Seven- or 21-day regimens of tenofovir/emtricitabine, zidovudine/lamivudine, or lopinavir/ritonavir after single-dose nevirapine (NVP) were effective in suppressing NVP resistance detected by population genotype. Allele-specific polymerase chain reaction revealed that the 21-day regimens were significantly better at preventing the emergence of minor NVP resistance.
Keywords: nevirapine, mother-to-child transmission, pregnancy, resistance, HIV
Abstract
Background. Nevirapine (NVP) resistance emerges in up to 70% of women exposed to single-dose (sd) NVP for prevention of mother-to-child transmission of human immunodeficiency virus (HIV).
Methods. HIV-infected pregnant women were randomized to receive sdNVP and either zidovudine/lamivudine (3TC), tenofovir/emtricitabine (FTC), or lopinavir/ritonavir for either 7 or 21 days. The primary endpoint was the emergence of new NVP resistance mutations as detected by standard population genotype at 2 and 6 weeks after treatment. Low-frequency NVP- or 3TC/FTC-resistant mutants at codons 103, 181, and 184 were sought using allele-specific polymerase chain reaction (ASP).
Results. Among 484 women randomized, 422 (87%) received study treatment. Four hundred twelve (98%) women had primary endpoint results available; of these, 5 (1.2%) had new NVP resistance detected by population genotype: 4 of 215 in the 7-day arms (1.9%; K103N in 4 women with Y181C, Y188C, or G190A in 3 of 4) and 1 of 197 (0.5%; V108I) in the 21-day arms (P = .37). Among women with ASP results, new NVP resistance mutations emerged significantly more often in the 7-day arms (13/74 [18%]) than in the 21-day arms (3/66 [5%], P = .019). 3TC/FTC-resistant mutants (M184V/I) emerged infrequently (7/134 [5%]), and their occurrence did not differ by arm.
Conclusions. Three short-term antiretroviral strategies, begun simultaneously with the administration of sdNVP, resulted in a low rate (1.2%) of new NVP-resistance mutations when assessed at 2 and 6 weeks following completion of study treatment by standard genotype. ASP revealed that 21-day regimens were significantly better than 7-day regimens at preventing the emergence of minor NVP resistance variants.
Clinical Trials Registration. NCT00099632.
A single intrapartum dose of nevirapine (sdNVP), a nonnucleoside reverse transcriptase inhibitor (NNRTI), significantly reduces the mother-to-child transmission of human immunodeficiency virus type 1 (HIV-1) [1], but not without consequence. Because of slow NVP metabolism, women are exposed to NVP monotherapy for a prolonged interval after sdNVP [2]. This facilitates selection of NVP resistance mutations detectable in 15%–45% of women by standard population genotype who received sdNVP [3–8]. The proportion of women who have new NVP resistance mutations detected after sdNVP is higher—up to 70% [9, 10]—when more sensitive assays such as allele-specific polymerase chain reaction (PCR), known as ASP, are used to detect minor viral populations (<20% of the circulating pool of virus). NVP resistance following sdNVP can compromise future maternal antiretroviral (ARV) treatment [11] and thus has significant public health implications [12].
Short-course (3–7 days) ARV strategies started at the time of sdNVP dosing have been implemented to minimize maternal exposure to NVP monotherapy, but a 7-day course of zidovudine (ZDV) and lamivudine (3TC) still allows the emergence of NVP resistance in up to 12% of women at 6 weeks postpartum using standard genotyping techniques [13] and up to 33% of women using ASP [14]. Our study was designed to compare 7- and 21-day ARV durations and evaluate alternate ARV strategies in the context of maternal intrapartum sdNVP, with the goal of reducing the emergence of NVP resistance to <2% of women. Lopinavir/ritonavir was chosen owing to its potential to block the emergence of NVP resistance, while avoiding resistance to nucleoside reverse transcriptase inhibitors (NRTIs) used in first-line ART regimens.
