Abstract
Background
Sleep disturbance may affect smoking cessation efforts. We describe sleep changes across three months among women in a smoking cessation program and tested whether sleep disturbances at baseline and 1 month post-quit attempt predicted smoking status at three months.
Methods
Participants (N = 322) were women in a randomized, clinical trial for smoking cessation. Sleep disturbances, as well as, insomnia, drowsiness, and sleep quality were evaluated prior to and during three months of cessation treatment. Repeated measures mixed models evaluated change in sleep over time by smoking outcome status. Logistic regression analyses determined whether sleep disturbances at baseline and 1 month post-quit were associated with smoking status at 3 months.
Results
Sleep disturbances were reported by more than 25% of women. Drowsiness, insomnia, and sleep quality changed over time. However, contrary to our hypotheses, none of the sleep variables at either baseline or 1 month post-quit attempt was associated with relapse (p′s > .05).
Conclusions
Although mild to severe drowsiness was reported by more women who relapsed than those who remained abstinent, none of the sleep disturbance symptoms predicted smoking relapse. Given high rates of sleep disturbances among women smokers, better prospective evaluations of the relationship of sleep disturbances to smoking cessation treatment outcome are needed.
Keywords: sleep quality, insomnia, smoking, drowsiness, women, cessation, withdrawal
1. Introduction
Cigarette smoking is the greatest cause of preventable morbidity and mortality in developed countries. Sleep disturbances are a primary symptom of nicotine withdrawal (Wetter et al., 1999a). However, unlike other withdrawal symptoms, sleep disturbances often do not subside within the first 21 days after cessation and thus may contribute to relapse (Jorenby et al., 1996). Specifically, poor sleep quality (Colrain, Trinder, & Swan, 2004) and daytime drowsiness (Hamidovic & de Wit H., 2009) are thought to interfere with the ability to quit smoking or maintain longer-term abstinence (Gourlay, Forbes, Marriner, & McNeil, 1999; Hamidovic and de Wit H., 2009). Nocturnal ‘sleep-disturbing nicotine cravings’ have also been reported to significantly impair success during a smoking cessation program (Riemerth, Kunze, & Groman, 2009). Understanding the relationship between sleep disturbances and smoking relapse may inform efforts to improve cessation interventions.
Accordingly, we were interested in subjective sleep disturbances among women smokers enrolled in a treatment program (Levine et al., 2010). In the present study, we sought to describe the sleep of women in a smoking cessation program across a three month period and test whether sleep disturbances at baseline or at 1 month after a quit attempt predict smoking status at three months post-quit.
1.1 Materials and Methods
1.1.1 Participants
The current study represents a secondary analysis. The original study protocol and results are described elsewhere (Levine et al. 2010). Briefly, participants were women smokers between the ages of 18 and 65 who smoked at least 10 cigarettes per day. Women were excluded if they had current major depressive disorder, suicidality, drug or alcohol dependence within the past year, uncontrolled hypertension, used bupropion for more than one week, were currently pregnant, or were using other smoking or weight loss treatments. A total of 322 women with sleep and outcome data were included in the current analyses. The study was approved by the University of Pittsburgh Institutional Review Board.
1.1.2. Measurements
Demographic information, smoking history and nicotine dependence were collected prior to randomization. Assessments of smoking, symptoms of nicotine withdrawal and depression were collected at baseline and weekly prior to each of the 12 group counseling sessions provided over three months. Questions used to determine sleep quality were administered at baseline (prior to a targeted quit date (TQD)), and at 1- and at 3 months post-TQD.
