Abstract
In this prospective cohort study, the presence of parasite-specific immunoglobulin A in breast milk was associated with protection of Bangladeshi infants from cryptosporidiosis and amebiasis. Our findings suggest that passive immunity could be harnessed for the prevention of Entamoeba histolytica and Cryptosporidium species infection in children living in endemic regions.
Keywords: diarrhea, Cryptosporidium species, Entamoeba histolytica, Giardia lamblia, breast milk
Enteric parasites make up a large burden of disease in young children worldwide [1, 2]. It is widely recognized that breastfeeding prevents infant mortality and protects from diarrheal illness [3]. In addition to being a rich source of nutrition, breast milk provides infants with nonspecific immune factors, such as lactoferrin, as well as antigen-specific secretory immunoglobulin A (IgA). In developing countries, breastfeeding has been shown to reduce mortality from diarrhea by >20-fold [4]. We hypothesized that passive immunity conferred by maternal parasite-specific IgA via breastfeeding could be a means to protect infants from enteric parasites. Here we used a prospective cohort study to investigate whether parasite-specific breast milk antibodies can protect infants from infection in the first year of life.
METHODS
The subjects studied were part of an ongoing community-based prospective cohort study of enteric infections in infants from a slum of Mirpur, in Dhaka, Bangladesh [1]. Infants were enrolled at birth and actively monitored in their homes for diarrhea. Details on the study population, enrollment, surveillance, and specific laboratory methods can be found in the Supplementary Data. Diarrheal and monthly stool samples were tested for Entamoeba histolytica, Cryptosporidium species, and Giardia lamblia using real-time polymerase chain reaction as previously published [5]. Diarrheal infection was defined by identification of a parasite in a diarrheal stool that had not been present in the preceding monthly surveillance stool.
Breast milk samples were collected from mothers 2–3 weeks after birth and tested by enzyme-linked immunosorbent assay (ELISA) for IgA to whole Cryptosporidium parvum oocysts, the carbohydrate recognition domain (CRD) of E. histolytica Gal/GalNac lectin protein, and Giardia recombinant cyst wall protein 1 (TechLab, Inc, Blacksburg, Virginia). During development of the ELISA, a titration step was performed to determine the optimal dilution needed to generate a linear range of values. Mothers were then divided into the top and bottom 50th percentiles for the quantity of each parasite-specific IgA in breast milk.
Statistical Analysis
Correlation between 2 continuous measures was assessed using Spearman rho or Pearson's R when appropriate. Comparison of continuous measures between low and high antibody levels was performed using independent t test or nonparametric tests. Time to the first infection was analyzed as time-to-event data under the survival analysis framework. The survival probabilities from infection were estimated using Kaplan-Meier methods, and survival differences of parasite-specific breast milk antibody levels in infection were evaluated with a log-rank test. Furthermore, the effect of antibody level on infection was analyzed using the Cox proportional hazards model, adjusting for potential confounding variables.
RESULTS
Between January 2008 to January 2009, 226 infants were enrolled and followed over the first year of life. Four mothers were unable to give breast milk samples. Most participating families had a median household expenditure of <US$100 per month, and almost 40% of mothers had no formal education. Nearly all households had access to the municipal water supply and employed safe food handling practices (Supplementary Table 1).
We found that by 4 months of age, approximately 50% of children were exclusively breastfed, and by age 1, no infants were exclusively breastfed. Notably, almost 100% of infants were partially breastfed throughout the entire first year of life (Supplementary Figure 1). There was no significant difference between duration of exclusive breastfeeding among infants with and without E. histolytica, Cryptosporidium species, and G. lamblia infections by t test (P = .54, .20, and .88, respectively; Supplementary Table 1).
Enteric Infection
Over the first year of life, infection by Cryptosporidium species, G. lamblia, and E. histolytica occurred in 40%, 80%, and 50% of infants, respectively (Supplementary Figure 2), and 13% had diarrhea from Cryptosporidium species during this period. These rates were similar to those of a subset of these children reported in a previous study [1]. Repeat infections with Giardia were common (86% infants had >1 Giardia infection) but were rare for Cryptosporidium species and E. histolytica.