METHODS
Study Design and Participants
This phase II, prospective, randomized, open-label study evaluated the effectiveness of 3 ARV strategies administered for 7 days (7-day arms) or 21 days (21-day arms) to HIV-1–infected pregnant women after sdNVP in 8 sites in sub-Saharan Africa, India, and Haiti. Prior to onset of labor, women were randomly assigned to receive sdNVP plus either zidovudine/lamivudine (ZDV/3TC; 300 mg of zidovudine and 150 mg of lamivudine) twice daily, tenofovir/emtricitabine (TDF/FTC; 300 mg of tenofovir and 200 mg of emtricitabine) daily, or lopinavir/ritonavir (LPV/r; 400 mg of lopinavir and 100 mg of ritonavir) twice daily. Participants were also randomized to short (7 days) or longer (21 days) treatment duration, in a factorial design. Randomization was stratified by whether ZDV had or was expected to be taken during the pregnancy. Infants received a single dose of study-supplied NVP within 48 hours of birth. Nevirapine tablets (200 mg) for the mothers and NVP suspension (50 mg/5 mL) for the infants were supplied through the study (Boehringer-Ingelheim). ZDV/3TC was supplied as co-formulated Combivir (GlaxoSmithKline). LPV/r was initially supplied as Kaletra capsules (Abbott) and changed to Aluvia tablets in April 2007. TDF/FTC was supplied as co-formulated Truvada (Gilead).
The targeted sample size for the study was 420 mothers receiving study treatment (and exposed infants). Women were randomized between 28 and 38 weeks of gestation, and were to start treatment intrapartum. Those who failed to initiate study treatment (eg, off-site delivery) were replaced. Primary endpoint evaluations were completed at 2 and 6 weeks after completion of randomized treatment (ie, weeks 3 and 7 after sdNVP for the 7-day arms and weeks 5 and 9 for the 21-day arms). The protocol required reporting of all signs, symptoms, and laboratory values of grade 3 or higher that occurred after starting study treatment, and any sign or symptom that led to a change in treatment.
The study was approved by sites and their affiliated US partner's institutional review boards. Written informed consent was obtained from participants. A data and safety monitoring board reviewed safety and efficacy data on a regular basis, and no safety issues were identified. The pharmaceutical sponsors (Abbott, Boehringer Ingelheim, Gilead, GlaxoSmithKline) were members of the study team and supplied drugs for the study, but did not participate in any of the data analysis. Plasma HIV-1 RNA levels were performed at AIDS Clinical Trials Group (ACTG)–certified laboratories using the Roche Amplicor Monitor HIV real-time polymerase chain reaction (RT-PCR) version 1.5 assay or the Abbott RealTime HIV-1 assay.
HIV-1 Resistance Testing and Subtyping
Resistance testing was undertaken using the US Food and Drug Administration–approved ViroSeq HIV-1 Genotyping System (version 2.0, Celera Diagnostics, Alameda, California) and performed in batches. Resistance testing was conducted at 3 ACTG-certified laboratories (Chennai, India; Johannesburg, South Africa; Pittsburgh, Pennsylvania). Samples that could not be amplified or adequately sequenced using standard ViroSeq primers were tested using Celera's alternate RT-PCR and/or sequencing primers (designated A3, A4, B4, C4, F1, G1, and H1). Samples with HIV-1 RNA levels ≤400 copies/mL were not tested and were assumed to have no resistance in the primary analysis. All sequencing product was resolved on an ABI 3100 Genetic Analyzer (Applied Biosystems, Foster City, California) and data were analyzed using ViroSeq version 2.7 or Sequencer version 4.8 software.
ASP testing on plasma samples with HIV-1 RNA ≥5000 copies/mL at baseline and 6 weeks after the completion of study treatment was undertaken at a single laboratory (Pittsburgh) using methods previously described [15] and performed in batches. The following mutants were sought: K103N (either ATT or AAC), Y181C, and M184V or I. The detection limit of ASP was 0.1% for K103N, 0.3% for Y181C, 0.3% for M184V, and 0.4% for M184I.
The primary endpoint of the study was the emergence of new NVP resistance mutants detected by standard population genotyping. Key secondary endpoints were the emergences of new NRTI and protease inhibitor resistance mutations as well as new NVP and 3TC/FTC resistance mutations as determined by ASP. The primary and secondary endpoints by standard genotyping were evaluated at 2 and 6 weeks after completion of randomized therapy. NVP and 3TC/FTC resistance mutations by ASP were evaluated at 6 weeks after completion of randomized treatment. The definition of drug resistance mutations (including considerations of different clades) was established prior to the first interim analysis according to the available literature at that time (International Antiviral Society-USA Table 2007). The term “new mutation” refers to a mutation detected after sdNVP that was not detected prior to sdNVP. When no resistance data were available prior to sdNVP, any drug resistance mutation detected was considered a new mutation. All wild-type/mutant mixtures were scored as resistant.
