyses (Losses to Follow-up Considered Cured) |
ITT Sensitivity Analyses (Losses to Follow-up Considered Failed) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical Cure |
Microbiologic Cure |
Clinical Cure |
Microbiologic Cure |
|||||||||
| AZM (N = 304) | DOX (N = 302) | P Value | AZM (N = 304) | DOX (N = 302) | P Value | AZM (N = 304) | DOX (N = 302) | P Value | AZM (N = 304) | DOX (N = 302) | P Value | |
| All participants | 253 (83.2) | 247 (81.8) | .64 | … | … | … | 194 (63.8) | 183 (60.6) | .41 | … | … | … |
| Chlamydia trachomatisa | 69 (90.8) | 56 (82.4) | .14 | 67 (90.5) | 63 (92.7) | .65 | 47 (61.8) | 39 (57.4) | .58 | 45 (60.8) | 46 (67.7) | .40 |
| Mycoplasma genitaliumb | 30 (66.7) | 20 (57.1) | .38 | 20 (44.4) | 15 (42.9) | .89 | 26 (57.8) | 13 (37.1) | .07 | 16 (35.6) | 8 (22.9) | .22 |
| Ureaplasma urealyticumc | 52 (80.0) | 60 (77.9) | .76 | 49 (75.4) | 59 (76.6) | .86 | 46 (70.8) | 44 (57.1) | .09 | 43 (66.2) | 43 (55.8) | .21 |
| Idiopathicd | 98 (83.8) | 113 (88.3) | .31 | … | … | … | 78 (66.7) | 94 (73.4) | .28 | … | … | … |
Abbreviations: AZM, azithromycin; DOX, doxycycline; ITT, intent to treat.
a Azithromycin: n = 76 for clinical cure and n = 74 for microbiologic cure; doxycycline: n = 68.
b Azithromycin: n = 45; doxycycline: n = 35.
c Azithromycin: n = 65; doxycycline: n = 77.
d Azithromycin: n = 117; doxycycline: n = 128.
Microbiologic Cure
In mITT analyses, the microbiologic cure rate was lower than the clinical cure rate and not significantly different by arm, with one exception. In the doxycycline arm, the microbiologic cure rate of C. trachomatis was higher than the clinical cure rate (90% vs 76%, P = .06; Table 2). Notably, the microbiologic cure rate was extremely low for men with M. genitalium: 40% for azithromycin versus 30% for doxycycline (P = .41).
Eradication of specific organisms did not differ between those returning after 2–3 or 3–5 weeks, nor between HIV-positive and HIV-negative men (data not shown).
Intent-to-treat analyses of microbiologic cure were similar to mITT analyses. There was no significant difference between azithromycin and doxycycline for men initially positive for C. trachomatis, M. genitalium, or U. urealyticum biovar 2. In sensitivity analyses, assuming men lost to follow-up had experienced clinical treatment failure, the microbiologic cure rate was substantially lower but still not significantly different by arm (Table 3).
Adjusting for unprotected sex between visits, prevalence ratios comparing azithromycin to doxycycline for clinical and microbiologic cure were similar to unadjusted results (Table 4).
Table 4.
Prevalence Ratios (Azithromycin Relative to Doxycycline) for Clinical and Microbiologic Cure at 3-Week Follow-up Visit, by Baseline Infecting Organism, Modified Intent-to-Treat Population
| Outcome | Crude Prevalence Ratio (95% CI)b | P Value | Adjusted Prevalence Ratioa (95% CI)b | P Value |
|---|---|---|---|---|
| Clinical cure | ||||
| All | 1.02 (.92–1.14) | .66 | 1.02 (.92–1.14) | .70 |
| C. trachomatis | 1.16 (.95–1.41) | .14 | 1.17 (.95–1.43) | .14 |
| M. genitalium | 1.20 (.74–1.95) | .73 | 1.12 (.59–2.13) | .72 |
| U. urealyticum | 1.10 (.89–1.36) | .37 | 1.13 (.90–1.40) | .30 |
| Idiopathic | .89 (.77–1.04) | .12 | .89 (.77–1.03) | .52 |
| Microbiologic cure | ||||
| C. trachomatis | .96 (.83–1.12) | .62 | .97 (.83–1.13) | .68 |
| M. genitalium | 1.18 (.54–2.57) | .69 | 1.36 (.64–2.91) | .43 |
| U. urealyticum | 1.08 (.85–1.38) | .54 | 1.09 (.86–1.37) | .51 |
Abbreviations: CI, confidence interval; C. trachomatis, Chlamydia trachomatis; M. genitalium, Mycoplasma genitalium; U. urealyticum; Ureaplasma urealyticum.