Breast Milk IgA and Protection From Infection
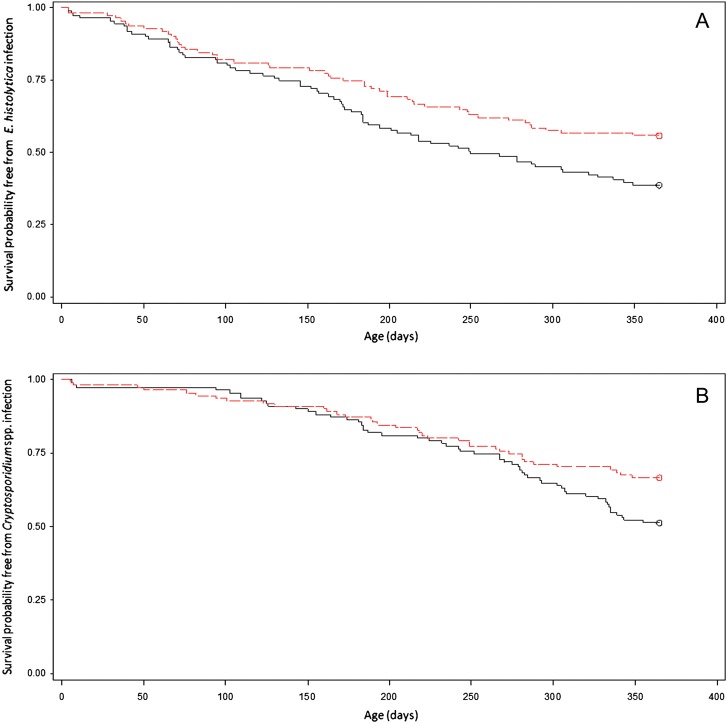
Infants of mothers in the top 50th percentile for anti-CRD breast milk IgA in mother's milk had a significantly higher probability of survival free of E. histolytica infection through the first year of life (log-rank test, P = .016; Figure 1A). Similarly, children whose mother's breast milk had high anti-Cryptosporidium IgA also had a significantly improved probability of survival free of Cryptosporidium infection (log-rank test, P = .039; Figure 1B). In contrast, there was no association between levels of anti-Giardia breast milk IgA and diarrheal or asymptomatic infection from Giardia, or occurrence of repeat infections (data not shown).
Figure 1.
Kaplan-Meier curve showing different probability of survival free of Entamoeba histolytica (A) or Cryptosporidium species (B) infection in year 1 of life among infants (N = 222) based on level of maternal parasite-specific breast milk immunoglobulin A (IgA). The y-axis represents the survival probability free from infection, and the x-axis is survival time in days, for the first 365 days of life. A, Infants of mothers in the top 50th percentile for maternal breast milk anti–carbohydrate recognition domain (red) had a significantly higher survival free of E. histolytica infection than infants whose mothers were in the lower 50th percentile (black) (log-rank χ2 test, P = .016). B, Infants of mothers in the top 50th percentile for anti-Cryptosporidium breast milk IgA (red) had a significantly higher survival free of infection compared to infants of mothers in the lower 50th percentile (black) (log-rank test, P = .039).
Infants exposed to high anti-CRD IgA had a 39% reduced risk of infection (hazard ratio [HR], 0.61; 95% confidence interval [CI], .423–.891; P = .010; Supplementary Table 2) and a 64% reduced risk of diarrheal disease from E. histolytica (HR, 0.356; 95% CI, .149–.849; P = .020). For cryptosporidiosis, infants exposed to high levels of IgA had a 38% reduced risk of Cryptosporidium species infection (HR, 0.622; 95% CI, .407–.952; P = .028; Supplementary Table 2) and a 64% reduced risk of Cryptosporidium species diarrhea (HR, 0.364; 95% CI, .165–.803; P =.01).