Data Analysis
The exact Cochran-Mantel-Haenszel tests were used to test for differences in proportions between treatment durations (7 vs 21 days) stratified by ARV regimen and the actual receipt of antenatal ZDV, among the 3 ARV regimens stratified by treatment duration and the actual receipt of antenatal ZDV, and between women with and without antenatal ZDV exposure stratified by treatment duration and ARV regimen. The analyses excluded women who were documented not to have taken sdNVP. All women were analyzed according to the actual receipt of antenatal ZDV. Exact confidence intervals for the proportion were calculated based on the binomial distribution. Self-reported adherence was summarized as the proportion of women who reported no missed dose on the day prior to the study visit at day 1 and over the 7 days prior to the study visit at weeks 1, 2, and 3 (weeks 2 and 3 for women assigned to 21-day duration only). All statistical tests were 2-sided at the 5% level of significance without adjustment for multiple testing (SAS version 9.1, StatXact version 8).
RESULTS
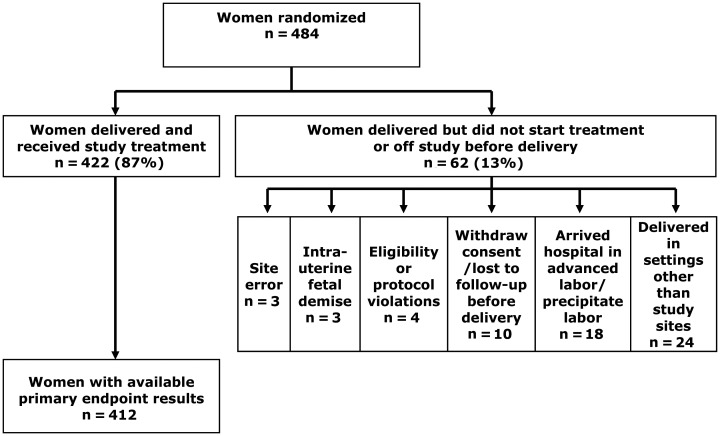
The first woman was randomized in January 2007 and the study closed to accrual in January 2010. A total of 484 women were randomized. Sixty-two (13%) women delivered but did not receive study treatment or were off study prior to delivery (Figure 1). Four hundred twenty-two (87%) received randomized study treatment, of whom 412 (98%) had primary endpoint data available.
Figure 1.
Flowchart of study participation.
Table 1 shows baseline characteristics of the participants. The median screening CD4+ count was 490 cells/mm3; the median HIV-1 RNA level was 3.50 log10 copies/mL (3162 copies/mL). At baseline (prior to sdNVP dosing), 83 (20%) women had HIV-1 RNA ≤400 copies/mL. Two hundred sixty-seven (63%) women reported receipt of ZDV during pregnancy for a median of 8 weeks. HIV-1 subtypes were available for participants with sufficient viremia to have had an HIV-1 resistance test performed. The majority were found to have clade C virus (62%, n = 237); other subtypes included A1 (15%, n = 56), A2 (n = 1), B (14%, n = 54), and D (8%, n = 32). Two women were found to have a complex recombinant subtype, and 1 could not be classified. Thirty-nine women had missing subtypes.
Table 1.
Maternal Baseline Characteristics by Study Treatment Duration
| Characteristic | Total (N = 422) | 7-Day Arm (n = 219) | 21-Day Arm (n = 203) | |
|---|---|---|---|---|
| Age at sdNVP dosing, y | Median (10%, 90%) | 26 (21, 34) | 26 (20, 35) | 25 (21,33) |
| Gestational age at sdNVP dosing, wk | Median (10%, 90%) | 38 (36, 41) | 38 (36, 41) | 38 (35, 41) |
| Race/ethnicity | Asian (other than Indian) | 1 (0%) | 1 (0%) | 0 (0%) |
| Black African | 260 (62%) | 137 (63%) | 123 (61%) | |
| Black of African origin | 58 (14%) | 31 (14%) | 27 (13%) | |
| Indian (Native of India) | 103 (24%) | 50 (23%) | 53 (26%) | |
| HIV-1 RNA, log10 copies/mL | Median (10%, 90%) | 3.50 (2.60, 4.83) | 3.51 (2.55, 4.81) | 3.50 (2.60, 4.85) |
| Mean (SD) | 3.56 (0.90) | 3.56 (0.91) | 3.57(0.90) | |
| HIV-1 RNA, copies/mL | ≤400 | 83 (20%) | 42 (19%) | 41 (20%) |
| 401–999 | 48 (11%) | 25 (11%) | 23 (11%) | |
| 1000–9999 | 148 (35%) | 79 (36%) | 69 (34%) | |
| 10 000–99 999 | 112 (27%) | 55 (25%) | 57 (28%) | |
| ≥100 000 | 29 (7%) | 17 (7%) | 12 (6%) | |
| Missing | 2 | 1 | 1 | |
| Screening CD4 count, cells/mm3 | Median (10%, 90%) | 490 (320, 861) | 468 (305, 777) | 508 (338, 888) |
| 250–349 | 69 (16%) | 43 (20%) | 26 (13%) | |
| 350–499 | 150 (36%) | 78 (36%) | 72 (35%) | |
| ≥500 | 203 (48%) | 98 (45%) | 105 (52%) | |
| Actual ZDV exposure during pregnancy | Yes | 267 (63%) | 136 (62%) | 131 (65%) |
| No | 155 (37%) | 83 (38%) | 72 (35%) | |
| HIV-1 subtype | A1 | 56 (15%) | 27 (13%) | 29 (16%) |
| A2 | 1 (0%) | 1 (0%) | 0 (0%) | |
| B | 54 (14%) | 29 (14%) | 25 (14%) | |
| C | 237 (62%) | 126 (62%) | 111 (62%) | |
| D | 32 (8%) | 19 (9%) | 13 (7%) | |
| Complex recombinant | 2 (1%) | 1 (0%) | 1 (1%) | |
| Unclassified | 1 (0%) | 0 (0%) | 1 (1%) | |
| Unknown | 39 | 16 | 23 |
Abbreviations: HIV-1, human immunodeficiency virus type 1; sdNVP, single-dose nevirapine; ZDV, zidovudine.
