Abstract
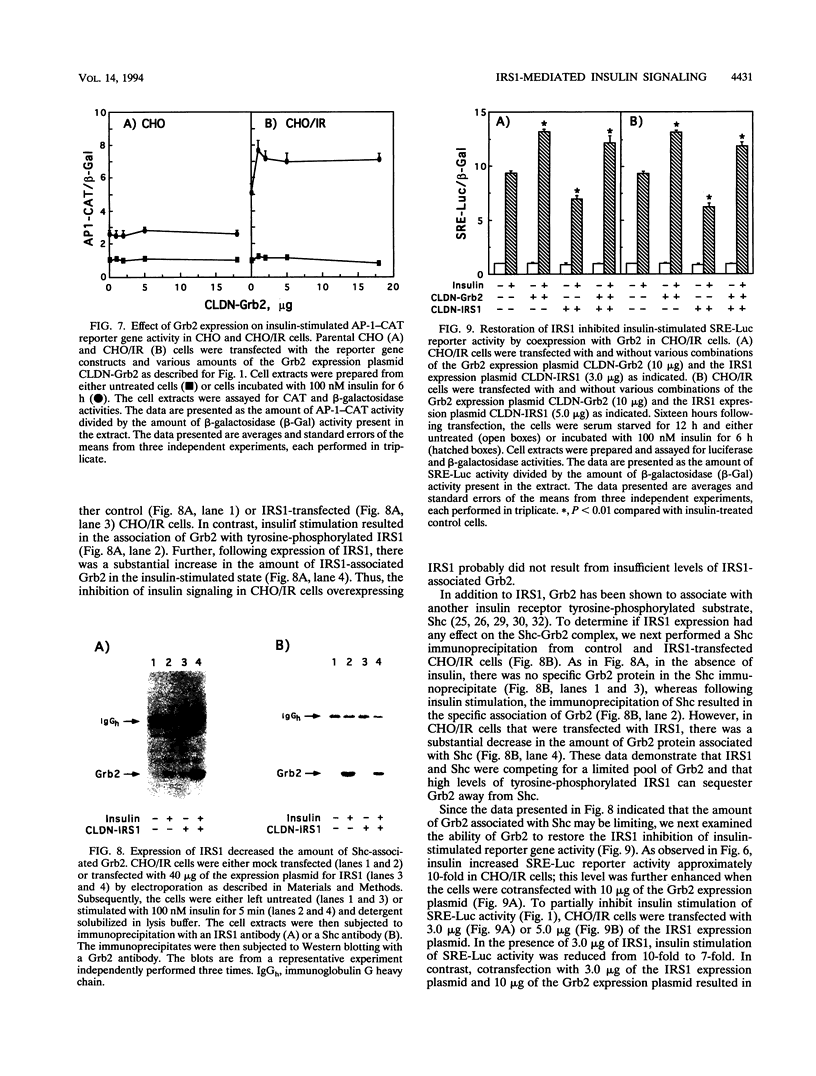
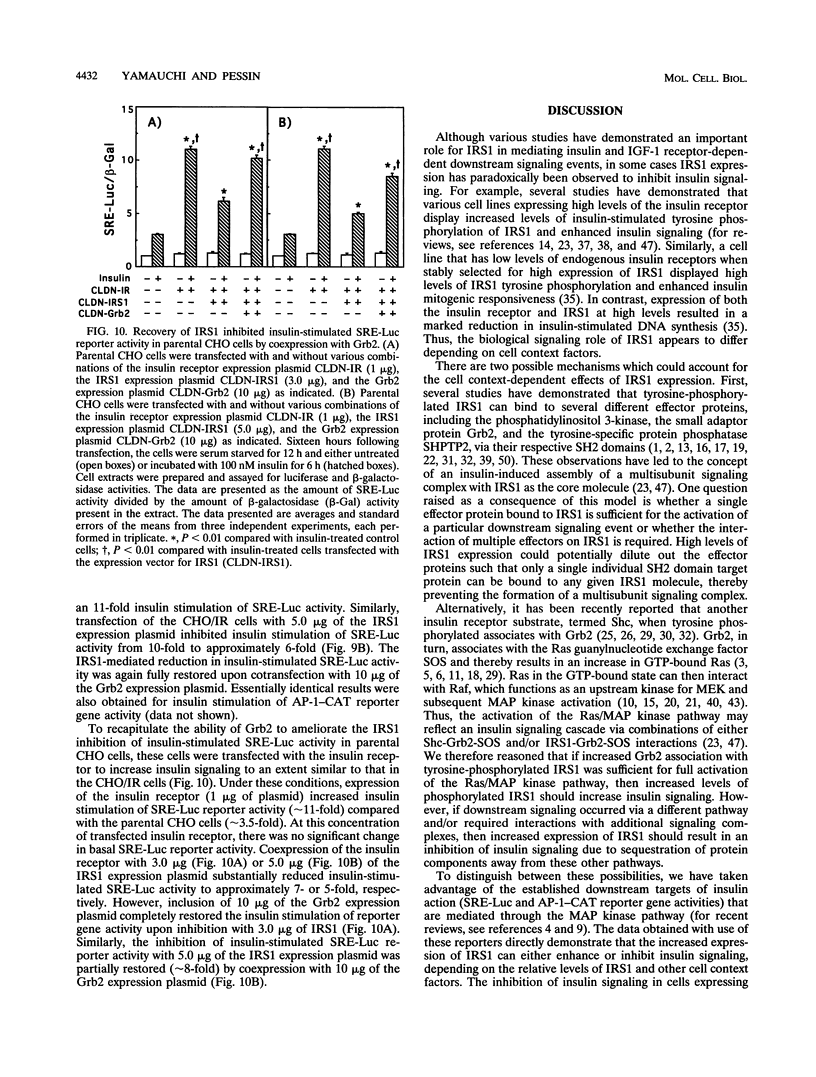
Insulin treatment of Chinese hamster ovary (CHO) cells expressing high levels of the insulin receptor (CHO/IR cells) activates both c-fos serum response element and activator protein 1 (AP-1) reporter genes approximately 10-fold. In contrast, parental CHO cells display only two- to threefold insulin stimulation of reporter gene activity. Transient transfection of parental CHO cells with an insulin receptor substrate 1 (IRS1) expression plasmid enhanced insulin downstream signaling in a biphasic manner, whereas IRS1 transfection of CHO/IR cells inhibited insulin signaling in a dose-dependent fashion. Further, expression of Grb2 in parental CHO cells had no effect on insulin signaling, whereas Grb2 increased insulin activation of reporter gene expression in CHO/IR cells. These data suggest that the expression levels of various effector molecules can either enhance or inhibit insulin downstream signaling events. To assess the relative effects of various insulin receptor, IRS1, and Grb2 levels on insulin signaling, parental CHO cells were transiently transfected with various combinations of expression plasmids encoding these proteins. Although expression of IRS1 resulted in a biphasic increase of insulin signaling in parental CHO cells, coexpression of IRS1 with the insulin receptor resulted in inhibition of signaling. This inhibition of insulin signaling directly correlated with an increased association of Grb2 with IRS1 and a concomitant sequestration of Grb2 away from Shc. Consistent with the Shc-Grb2 pathway as the major route for insulin-stimulated c-Fos and AP-1 transcriptional activation, the IRS1-mediated inhibition was reversed by transfection with an expression plasmid for Grb2. These data demonstrate that the extent of insulin-stimulated downstream signaling was dependent not only on the levels of individual signaling molecules but also on the formation of multiprotein complexes with specific stoichiometries.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backer J. M., Myers M. G., Jr, Shoelson S. E., Chin D. J., Sun X. J., Miralpeix M., Hu P., Margolis B., Skolnik E. Y., Schlessinger J. Phosphatidylinositol 3'-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 1992 Sep;11(9):3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer J. M., Myers M. G., Jr, Sun X. J., Chin D. J., Shoelson S. E., Miralpeix M., White M. F. Association of IRS-1 with the insulin receptor and the phosphatidylinositol 3'-kinase. Formation of binary and ternary signaling complexes in intact cells. J Biol Chem. 1993 Apr 15;268(11):8204–8212. [PubMed] [Google Scholar]
- Baltensperger K., Kozma L. M., Cherniack A. D., Klarlund J. K., Chawla A., Banerjee U., Czech M. P. Binding of the Ras activator son of sevenless to insulin receptor substrate-1 signaling complexes. Science. 1993 Jun 25;260(5116):1950–1952. doi: 10.1126/science.8391166. [DOI] [PubMed] [Google Scholar]
- Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buday L., Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 1993 May 7;73(3):611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- Chardin P., Camonis J. H., Gale N. W., van Aelst L., Schlessinger J., Wigler M. H., Bar-Sagi D. Human Sos1: a guanine nucleotide exchange factor for Ras that binds to GRB2. Science. 1993 May 28;260(5112):1338–1343. doi: 10.1126/science.8493579. [DOI] [PubMed] [Google Scholar]
- Chuang L. M., Myers M. G., Jr, Backer J. M., Shoelson S. E., White M. F., Birnbaum M. J., Kahn C. R. Insulin-stimulated oocyte maturation requires insulin receptor substrate 1 and interaction with the SH2 domains of phosphatidylinositol 3-kinase. Mol Cell Biol. 1993 Nov;13(11):6653–6660. doi: 10.1128/mcb.13.11.6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang L. M., Myers M. G., Jr, Seidner G. A., Birnbaum M. J., White M. F., Kahn C. R. Insulin receptor substrate 1 mediates insulin and insulin-like growth factor I-stimulated maturation of Xenopus oocytes. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5172–5175. doi: 10.1073/pnas.90.11.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. J. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993 Jul 15;268(20):14553–14556. [PubMed] [Google Scholar]
- Egan S. E., Giddings B. W., Brooks M. W., Buday L., Sizeland A. M., Weinberg R. A. Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature. 1993 May 6;363(6424):45–51. doi: 10.1038/363045a0. [DOI] [PubMed] [Google Scholar]
- Frattali A. L., Treadway J. L., Pessin J. E. Evidence supporting a passive role for the insulin receptor transmembrane domain in insulin-dependent signal transduction. J Biol Chem. 1991 May 25;266(15):9829–9834. [PubMed] [Google Scholar]
- Giorgetti S., Ballotti R., Kowalski-Chauvel A., Tartare S., Van Obberghen E. The insulin and insulin-like growth factor-I receptor substrate IRS-1 associates with and activates phosphatidylinositol 3-kinase in vitro. J Biol Chem. 1993 Apr 5;268(10):7358–7364. [PubMed] [Google Scholar]
- Goldfine I. D. The insulin receptor: molecular biology and transmembrane signaling. Endocr Rev. 1987 Aug;8(3):235–255. doi: 10.1210/edrv-8-3-235. [DOI] [PubMed] [Google Scholar]
- Koide H., Satoh T., Nakafuku M., Kaziro Y. GTP-dependent association of Raf-1 with Ha-Ras: identification of Raf as a target downstream of Ras in mammalian cells. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8683–8686. doi: 10.1073/pnas.90.18.8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhné M. R., Pawson T., Lienhard G. E., Feng G. S. The insulin receptor substrate 1 associates with the SH2-containing phosphotyrosine phosphatase Syp. J Biol Chem. 1993 Jun 5;268(16):11479–11481. [PubMed] [Google Scholar]
- Lavan B. E., Kuhné M. R., Garner C. W., Anderson D., Reedijk M., Pawson T., Lienhard G. E. The association of insulin-elicited phosphotyrosine proteins with src homology 2 domains. J Biol Chem. 1992 Jun 5;267(16):11631–11636. [PubMed] [Google Scholar]