The association of parasite-specific IgA with protection remained significant for both E. histolytica and Cryptosporidium species when adjusted for maternal age, body mass index, infant height-for-age z score (HAZ), and days of exclusive breastfeeding using Cox proportional hazards. Higher HAZ at birth was protective for amebiasis, with a 1-unit increase in HAZ conferring 15% reduced risk for E. histolytica infection (HR, 0.848; 95% CI, .721–.998; P = .047), as previously seen [1].
Immunofluorescence performed on C. parvum oocysts demonstrated the presence of IgA targeting the surface of oocysts in breast milk (Figure 2). Similarly, previous work had demonstrated that antibodies to the CRD domain of the Gal/GalNAc lectin localized to the E. histolytica trophozoite surface [6].
Figure 2.
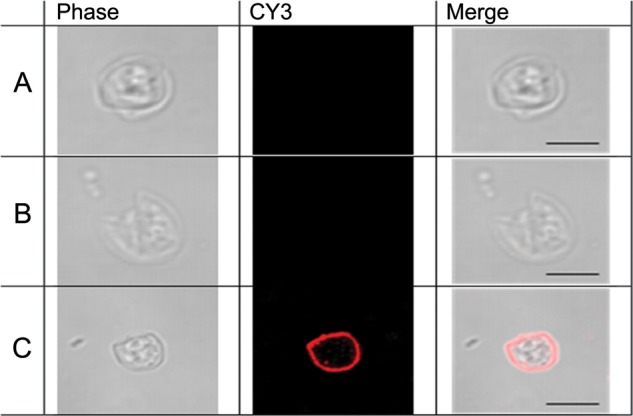
Immunofluorescent staining of Cryptosporidium parvum oocysts with parasite-specific breast milk immunoglobulin A (IgA). Cryptosporidium oocysts treated without a primary antibody (A) or with breast milk (B) from mothers in the lower 50th percentile did not stain with CY3-conjugated goat antihuman serum IgA. C, Oocysts treated with high anti-Cryptosporidium IgA breast milk did stain with the CY3-conjugated secondary antibody, demonstrating the presence of IgA directed at Cryptosporidium oocyst within breast milk. Scale bar = 5 µm.
Finally, to assess whether concentration of breast milk IgA remained constant over the duration of the breastfeeding period, we tested E. histolytica CRD IgA in serial breast milk samples. Anti-CRD breast milk IgA at month 1 correlated significantly with levels at month 3 and month 5 (Spearman rho = 0.44 and 0.53, respectively, P < .01).
DISCUSSION
The most important conclusion of this study was that parasite-specific breast milk IgA was associated with protection of infants from E. histolytica and Cryptosporidium species infection. Our findings are consistent with passive immunity being transferred via breast milk IgA. There was no protection from Giardia infection observed in children from mothers with high anti-Giardia IgA, consistent with earlier work [7, 8]. Potential explanations include the ability of Giardia to antigenically vary its surface coat, or the extremely high exposure to this parasite in this community. Differences in genetic susceptibility and environmental exposure may also contribute to protection from infection.
The association of anti-CRD breast milk IgA with protection from E. histolytica infection offers added support for the use of the E. histolytica Gal/GalNAc lectin as a vaccine against amebiasis, in this context as a maternally administered vaccine with the goal of boosting breast milk IgA. The CRD domain contains all of the neutralizing antibody epitopes of the Gal/GalNAc lectin that block amebic adherence to and killing of host cells [6, 9, 10].
Historically, immunity to Cryptosporidium species has been thought to be primarily cell mediated. The present studies support a role for humoral immunity, consistent with our previous work showing increased susceptibility associated with specific human leukocyte antigen class II alleles [11], and prior data of humoral immune responses against Cryptosporidium species [12–16]. Our findings differ from a Peruvian study of Cryptosporidium IgA, likely because of different epidemiology and use of more sensitive diagnostic methods [17]. Interestingly, almost half of infants in our study were infected with Cryptosporidium species by age 1, with infection rare prior to 6 months, and with a male preponderance as previously reported [18, 19].