Sleep Measures
We used a single item from the Beck Depression Inventory (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) as an assessment of disturbed sleep. Choices include: 0 = I can sleep as well as usual, 1 = I don't sleep as well as I used to, 2 = I wake up 1-2 hours earlier than usual and find it hard to go back to sleep, 3 = I wake up several hours earlier than I used to and cannot get back to sleep. We dichotomized this variable into “0” = undisturbed/no complaints with sleep and “1” = disturbed/complaints with sleep (which encompassed options 1-3). We used two items (“drowsiness” and “insomnia”) from a 12-item visual analogue scale (VAS) that assessed withdrawal symptoms in the previous 24 hours. Scores ranged from 0 (“not at all”) to 100 (“severe”). The median was ∼30, therefore a value of 30 or greater was considered as positive endorsement of the symptom. The final sleep variable was derived from five items from the Wisconsin Smoking Withdrawal Scale (WWS), a 28-item scale measuring the common withdrawal symptoms of smoking cessation (Welsch et al., 1999), as a measure of sleep quality. The five items were: 1. “I am getting restful sleep”; 2. I awaken from sleep frequently during the night?”; 3. I am satisfied with my sleep”; 4. I feel that I am getting enough sleep; 5. “My sleep has been troubled”. Each item was scored on a Likert scale 0 = strongly disagree, 1 = Disagree, 2 = Feel neutral, 3= Agree, 4 = Strongly Agree. The second and fifth items were reversed scored. Thus, the possible range of scores on this measure of sleep quality was 0-20, with higher numbers reflecting better sleep quality.
1.1.3. Abstinence
As described in detail elsewhere (Levine et al., 2010), women were interviewed about smoking, expired-air carbon monoxide (CO) was collected using a Vitalograph® BreathCO carbon monoxide monitor, and salivary samples were collected to assay cotinine levels. Women who dropped out of treatment were considered to have relapsed. Three month abstinence is defined by the self-report of no smoking during the seven days prior to the assessment and an expired-air carbon monoxide (CO) reading of 8 or less at 1 and 3-month after the target quit date.
1.1.4 Statistical Analyses
Descriptive statistics were conducted to describe the demographic and clinical characteristics of the sample. Square root transformations were made on the insomnia and drowsiness variables prior to statistical comparison. Repeated measures mixed effect models were conducted to evaluate change in sleep symptom measures (logistic for BDI item and linear for drowsiness and insomnia and sleep quality (WWS score) across the 3 months of treatment. The models included an interaction of smoking status by time and covaried for drug status because drug alone was significantly associated with abstinence at 3 months after TQD. We also tested whether baseline sleep (prior to randomization), as well as sleep complaints at month 1 after TQD, covarying for baseline sleep, were associated with relapse at 3 months of treatment. Finally, time to relapse was tested in a Cox proportional hazards model with sleep measures as a time dependent covariates in separate models. Analyses were conducted using SAS 9.2.
2. Results
Table 1 shows the demographic and clinical characteristics as well as the descriptives by smoking status at 3 months. Prior to treatment, the BDI sleep question was positively endorsed by 136 (42%) of the women, indicating complaints of poor sleep quality or difficulty maintaining sleep before quitting. Participants also reported slight drowsiness (mean on VAS = 23.1) and insomnia (mean on VAS = 13.8). Finally, the composite sleep quality score (mean = 11.9) indicated that participants were moderately satisfied with their sleep. When considering women by smoking status at 3 months, there were no differences between women who were and were not smoking on any of the four sleep items evaluated at baseline (p′s > .30).
Table 1. Demographic and clinical measures for entire cohort as well as by outcome status at 3 months.