The proportion of women with HIV-1 RNA ≤400 copies/mL was similar for both durations at 2 weeks after the end of treatment (39% at week 3 for the 7-day vs 40% at week 5 for the 21-day arms) and at 6 weeks after the end of treatment (9% at week 7 for the 7-day vs 11% at week 9 for the 21-day arms). When pooled over the 7- and 21-day arms, more women receiving TDF/FTC or ZDV/3TC had HIV-1 RNA ≤400 copies/mL at 2 weeks after treatment than those receiving LPV/r (57% vs 44% vs 17%, P < .001). However, the proportions of women with HIV-1 RNA ≤400 copies/mL at 6 weeks after treatment were similar for the 3 ARV strategies (12% vs 11% vs 8%).
Population Genotype Analysis
Ten (2%) women were excluded from the analyses due to lack of primary endpoint data; specimens were lacking for 8 women (3 each in the 7-day TDF/FTC arm and the 21-day LPV/r arm; 1 each in the 21-day ZDV/3TC arm and the 21-day TDF/FTC arm), and assay failures occurred for 2 women (1 each in the 7-day and 21-day TDF/FTC arms) at both primary endpoint weeks.
Of the 412 women with primary endpoint results available, only 5 (1.2%) women had at least 1 new NVP-resistant mutation: 1 in the 7-day LPV/r arm developed K103N; 1 in the 7-day LPV/r arm developed both K103N and Y181C; 1 in the 7-day LPV/r arm developed K103N and Y188C; 1 in the 7-day ZDV/3TC arm developed both K103N and G190A; and 1 in the 21-day LPV/r arm developed V108I (Table 2). This corresponds to 4 (1.9%) women with new NVP-resistant mutations for the 7-day and 1 (0.5%) for 21-day arms. Of the 5 women developing NVP resistance, 1 received ZDV/3TC, and 4 received LPV/r. None of the women in the TDF/FTC arms developed new NVP-resistant mutations. Three of the 5 women with new NVP-resistant mutations had antenatal ZDV exposure and 2 reported no ZDV exposure.
Table 2.
Poststudy Treatment Nevirapine and Nucleoside Reverse Transcriptase Resistance Mutations Detected by Population-Based Genotyping
| Mutation(s) | Overall (n = 412) | 7-Day, ZDV/3TC (n = 73) | 21-Day, ZDV/3TC (n = 67) | 7-Day, TDF/FTC (n = 71) | 21-Day, TDF/FTC (n = 65) | 7-Day, LPV/r (n = 71) | 21-Day, LPV/r (n = 65) |
|---|---|---|---|---|---|---|---|
| New NVP-resistant mutation(s) detected | |||||||
| None | 407 (98.8%) | 72 (98.6%) | 67 (100.0%) | 71 (100.0%) | 65 (100.0%) | 68 (95.8%) | 64 (98.5%) |
| Detected | 5 (1.2%) | 1 (1.4%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 3 (4.2%) | 1 (1.5%) |
| K103N | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| K103N, Y181C | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| K103N, Y188C | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| K103N, G190A | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| V108I | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| New NRTI-resistant mutation(s) detected | |||||||
| None | 408 (99.0%) | 73 (100.0%) | 66 (98.5%) | 70 (98.6%) | 64 (98.5%) | 70 (98.6%) | 65 (100.0%) |
| Detected | 4 (1.0%) | 0 (0.0%) | 1 (1.5%) | 1 (1.4%) | 1 (1.5%) | 1 (1.4%) | 0 (0.0%) |
| M41L | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| M41L, K70R | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| K70R | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| M184I | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
Abbreviations: 3TC, lamivudine; FTC, emtricitabine; LPV/r, lopinavir/ritonavir; NRTI, nucleoside reverse transcriptase inhibitor; NVP, nevirapine; TDF, tenofovir; ZDV, zidovudine.