A limitation of our work is that only breast milk from the first month of life was tested for all antibodies, with this data extrapolated for the entire first year of life. However, studies have shown that with the exception of antibody-rich colostrum, there is not a significant decrease in antigen-specific secretory IgA over the first few months [7]. Consistent with this, E. histolytica CRD IgA levels correlated significantly from month 1 to months 3 and 5. Further studies are needed to validate these epidemiologic findings.
We hypothesize that once ingested by the infant, IgA in breast milk coats infective stages of Cryptosporidium species and E. histolytica, and may prevent excystation, attachment, or invasion of host epithelial cells. A number of antigenic targets have been identified on the infective sporozoite stage of Cryptosporidium species, and it remains to be seen whether antibodies to these epitopes can interfere with infection [16, 20].
The concept of harnessing passive immunity to protect infants is not novel. Antenatal maternal immunization with influenza vaccine prevents influenza in both mothers and infants <6 months of age [21]. While immunity to influenza is likely transferred via placental IgG, our study shows that breast milk IgA may be an unrecognized source of protection from enteric protozoa. Our findings suggest that passive immunity may play a significant role in defense against Cryptosporidium species and E. histolytica during the first year of life. Most importantly, our findings highlight the need and urgency to focus on vaccine development for enteric protozoa. Immunization of women of childbearing age and subsequent promotion of breastfeeding may be one way to protect infants in low-income countries from Cryptosporidium species and E. histolytica infection, and thereby reduce the global burden of diarrheal disease.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the parents and children of Mirpur for their participation in this study and Drs Joel Herbein and David Lyerly of TechLab, Inc, for the provision of reagents for testing parasite-specific IgA.
Financial support. This work was supported by the National Institutes of Health (grant number 5R01 AI043596 to W. A. P.) and by the Bill & Melinda Gates Foundation.
Potential conflicts of interest. W. A. P. receives licensing fees from TechLab, Inc, for amebiasis diagnostics; these fees are donated in their entirety to the American Society of Tropical Medicine and Hygiene. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Mondal D, Minak J, Alam M, et al. Contribution of enteric infection, altered intestinal barrier function, and maternal malnutrition to infant malnutrition in Bangladesh. Clin Infect Dis. 2012;54:185–91. doi: 10.1093/cid/cir807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guerrant DI, Moore SR, Lima AA, Patrick PD, Schorling JB, Guerrant RL. Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function four–seven years later in a poor urban community in northeast Brazil. Am J Trop Med Hyg. 1999;61:707–13. doi: 10.4269/ajtmh.1999.61.707. [DOI] [PubMed] [Google Scholar]
- 3.Arifeen S, Black RE, Antelman G, Baqui A, Caulfield L, Becker S. Exclusive breastfeeding reduces acute respiratory infection and diarrhea deaths among infants in Dhaka slums. Pediatrics. 2001;108:e7. doi: 10.1542/peds.108.4.e67. [DOI] [PubMed] [Google Scholar]
- 4.Feachem RG, Koblinsky MA. Interventions for the control of diarrhoeal diseases among young children: promotion of breast-feeding. Bull World Health Organ. 1984;62:271–91. [PMC free article] [PubMed] [Google Scholar]
- 5.Haque R, Roy S, Siddique A, et al. Multiplex real-time PCR assay for detection of Entamoeba histolytica, Giardia lamblia and Cryptosporidium spp. Am J Trop Med Hyg. 2007;76:713–7. [PubMed] [Google Scholar]