a Prevalence ratios were adjusted for unprotected sex between visits. Unprotected sex was defined as any sexual encounter without a condom. Not having unprotected sex was defined as anyone who did not have sex between visits, or had sex with a condom for each sexual encounter. Twenty participants were missing values for unprotected sex between visits; these participants are not included in the adjusted analysis. Prevalence ratios, 95% CIs, and P values were obtained from multivariate log binomial regression models with robust standard errors, with either clinical cure at visit 2 or microbiologic cure at visit 2 as the outcome.
b Referent group is doxycycline.
There were no important harms or unintended effects. Expected adverse events (nausea, vomiting, diarrhea, rash) were experienced by 53 men randomized to azithromycin and 56 randomized to doxycycline; nearly all were mild. In the azithromycin arm, 75% of such events were related to the study drug as were 66% in the doxycycline arm. One severe adverse event occurred in the azithromycin group and 3 occurred in the doxycycline group; none were related to study drugs.
DISCUSSION
In this double-blind randomized trial, rates of clinical and microbiologic cure of NGU after CDC-recommended therapy were ≤80%. There were no significant differences in efficacy between azithromycin and doxycycline, and this was true for both clinical and microbiologic cure. Chlamydia trachomatis, U. urealyticum biovar 2, and idiopathic NGU remained relatively sensitive to standard therapies, but M. genitalium was not. The clinical cure rate of M. genitalium–associated NGU, which may account for up to a quarter of cases, was <70% irrespective of the drug used, and the microbiologic cure rate was <40%.
These results were similar to the Stamm et al trial in the mid-1990s [10]. Stamm demonstrated no difference in clinical cure rates after azithromycin or doxycycline treatment (90% and 89%, respectively), but cure rates were 10–15 percentage points higher than ours, as were clearance rates for C. trachomatis (95% for azithromycin; 93% for doxycycline). More recently, Schwebke et al [12] reported significantly lower clearance of C. trachomatis after azithromycin compared to doxycycline (77% vs 95%, P = .01), somewhat lower clinical efficacy of azithromycin for all-cause NGU (69% vs 75%, not significant), and significantly higher efficacy of azithromycin compared to doxycycline for M. genitalium (67% vs 31%, P < .01).
A number of differences in trial design and analytic strategy may contribute to these differences. First, ours was a single-site trial, whereas the others were multisite studies. Second, we measured clinical and microbiologic cure 3 weeks after treatment (to exclude detection of residual DNA [19–21]), whereas Stamm et al and Schwebke et al assessed men at 2 and 5 weeks after therapy, reporting 2-week clinical cure and cumulative rates for C. trachomatis eradication. Third, the Stamm et al trial assessed C. trachomatis by culture, whereas both other trials used NAATs. Finally, the Schwebke et al trial classified men who did not return as having clinical and/or microbiologic treatment failure [12] (comparable to our ITT sensitivity analysis), whereas the Stamm et al trial excluded them from analyses [10] (comparable to our mITT analysis). Because our ITT results were highly sensitive to the classification of men lost to follow-up, and true ITT analyses become impractical if the outcome cannot be ascertained when subjects fail to return [22], we believe our mITT results are most relevant.