No significant difference in the proportion of women with new NVP-resistant mutations was detected between regimen durations (1.9% vs 0.5%, P = .37), among ARV regimens (0.7% vs 0% vs 2.9%, P = .091), or between women with and without antenatal ZDV use (1.1% vs 1.4%, P = 1.00) based on standard population genotyping. The 95% exact confidence intervals (CIs) for the proportion of women with new NVP-resistance mutations were .5%–4.7% for the 7-day arms and 0%–2.8% for the 21-day arms; 0%–3.9% for ZDV/3TC, 0%–2.7% for TDF/FTC, and .8%–7.4% for LPV/r; .2%–3.3% for women with antenatal ZDV use, and .2%–4.8% for women with no antenatal ZDV use.
Four (1%) women were found to have at least 1 new NRTI resistance mutation detected by population genotype: 1 woman in the 21-day ZDV/3TC arm had M41L, 1 in the 7-day TDF/FTC arm had M41L and K70R, 1 in the 7-day LPV/r arm had K70R, and 1 in the 21-day TDF/FTC arm had M184I (Table 2). All 3 women with M41L and/or K70R mutations received antenatal ZDV. This corresponds to 2 for the 7-day and 2 for the 21-day; 1 for ZDV/3TC, 2 for TDF/FTC, and 1 for LPV/r; 3 for women with antenatal ZDV use, and 1 for women with no antenatal ZDV use. No significant difference in the proportion of women with new NRTI resistance mutations was detected between treatment durations (0.9% [95% exact CI, .1%–3.3%] vs 1.0% [95% CI, .1%–3.6%]), among ARV regimens (0.7% [95% CI, 0%–3.9%] vs 1.5% [95% CI, .2%–5.2%] vs 0.7% [95% CI, 0%–4.0%]), or between women with and without antenatal ZDV use (1.1% [95% CI, .2%–3.3%] vs 0.7% [95% CI, 0%–3.7%]), all P ≥ .11.
No women had a new protease inhibitor mutation detected by population genotype at the primary endpoint weeks in the 7- or 21-day arms.
Allele-Specific PCR
One hundred fifty women met the criteria for ASP testing requiring HIV-1 RNA ≥5000 copies/mL (80 in the 7-day arms vs 70 in the 21-day arms; 48 women received ZDV/3TC, 46 received TDF/FTC, and 56 received LPV/r; 60 women received antenatal ZDV and 90 did not), of whom 139 (93%) and 136 (91%) had results available at baseline and 6 weeks posttreatment for K103N and Y181C, respectively. ASP testing identified K103N mutations in 13 (9%) baseline samples from before sdNVP and Y181C mutations in 1 (1%) baseline sample. New K103N mutations emerged significantly more often with the 7-day arms compared to the 21-day arms (11 [17%] vs 2 [3%], P = .016, Table 3). Among the 13 women who developed new K103N mutations, 3 (in the 7-day arms) had mutant frequencies ≥1% of the subject's virus population. The Y181C mutation was identified in 3 (2%) women with one having a frequency ≥1% of the virus population. There was no significant difference in new Y181C mutations between the 7-day and 21-day arms. New NVP mutations were found at 6 weeks following completion of study therapy in 13 (18%) of 74 women in the 7-day arms and 3 (5%) of 66 women in the 21-day arms (P = .019), most commonly the K103N mutation (13/16, 81%). Four women had new NVP resistance mutant frequencies >1% of their virus populations at 6 weeks poststudy treatment; all had received 7-day regimens. The median mutation frequency was higher for Y181C at 0.6% than K103N at 0.4%.
Table 3.