- 6.Dodson JM, Lenkowski PW, Jr, Eubanks AC, et al. Infection and immunity mediated by the carbohydrate recognition domain of the Entamoeba histolytica Gal/GalNAc lectin. J Infect Dis. 1999;179:460–6. doi: 10.1086/314610. [DOI] [PubMed] [Google Scholar]
- 7.Miotti PG, Gilma RH, Pickering LK, Ruiz-Palacios GM, Park HS, Yolken RH. Prevalence of serum and milk antibodies to Giardia lamblia in different populations of lactating women. J Infect Dis. 1985;152:1025–31. doi: 10.1093/infdis/152.5.1025. [DOI] [PubMed] [Google Scholar]
- 8.Walterspiel JN, Morrow AL, Guerrero ML, Ruiz-Palacios GM, Pickering LK. Secretory anti-Giardia lamblia antibodies in human milk: protective effect against diarrhea. Pediatrics. 1994;93:28–31. [PubMed] [Google Scholar]
- 9.Haque R, Mondal D, Duggal P, et al. Entamoeba histolytica infection in children and protection from subsequent amebiasis. Infect Immun. 2006;74:904–9. doi: 10.1128/IAI.74.2.904-909.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Islam A, Stoll BJ, Ljungstrom I, Biswas J, Nazrul H, Huldt G. The prevalence of Entamoeba histolytica in lactating women and their infants in Bangladesh. Trans R Soc Trop Med Hyg. 1988;82:99–103. doi: 10.1016/0035-9203(88)90276-3. [DOI] [PubMed] [Google Scholar]
- 11.Kirkpatrick BD, Haque R, Duggal P, et al. Association between Cryptosporidium infection and human leukocyte antigen class I and class II alleles. J Infect Dis. 2008;197:474–8. doi: 10.1086/525284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okhuysen PC, Chappell CL, Crabb J, Valdez LM, Douglass ET, DuPont HL. Prophylactic effect of bovine anti-Cryptosporidium hyperimmune colostrum immunoglobulin in healthy volunteers challenged with Cryptosporidium parvum. Clin Infect Dis. 1998;6:1324–9. doi: 10.1086/516374. [DOI] [PubMed] [Google Scholar]
- 13.Nord J, Ma P, DiJohn D, Tzipori S, Tacket CO. Treatment with bovine hyperimmune colostrum of cryptosporidial diarrhea in AIDS patients. AIDS. 1990;4:581–4. doi: 10.1097/00002030-199006000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Jenkins MC, O'Brien C, Trout J, Guidry A, Fayer R. Hyperimmune bovine colostrum specific for recombinant Cryptosporidium parvum antigen confers partial protection against cryptosporidiosis in immunosuppressed adult mice. Vaccine. 1999;17:2453–60. doi: 10.1016/s0264-410x(98)00369-7. [DOI] [PubMed] [Google Scholar]
- 15.Kaushik K, Khurana S, Wanchu A, Malla N. Serum immunoglobulin G, M and A response to Cryptosporidium parvum in Cryptosporidium-HIV co-infected patients. BMC Infect Dis. 2009;9:179. doi: 10.1186/1471-2334-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allison GM, Rogers KA, Borad A, et al. Antibody responses to the immunodominant Cryptosporidium gp15 antigen and gp15 polymorphisms in a case-control study of cryptosporidiosis in children in Bangladesh. Am J Trop Med Hyg. 2011;85:97–104. doi: 10.4269/ajtmh.2011.11-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterling CR, Gilman RH, Sinclair NA, Cama V, Castillo R, Diaz F. The role of breast milk in protecting urban Peruvian children against cryptosporidiosis. J Protozool. 1991;38:23S–5. [PubMed] [Google Scholar]
- 18.Khan WA, Rogers KA, Karim MM, et al. Cryptosporidiosis among Bangladeshi children with diarrhea: a prospective, matched, case-control study of clinical features, epidemiology and systemic antibody responses. Am J Trop Med Hyg. 2004;71:412–9. [PubMed] [Google Scholar]
- 19.Newman RD, Sears CL, Moore SR, et al. Longitudinal study of Cryptosporidium infection in children in northeastern Brazil. J Infect Dis. 1999;180:167–75. doi: 10.1086/314820. [DOI] [PubMed] [Google Scholar]
- 20.Cevallos AM, Zhang X, Waldor MK, et al. Molecular cloning and expression of a gene encoding Cryptosporidium parvum glycoproteins gp40 and gp15. Infect Immun. 2000;68:4108–16. doi: 10.1128/iai.68.7.4108-4116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359:1555–64. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.