The efficacy of azithromycin and doxycycline for all-cause and C. trachomatis–associated NGU that we observed was somewhat lower than in the mid-1990s [10], but not dramatically so. However, the extremely low efficacy of both drugs against M. genitalium is concerning. Whereas previous studies have consistently shown low cure rates for M. genitalium after treatment with doxycycline [11, 12, 16, 23], the low cure rates for azithromycin were unexpected. In all previous comparisons, azithromycin was significantly more effective than doxycycline [11, 12, 23]. Our findings suggest that susceptibility of M. genitalium to azithromycin is especially low in Seattle, declining over time, or both. Comparing 3 US trials, the microbiologic cure rate of M. genitalium following azithromycin declined from 77% in New Orleans (2002–2004) [11], to 67% in the eastern and southern United States (2006–2009) [12], to 40% in our Seattle-based study (2007–2011). Similar time trends occurred in Australia where azithromycin cure rates declined from 84% (2005–2007) to 69% (2007–2009). Pretreatment specimens from half the Australian participants who failed treatment were susceptible to azithromycin, suggesting that therapy selected for resistant isolates [24] and resistance may increase in areas of high usage. Antimicrobial susceptibility testing of isolates recovered in this trial is ongoing.
These results raise a number of questions about NGU treatment. Their relative consistency with the initial 1990s trial suggests that azithromycin remains an effective therapy for NGU overall, and for C. trachomatis specifically, though efficacy may be declining. Nevertheless, the reasons for the lower efficacy of azithromycin in the well-designed Schwebke et al trial merit further investigation. In contrast, these findings, along with other studies of M. genitalium and NGU, suggest that treatment guidelines for persistent NGU may require revision. Current guidelines recommend that men originally treated with doxycycline receive azithromycin and metronidazole or tinidazole for T. vaginalis. Here, 30% of men with persistent urethritis had M. genitalium and neither azithromycin nor doxycycline was particularly effective. There are no commercial assays for M. genitalium in the United States and, although a number of correlates of M. genitalium have been identified, few differentiate M. genitalium–associated NGU from other etiologies [25], making it challenging to identify these infections. Moxifloxacin (400 mg × 7 days) has been highly effective against M. genitalium in a number of settings [26–29] and our Seattle STD clinic now treats persistent NGU with moxifloxacin. However, this must be balanced with the potential for moxifloxacin-associated hepatotoxicity.
This double-blind randomized trial was well controlled and used sensitive NAATs to detect pathogens, and losses to follow-up were relatively low. Nevertheless, results may not be entirely generalizable; antimicrobial resistance patterns vary regionally and may differ in other locations. Despite a robust sample size, we had smaller numbers in some subgroup analyses and pathogen-specific estimates may be less stable.
In this population, azithromycin and doxycycline were similarly effective against NGU, but clinical and microbiologic cure rates showed some decline from the 1990s. The exception was M. genitalium, which accounts for 10%–25% of NGU cases and responded poorly to both regimens. The absence of a commercially available assay for M. genitalium, along with treatment failure up to 70%, presents substantial challenges for the clinical management of NGU. Development of commercially available assays, monitoring of M. genitalium susceptibility to azithromycin, and new antibiotic regimens for NGU are needed.
Notes
Acknowledgments. The authors would like to thank the men who participated in the trial, as well as the clinicians and staff in the Public Health–Seattle & King County Sexually Transmitted Diseases Clinic (Yolanda Bantolino, Sylvia Berry, Irene King, Eduardo Muñoz, Victory Murphy, Sally Pendras, Sue Szabo, Michael Verdon, Fred Koch, Roxanne Kerani, Barbara Krekeler); study staff (Sarah McDougal, Noa Kay, Dwyn Dithmer-Schreck); George Kenny, Sabina Astete, Lisa Lowenstein, and Linda Arnesen in the Totten Laboratory; Linda Cles in the UW Chlamydia Laboratory; Gen-Probe, Inc for reagents; Ana-Maria Xet-Mull and William Whittington for trichomonas testing at the University of Washington; HMC IDS (Jeffrey Purcell, Bao Chau Vo, Asaad Awan, Kelly Nguyen); and the data safety and monitoring board (Edward W. Hook III, David H. Martin, H. Hunter Handsfield, Sarah Holte). We also thank Carolyn Deal, Elizabeth Rogers, and Peter Wolff at the Division of Microbiology and Infectious Diseases at the National Institutes of Health, and Pfizer, Inc, for supplying study drugs. Finally, we extend special thanks to King K. Holmes for guidance and support.