Poststudy Treatment K103N and Y181C Resistance Mutations Detected by Allele-Specific Polymerase Chain Reaction
| Mutation | Total (n = 150) | 7-Day Arm (n = 80) | 21-Day Arm (n = 70) | P Value |
|---|---|---|---|---|
| New K103N mutation frequency (%) | ||||
| No. | 127 | 66 | 61 | |
| No new mutation | 114 (90%) | 55 (83%) | 59 (97%) | .016 |
| New mutation | 13 (10%) | 11 (17%) | 2 (3%) | |
| <1% | 10 | 8 | 2 | |
| ≥1% | 3 | 3 | 0 | |
| New Y181C mutation frequency (%) | ||||
| No. | 135 | 72 | 63 | |
| No new mutation | 132 (98%) | 70 (97%) | 62 (98%) | 1.000 |
| New mutation | 3 (2%) | 2 (3%) | 1 (2%) | |
| <1% | 2 | 1 | 1 | |
| ≥1% | 1 | 1 | 0 | |
| Any new mutation (%) | ||||
| No. | 140 | 74 | 66 | |
| No | 124 (89%) | 61 (82%) | 63 (95%) | .019 |
| Yes | 16 (11%) | 13 (18%) | 3 (5%) | |
Table 4.
Poststudy Treatment M184V/I Resistance Mutations Detected by Allele-Specific Polymerase Chain Reaction
| Mutation | Total (n = 150) | ZDV/3TC (n = 48) | TDF/FTC (n = 46) | LPV/r (n = 56) | P Value |
|---|---|---|---|---|---|
| New M184V mutation frequency (%) | |||||
| No. | 134 | 43 | 40 | 51 | .181 |
| No new mutation | 132 (99%) | 41 (95%) | 40 (100%) | 51 (100%) | |
| New mutation | 2 (1%) | 2 (5%) | 0 (0%) | 0 (0%) | |
| <1% | 2 | 2 | 0 | 0 | |
| New M184I mutation frequency (%) | |||||
| No. | 119 | 40 | 34 | 45 | .735 |
| No new mutation | 114 (96%) | 38 (95%) | 32 (94%) | 44 (98%) | |
| New mutation | 5 (4%) | 2 (5%) | 2 (6%) | 1 (2%) | |
| <1% | 5 | 2 | 2 | 1 | |
| Any new M184 mutation (%) | |||||
| No. | 134 | 43 | 40 | 51 | .344 |
| No new mutation | 127 (95%) | 39 (91%) | 38 (95%) | 50 (98%) | |
| New mutation | 7 (5%) | 4 (9%) | 2 (5%) | 1 (2%) | |
| <1% | 7 | 4 | 2 | 1 |
Abbreviations: 3TC, lamivudine; FTC, emtricitabine; LPV/r, lopinavir/ritonavir; TDF, tenofovir; ZDV, zidovudine.
No significant difference in new NVP resistance mutations was detected among the 3 ARV regimens nor associated with antenatal ZDV use. In contrast to the other arms, no woman who received 21 days of TDF/FTC developed new NVP-resistance mutations by standard population genotyping or ASP.
Safety and Adherence
All regimens were well tolerated, and there was no difference between study regimens and treatment durations with regard to grade 3–4 laboratory abnormalities. When compared among regimens, a significantly higher proportion reported complete adherence at week 1 for TDF/FTC than ZDV/3TC and LPV/r (93% vs 84% vs 85%, P = .038). Women with antenatal ZDV use reported significantly lower adherence than women with no antenatal ZDV use at week 1 visit (82% vs 97%, P < .001).
DISCUSSION
In this study, very few women (1.2%) developed NVP resistance as assessed by standard population genotyping at 2 and 6 weeks following completion of short-term ARV use, begun simultaneously with the administration of sdNVP for the prevention of mother-to-child transmission (pMTCT). The percentage of women developing resistance was low for both the 7- and 21-day arms (1.9% and 0.5%). The more sensitive ASP assay showed that the 21-day regimen was significantly better at preventing the emergence of NVP resistance mutations (5% vs 18%, P = .019). Furthermore, all 4 women with K103N or Y181C mutant frequencies ≥1% of the total population were in the 7-day arms. Emergence of NRTI resistance was rare, as demonstrated by both standard population genotype (1%) and ASP (5%), and was not more common with 21-day regimens (Table 4). Taken together, these findings suggest that a 21-day regimen will optimally suppress the emergence of NVP resistance after sdNVP without increasing the emergence of 3TC/FTC resistance. A low occurrence of NVP resistance was also recently reported with 7 days of triple drug therapy or 30 days of dual drug therapy after sdNVP in 169 Thai women in the IMPAACT P1032 trial [16].