- Li N., Batzer A., Daly R., Yajnik V., Skolnik E., Chardin P., Bar-Sagi D., Margolis B., Schlessinger J. Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signalling. Nature. 1993 May 6;363(6424):85–88. doi: 10.1038/363085a0. [DOI] [PubMed] [Google Scholar]
- Lowenstein E. J., Daly R. J., Batzer A. G., Li W., Margolis B., Lammers R., Ullrich A., Skolnik E. Y., Bar-Sagi D., Schlessinger J. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 1992 Aug 7;70(3):431–442. doi: 10.1016/0092-8674(92)90167-b. [DOI] [PubMed] [Google Scholar]
- Macdonald S. G., Crews C. M., Wu L., Driller J., Clark R., Erikson R. L., McCormick F. Reconstitution of the Raf-1-MEK-ERK signal transduction pathway in vitro. Mol Cell Biol. 1993 Nov;13(11):6615–6620. doi: 10.1128/mcb.13.11.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodie S. A., Willumsen B. M., Weber M. J., Wolfman A. Complexes of Ras.GTP with Raf-1 and mitogen-activated protein kinase kinase. Science. 1993 Jun 11;260(5114):1658–1661. doi: 10.1126/science.8503013. [DOI] [PubMed] [Google Scholar]
- Myers M. G., Jr, Backer J. M., Sun X. J., Shoelson S., Hu P., Schlessinger J., Yoakim M., Schaffhausen B., White M. F. IRS-1 activates phosphatidylinositol 3'-kinase by associating with src homology 2 domains of p85. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10350–10354. doi: 10.1073/pnas.89.21.10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M. G., Jr, White M. F. The new elements of insulin signaling. Insulin receptor substrate-1 and proteins with SH2 domains. Diabetes. 1993 May;42(5):643–650. doi: 10.2337/diab.42.5.643. [DOI] [PubMed] [Google Scholar]
- Ohmichi M., Matuoka K., Takenawa T., Saltiel A. R. Growth factors differentially stimulate the phosphorylation of Shc proteins and their association with Grb2 in PC-12 pheochromocytoma cells. J Biol Chem. 1994 Jan 14;269(2):1143–1148. [PubMed] [Google Scholar]
- Pelicci G., Lanfrancone L., Grignani F., McGlade J., Cavallo F., Forni G., Nicoletti I., Grignani F., Pawson T., Pelicci P. G. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell. 1992 Jul 10;70(1):93–104. doi: 10.1016/0092-8674(92)90536-l. [DOI] [PubMed] [Google Scholar]
- Pronk G. J., McGlade J., Pelicci G., Pawson T., Bos J. L. Insulin-induced phosphorylation of the 46- and 52-kDa Shc proteins. J Biol Chem. 1993 Mar 15;268(8):5748–5753. [PubMed] [Google Scholar]
- Rice K. M., Lienhard G. E., Garner C. W. Regulation of the expression of pp160, a putative insulin receptor signal protein, by insulin, dexamethasone, and 1-methyl-3-isobutylxanthine in 3T3-L1 adipocytes. J Biol Chem. 1992 May 15;267(14):10163–10167. [PubMed] [Google Scholar]
- Rose D. W., Saltiel A. R., Majumdar M., Decker S. J., Olefsky J. M. Insulin receptor substrate 1 is required for insulin-mediated mitogenic signal transduction. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):797–801. doi: 10.1073/pnas.91.2.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozakis-Adcock M., Fernley R., Wade J., Pawson T., Bowtell D. The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator mSos1. Nature. 1993 May 6;363(6424):83–85. doi: 10.1038/363083a0. [DOI] [PubMed] [Google Scholar]