Financial support. This work was supported by the National Institutes of Health: National Institute of Allergy and Infectious Diseases (U19 AI31448, R01 AI072728, and T32 AI07140 trainee support to C. W. G.) and the National Cancer Institute (R25 CA094880 trainee support to D. V. C.). Pfizer, Inc, provided study drugs (active azithromycin, active doxycycline, and placebo azithromycin placebo). The HMC IDS provided placebo doxycycline.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Schaeffer EM. Re: demographic, behavioral, and clinical characteristics of men with nongonococcal urethritis differ by etiology: a case-comparison study. J Urol. 2012;187:1003. doi: 10.1016/j.juro.2011.11.052. [DOI] [PubMed] [Google Scholar]
- 2.Dunlop EM, Harper IA, al-Hussaini MK, et al. Relation of TRIC agent to “non-specific genital infection.”. Br J Vener Dis. 1966;42:77–87. doi: 10.1136/sti.42.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: from Chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011;24:498–514. doi: 10.1128/CMR.00006-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deguchi T, Yoshida T, Miyazawa T, et al. Association of Ureaplasma urealyticum (biovar 2) with nongonococcal urethritis. Sex Transm Dis. 2004;31:192–5. doi: 10.1097/01.olq.0000114653.26951.71. [DOI] [PubMed] [Google Scholar]
- 5.Wetmore CM, Manhart LE, Lowens MS, et al. Ureaplasma urealyticum is associated with nongonococcal urethritis among men with fewer lifetime sexual partners: a case-control study. J Infect Dis. 2011;204:1274–82. doi: 10.1093/infdis/jir517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ondondo RO, Whittington WL, Astete SG, Totten PA. Differential association of Ureaplasma species with non-gonococcal urethritis in heterosexual men. Sex Transm Infect. 2010;86:271–5. doi: 10.1136/sti.2009.040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin DH. Urethritis in males. In: Holmes KK, Sparling PF, Stamm WE, et al., editors. Sexually transmitted diseases. 4th ed. New York: McGraw-Hill; 2008. pp. 1107–26. [Google Scholar]
- 8.Holmes KK, Stamm WE, Sobel JD. Lower genital tract infection syndromes in women. In: Holmes KK, Sparling PF, Stamm WE, editors. Sexually transmitted diseases. 4th ed. New York: McGraw-Hill; 2008. pp. 987–1016. [Google Scholar]
- 9.Centers for Disease Control and Prevention. Updated recommended treatment regimens for gonococcal infections and associated conditions—United States, April 2007. Available at http://www.cdc.gov/std/treatment/2006/updated-regimens.htm. Accessed 12 June 2012. [Google Scholar]
- 10.Stamm WE, Hicks CB, Martin DH, et al. Azithromycin for empirical treatment of the nongonococcal urethritis syndrome in men. A randomized double-blind study. JAMA. 1995;274:545–9. [PubMed] [Google Scholar]
- 11.Mena LA, Mroczkowski TF, Nsuami M, Martin DH. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium-positive urethritis in men. Clin Infect Dis. 2009;48:1649–54. doi: 10.1086/599033. [DOI] [PubMed] [Google Scholar]
- 12.Schwebke JR, Rompalo A, Taylor S, et al. Re-evaluating the treatment of nongonococcal urethritis: emphasizing emerging pathogens—a randomized clinical trial. Clin Infect Dis. 2011;52:163–70. doi: 10.1093/cid/ciq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen JS, Bradshaw CS, Tabrizi SN, Fairley CK, Hamasuna R. Azithromycin treatment failure in Mycoplasma genitalium-positive patients with nongonococcal urethritis is associated with induced macrolide resistance. Clin Infect Dis. 2008;47:1546–53. doi: 10.1086/593188. [DOI] [PubMed] [Google Scholar]