The OCTANE/ACTG A5208 study revealed that the persistence of NVP-resistant mutants at frequencies >1% of the virus population adversely affects response to initial, NVP-based antiretroviral therapy (ART) [15]. OCTANE was designed to compare the efficacy of a protease inhibitor–based regimen to an NVP-based regimen in women previously exposed to sdNVP >6 months earlier [11]. Women were significantly more likely to experience virologic failure or death after starting the NVP-containing ART arm compared to the LPV/r-containing ART. ASP testing revealed that virologic failure or death among women receiving NVP-based ART was significantly associated with K103N or Y181C resistance mutations at virus population frequencies >1% in pre-ART samples. In the current study, only 4 (2.7%) women had >1% NVP-resistant mutants detected after completion of the regimens. It is not known how long such mutants persist at frequencies >1% and whether they would affect response to NNRTI-based ART when it is initiated. Nevertheless, our study was designed to identify well-tolerated, affordable, effective strategies to maintain future ART options for women who receive sdNVP. A greater effect of 21-day regimens was clearly shown in preventing the emergence of low-frequency NVP-resistant mutants, although the long-term clinical significance of this effect is uncertain.
Several additional points should be made regarding this study. It was a complex design successfully conducted in resource-constrained sites, in a variety of care settings with differing levels of antenatal care, and enrolled participants infected with multiple subtypes. The completeness of maternal HIV-1 resistance data for the primary endpoint was excellent (98%). Participants were allowed antenatal ZDV per local standard of care, and were randomized regardless of HIV-1 RNA at entry. All ARV treatment strategies were well-tolerated. Adherence with the 3 ARV strategies during the first week of treatment was significantly better in women randomized to receive once-daily TDF/FTC than twice-daily interventions of ZDV/3TC or LPV/r. NVP resistance was not detected in any women who received TDF/FTC for 21 days by standard population genotyping or ASP.
This study opened in 2006, when World Health Organization (WHO) guidelines recommended sdNVP in the minimum standard-of-care package for HIV-infected women. Owing to the challenges of pMTCT scale-up, there is a continuing need for effective strategies to prevent the emergence of NVP resistance. As of May 2011, women in 29 countries were still receiving sdNVP for pMTCT. Our findings should help to inform approaches to pMTCT for HIV-1–infected women who may not have access to extended postpartum ART (recommended in WHO Option B [11]). Under these circumstances, a 21-day ARV strategy should be strongly considered to preserve future treatment options.
Notes
Acknowledgments. We thank the women who participated in the study and the following members of the A5207 AIDS Clinical Trials Group Study Team: David Haas, Elaine Ferguson, Scharla Estep, William Kabat, Michael Klebert, and Robert T. Schooley. The study was made possible by the diligent efforts of the site investigators and staff at the 8 study sites in Africa, Haiti, and India: Dr Hilda Kizito and Phyllis Rubondo Mwesigwa, Joint Clinical Research Centre, Kampala, Uganda (AI069501); Dr Newton Kumwenda and Stacey Hurst, College of Medicine JHU CRS at Queen Elizabeth Central Hospital, Blantyre, Malawi (AI069518); Dr Poongulali Selvamuthu and Ms Jabin Sharma, YRG CARE Medical Centre, VHS Chennai CRS, Chennai, India (AI069432); Dr Jean William Pape and Dr Patrice Severe, Les Centres GHESKIO CRS, Port-au-Prince, Haiti (AI069421); Durban CRS investigators and staff, Durban Adult HIV CRS, Durban, South Africa (AI069426–01); Dr Francesca Conradie and Pauline Vunandlala, WITS HIV CRS, Johannesburg, South Africa (AI38858, AI69463); Dr Olola Oneko and Julitha Kimbi, Kilimanjaro Christian Medical Centre, Moshi, Tanzania (AI069484); and BJ Medical College CRS investigators and staff, Pune, India (AI069417). Pharmaceutical support was provided by Abbott Laboratories, Boehringer Ingelheim, Gilead Sciences, and GlaxoSmithKline.
Financial support. The study was supported by the AIDS Clinical Trials Group funded by the US National Institute of Allergy and Infectious Diseases, National Institutes of Health (ACTG network leadership grant 1U01AI068636-01; ACTG statistical and data analysis center grant 1U01AI068634-01); the National Institute of Allergy and Infectious Diseases (University of Pittsburgh clinical trials unit grant 1U01 AI069494-01); and the Virology Support Laboratory Subcontract 204VC009 of the AIDS Clinical Trials Group Central Group (grant 1 U01A10 68636-01).