- Ruff-Jamison S., McGlade J., Pawson T., Chen K., Cohen S. Epidermal growth factor stimulates the tyrosine phosphorylation of SHC in the mouse. J Biol Chem. 1993 Apr 15;268(11):7610–7612. [PubMed] [Google Scholar]
- Skolnik E. Y., Batzer A., Li N., Lee C. H., Lowenstein E., Mohammadi M., Margolis B., Schlessinger J. The function of GRB2 in linking the insulin receptor to Ras signaling pathways. Science. 1993 Jun 25;260(5116):1953–1955. doi: 10.1126/science.8316835. [DOI] [PubMed] [Google Scholar]
- Skolnik E. Y., Lee C. H., Batzer A., Vicentini L. M., Zhou M., Daly R., Myers M. J., Jr, Backer J. M., Ullrich A., White M. F. The SH2/SH3 domain-containing protein GRB2 interacts with tyrosine-phosphorylated IRS1 and Shc: implications for insulin control of ras signalling. EMBO J. 1993 May;12(5):1929–1936. doi: 10.1002/j.1460-2075.1993.tb05842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpo D. J., Stewart T. N., Gilman M. Z., Blackshear P. J. Identification of c-fos sequences involved in induction by insulin and phorbol esters. J Biol Chem. 1988 Feb 5;263(4):1611–1614. [PubMed] [Google Scholar]
- Sun X. J., Crimmins D. L., Myers M. G., Jr, Miralpeix M., White M. F. Pleiotropic insulin signals are engaged by multisite phosphorylation of IRS-1. Mol Cell Biol. 1993 Dec;13(12):7418–7428. doi: 10.1128/mcb.13.12.7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X. J., Miralpeix M., Myers M. G., Jr, Glasheen E. M., Backer J. M., Kahn C. R., White M. F. Expression and function of IRS-1 in insulin signal transmission. J Biol Chem. 1992 Nov 5;267(31):22662–22672. [PubMed] [Google Scholar]
- Sun X. J., Rothenberg P., Kahn C. R., Backer J. M., Araki E., Wilden P. A., Cahill D. A., Goldstein B. J., White M. F. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991 Jul 4;352(6330):73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- Taylor R. Insulin action 1991. Clin Endocrinol (Oxf) 1991 Feb;34(2):159–171. doi: 10.1111/j.1365-2265.1991.tb00287.x. [DOI] [PubMed] [Google Scholar]
- Taylor S. I., Cama A., Accili D., Barbetti F., Quon M. J., de la Luz Sierra M., Suzuki Y., Koller E., Levy-Toledano R., Wertheimer E. Mutations in the insulin receptor gene. Endocr Rev. 1992 Aug;13(3):566–595. doi: 10.1210/edrv-13-3-566. [DOI] [PubMed] [Google Scholar]
- Tobe K., Matuoka K., Tamemoto H., Ueki K., Kaburagi Y., Asai S., Noguchi T., Matsuda M., Tanaka S., Hattori S. Insulin stimulates association of insulin receptor substrate-1 with the protein abundant Src homology/growth factor receptor-bound protein 2. J Biol Chem. 1993 May 25;268(15):11167–11171. [PubMed] [Google Scholar]