- 14.Dutro SM, Hebb JK, Garin CA, Hughes JP, Kenny GE, Totten PA. Development and performance of a microwell-plate-based polymerase chain reaction assay for Mycoplasma genitalium. Sex Transm Dis. 2003;30:756–63. doi: 10.1097/01.OLQ.0000078821.27933.88. [DOI] [PubMed] [Google Scholar]
- 15.Kenny GE. Mycoplasmata. In: Lenette E, Balows A, Hausler WJ, Truant J, editors. Manual of clinical microbiology. 3rd ed. Washington, DC: American Society for Microbiology; 1980. pp. 365–70. [Google Scholar]
- 16.Falk L, Fredlund H, Jensen JS. Tetracycline treatment does not eradicate Mycoplasma genitalium. Sex Transm Infect. 2003;79:318–9. doi: 10.1136/sti.79.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gambini D, Decleva I, Lupica L, Ghislanzoni M, Cusini M, Alessi E. Mycoplasma genitalium in males with nongonococcal urethritis: prevalence and clinical efficacy of eradication. Sex Transm Dis. 2000;27:226–9. doi: 10.1097/00007435-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–56. [PubMed] [Google Scholar]
- 19.Workowski KA, Lampe MF, Wong KG, Watts MB, Stamm WE. Long-term eradication of Chlamydia trachomatis genital infection after antimicrobial therapy. Evidence against persistent infection. JAMA. 1993;270:2071–5. [PubMed] [Google Scholar]
- 20.Gaydos CA, Crotchfelt KA, Howell MR, Kralian S, Hauptman P, Quinn TC. Molecular amplification assays to detect chlamydial infections in urine specimens from high school female students and to monitor the persistence of chlamydial DNA after therapy. J Infect Dis. 1998;177:417–24. doi: 10.1086/514207. [DOI] [PubMed] [Google Scholar]
- 21.Morré SA, Sillekens PT, Jacobs MV, et al. Monitoring of Chlamydia trachomatis infections after antibiotic treatment using RNA detection by nucleic acid sequence based amplification. Mol Pathol. 1998;51:149–54. doi: 10.1136/mp.51.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedman LM, Furbert CD, DeMets DL. Fundamentals of clinical trials. 3rd ed. New York: Springer-Verlag; 1998. [Google Scholar]
- 23.Bjornelius E, Anagrius C, Bojs G, et al. Antibiotic treatment of symptomatic Mycoplasma genitalium infection in Scandinavia: a controlled clinical trial. Sex Transm Infect. 2008;84:72–6. doi: 10.1136/sti.2007.027375. [DOI] [PubMed] [Google Scholar]
- 24.Twin J, Jensen JS, Bradshaw CS, et al. Transmission and selection of macrolide resistant Mycoplasma genitalium infections detected by rapid high resolution melt analysis. PLoS One. 2012;7:e35593. doi: 10.1371/journal.pone.0035593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wetmore CM, Manhart LE, Lowens MS, et al. Demographic, behavioral, and clinical characteristics of men with nongonococcal urethritis differ by etiology: a case-comparison study. Sex Transm Dis. 2011;38:180–6. doi: 10.1097/OLQ.0b013e3182040de9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradshaw CS, Chen MY, Fairley CK. Persistence of Mycoplasma genitalium following azithromycin therapy. PLoS One. 2008;3:e3618. doi: 10.1371/journal.pone.0003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradshaw CS, Jensen JS, Tabrizi SN, et al. Azithromycin failure in Mycoplasma genitalium urethritis. Emerg Infect Dis. 2006;12:1149–52. doi: 10.3201/eid1207.051558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jernberg E, Moghaddam A, Moi H. Azithromycin and moxifloxacin for microbiological cure of Mycoplasma genitalium infection: an open study. Int J STD AIDS. 2008;19:676–9. doi: 10.1258/ijsa.2008.008038. [DOI] [PubMed] [Google Scholar]
- 29.Ross JD, Cronje HS, Paszkowski T, et al. Moxifloxacin versus ofloxacin plus metronidazole in uncomplicated pelvic inflammatory disease: results of a multicentre, double blind, randomised trial. Sex Transm Infect. 2006;82:446–51. doi: 10.1136/sti.2005.019109. [DOI] [PMC free article] [PubMed] [Google Scholar]