Potential conflicts of interest. J. W. M. is a consultant to Gilead Sciences and RFS Pharmaceuticals and owns share options in RFS Pharmaceuticals. M. D. H. is a paid data monitoring committee member for Boehringer Ingelheim, Medicines Development, Pfizer, and Tibotec. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose NVP compared with zidovudine for prevention of mother-to-infant transmission of HIV-1 in Kampala, Uganda: HIVNET-012 randomised trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 2.Mirochnick M, Fenton T, Gagnier P, et al. Pharmacokinetics of NVP in human immunodeficiency virus type 1-infected pregnant women and their neonates. J Infec Dis. 1998;178:368–74. doi: 10.1086/515641. [DOI] [PubMed] [Google Scholar]
- 3.Eshleman SH, Mracna M, Guay LA, et al. Selection and fading of resistance mutations in women and infants receiving nevirapine to prevent HIV-1 vertical transmission (HIVNET 012) AIDS. 2001;15:1951–7. doi: 10.1097/00002030-200110190-00006. [DOI] [PubMed] [Google Scholar]
- 4.Beckerman KP. Long term findings of HIVNET 012: the next steps. Lancet. 2003;362:842–3. doi: 10.1016/S0140-6736(03)14345-0. [DOI] [PubMed] [Google Scholar]
- 5.Eshleman SH, Guay LA, Mwatha A, et al. Characterization of nevirapine resistance mutations in women with subtype A vs. D HIV-1 6-8 weeks after single-dose nevirapine (HIVNET 012) J Acquir Immune Defic Syndr. 2004;35:126–30. doi: 10.1097/00126334-200402010-00004. [DOI] [PubMed] [Google Scholar]
- 6.Flys TS, Chen S, Jones DC, et al. Quantitative analysis of HIV-1 variants with the K103N resistance mutation after single-dose nevirapine in women with HIV-1 subtypes A, C, and D. J Acquir Immune Defic Syndr. 2006;42:610–3. doi: 10.1097/01.qai.0000221686.67810.20. [DOI] [PubMed] [Google Scholar]
- 7.Kassaye S, Lee E, Kantor R, et al. Drug resistance in plasma and breast milk after single-dose nevirapine in subtype C HIV-1: population and clonal sequence analysis. AIDS Res Hum Retroviruses. 2007;23:1055–61. doi: 10.1089/aid.2007.0045. [DOI] [PubMed] [Google Scholar]
- 8.Loubser S, Balfe P, Sherman G, et al. Decay of K103N mutants in cellular DNA and plasma RNA after single-dose nevirapine to reduce mother-to-child transmission. AIDS. 2006;20:995–1002. doi: 10.1097/01.aids.0000222071.60620.1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson JA, Li JF, Morris L, et al. Emergence of drug-resistant HIV-1 after intrapartum administration of single-dose nevirapine is substantially underestimated. J Infect Dis. 2005;192:16–23. doi: 10.1086/430741. [DOI] [PubMed] [Google Scholar]
- 10.Palmer S, Boltz V, Martinson N, et al. Persistence of nevirapine-resistant HIV-1 in women after single-dose nevirapine therapy for prevention of maternal-to-fetal HIV-1 transmission. Proc Natl Acad Sci U S A. 2006;103:7094–9. doi: 10.1073/pnas.0602033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lockman S, Hughes M, McIntyre J, et al. the OCTANE 5208 Study Team. Antiretroviral therapies in women after single-dose nevirapine exposure. N Engl J Med. 2010;363:1499–509. doi: 10.1056/NEJMoa0906626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: recommendations for a public health approach—2010 version. Geneva, Switzerland: World Health Organization; 2010. [PubMed] [Google Scholar]
- 13.McIntyre JA, Hopley M, Moodley D, et al. Efficacy of short-course AZT plus 3TC to reduce nevirapine resistance in the prevention of mother-to-child HIV transmission: a randomized clinical trial. PLoS Med. 2009;6:e1000172. doi: 10.1371/journal.pmed.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmer S, Boltz V, Chow J, et al. Short-course combivir after single-dose nevirapine reduces but does not eliminate the emergence of nevirapine resistance in women. Antiviral Therapy. 2012;17:327–336. doi: 10.3851/IMP1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boltz VF, Zheng Y, Lockman S, et al. Role of low-frequency HIV-1 variants in failure of nevirapine-containing antiviral therapy in women previously exposed to single-dose nevirapine. Proc Natl Acad Sci U S A. 2011;108:9202–7. doi: 10.1073/pnas.1105688108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Dyke RB, Ngo-Giang-Huong N, Shapiro DE, et al. A comparison of 3 regimens to prevent nevirapine resistance mutations in HIV-infected pregnant women receiving a single intrapartum dose of nevirapine. Clin Infect Dis. 2012;54:285–93. doi: 10.1093/cid/cir798. [DOI] [PMC free article] [PubMed] [Google Scholar]