- Van Aelst L., Barr M., Marcus S., Polverino A., Wigler M. Complex formation between RAS and RAF and other protein kinases. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6213–6217. doi: 10.1073/pnas.90.13.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtek A. B., Hollenberg S. M., Cooper J. A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993 Jul 16;74(1):205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- Wang L. M., Keegan A. D., Li W., Lienhard G. E., Pacini S., Gutkind J. S., Myers M. G., Jr, Sun X. J., White M. F., Aaronson S. A. Common elements in interleukin 4 and insulin signaling pathways in factor-dependent hematopoietic cells. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4032–4036. doi: 10.1073/pnas.90.9.4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. M., Myers M. G., Jr, Sun X. J., Aaronson S. A., White M., Pierce J. H. IRS-1: essential for insulin- and IL-4-stimulated mitogenesis in hematopoietic cells. Science. 1993 Sep 17;261(5128):1591–1594. doi: 10.1126/science.8372354. [DOI] [PubMed] [Google Scholar]
- Waters S. B., Yamauchi K., Pessin J. E. Functional expression of insulin receptor substrate-1 is required for insulin-stimulated mitogenic signaling. J Biol Chem. 1993 Oct 25;268(30):22231–22234. [PubMed] [Google Scholar]
- White M. F., Kahn C. R. The insulin signaling system. J Biol Chem. 1994 Jan 7;269(1):1–4. [PubMed] [Google Scholar]
- White M. F., Maron R., Kahn C. R. Insulin rapidly stimulates tyrosine phosphorylation of a Mr-185,000 protein in intact cells. Nature. 1985 Nov 14;318(6042):183–186. doi: 10.1038/318183a0. [DOI] [PubMed] [Google Scholar]
- Yamauchi K., Holt K., Pessin J. E. Phosphatidylinositol 3-kinase functions upstream of Ras and Raf in mediating insulin stimulation of c-fos transcription. J Biol Chem. 1993 Jul 15;268(20):14597–14600. [PubMed] [Google Scholar]
- Yonezawa K., Ueda H., Hara K., Nishida K., Ando A., Chavanieu A., Matsuba H., Shii K., Yokono K., Fukui Y. Insulin-dependent formation of a complex containing an 85-kDa subunit of phosphatidylinositol 3-kinase and tyrosine-phosphorylated insulin receptor substrate 1. J Biol Chem. 1992 Dec 25;267(36):25958–25965. [PubMed] [Google Scholar]
- Zhang X. F., Settleman J., Kyriakis J. M., Takeuchi-Suzuki E., Elledge S. J., Marshall M. S., Bruder J. T., Rapp U. R., Avruch J. Normal and oncogenic p21ras proteins bind to the amino-terminal regulatory domain of c-Raf-1. Nature. 1993 Jul 22;364(6435):308–313. doi: 10.1038/364308a0. [DOI] [PubMed] [Google Scholar]
- de Vries-Smits A. M., Burgering B. M., Leevers S. J., Marshall C. J., Bos J. L. Involvement of p21ras in activation of extracellular signal-regulated kinase 2. Nature. 1992 Jun 18;357(6379):602–604. doi: 10.1038/357602a0. [DOI] [PubMed] [Google Scholar]
- van der Burg B., de Groot R. P., Isbrücker L., Kruijer W., de Laat S. W. Oestrogen directly stimulates growth factor signal transduction pathways in human breast cancer cells. J Steroid Biochem Mol Biol. 1991;40(1-3):215–221. doi: 10.1016/0960-0760(91)90185-8. [DOI] [PubMed] [Google Scholar]
- van der Burg B., de Groot R. P., Isbrücker L., Kruijer W., de Laat S. W. Stimulation of TPA-responsive element activity by a cooperative action of insulin and estrogen in human breast cancer cells. Mol Endocrinol. 1990 Nov;4(11):1720–1726. doi: 10.1210/mend-4-11-1720. [DOI] [PubMed] [Google Scholar]