| Whole group =322 |
Relapse N=228 |
Nonrelapsers N=94 |
t or χ2 |
df | p | |
|---|---|---|---|---|---|---|
|
| ||||||
| Age (years) | 41.9 (10.2) | 42.0 (10.2) | 41.5 (10.2) | 0.40 | 320 | .69 |
|
| ||||||
| College educated | 34% (109) | 32% (73) | 39% (37) | 1.80 | 1 | .17 |
|
| ||||||
| Treatment group: | 13.59 | 3 | .004 | |||
| Drug+CBT | 29% (94) | 25% | 39% | |||
| Drug+STD | 26% (85) | 24% | 32% | |||
| Placebo+CBT | 26% (84) | 30% | 17% | |||
| Placebo+STD | 18% (59) | 21% | 12% | |||
|
| ||||||
| BDI score minus the sleep item | 6.9 (6.4) | 7.3 (6.8) | 6.2 (5.3) | 1.32 | 314 | .19 |
|
| ||||||
| % positive endorsement of BDI sleep item only | 42% (136) | 43% (98) | 39% (37) | 0.45 | 1 | .50 |
|
| ||||||
| WWS total: 5-items | 11.9 (4.7) | 11.8 (4.6) | 12.4 (4.9) | 1.05 | 314 | .30 |
|
| ||||||
| Insomnia | 13.8 (23.3) | 14.5 (24.1) | 12.2 (21.2) | 0.78 | 319 | .44 |
|
| ||||||
| Drowsiness | 23.1 (25.5) | 22.3 (25.0) | 24.9 (26.6) | 0.83 | 319 | .41 |
Values are presented at mean (SD) or percent (N). t-tests or chi-square analyses were used as appropriate.
Drug = bupropion or placebo
CBT = cognitive behavioral therapy
STD = standard treatment of care
WWS = Wisconsin Withdrawal Scale. Range 0=20 with higher scores representing better sleep quality
Insomnia and Drowsiness measure = Range 0-100. Scores > 20 indicate at least slight endorsement
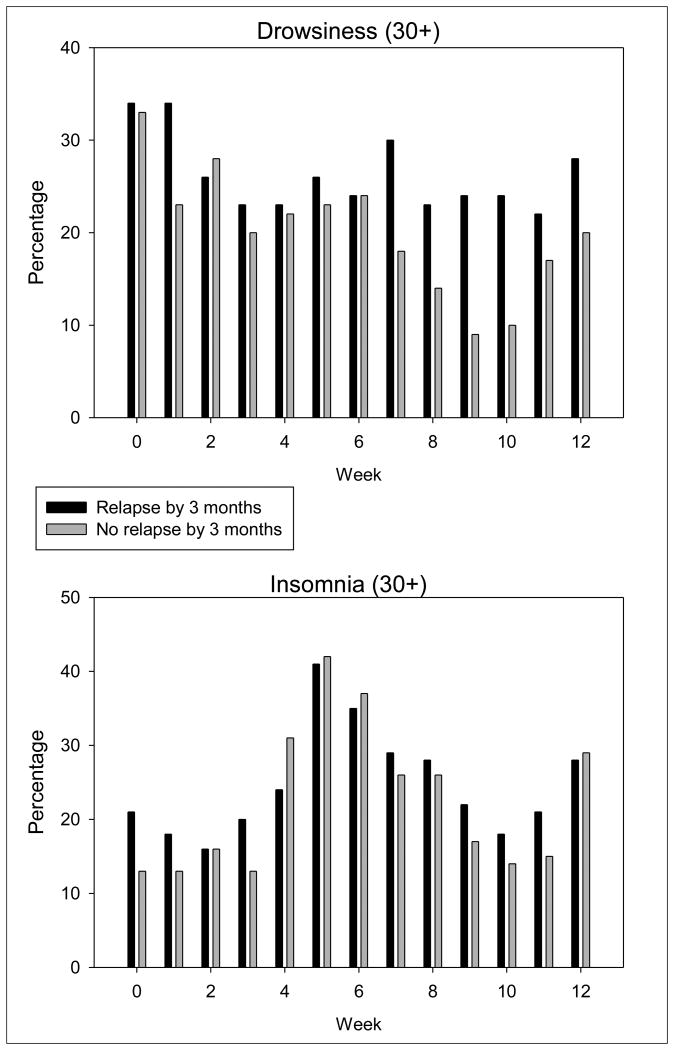
We examined the proportion of women who endorsed sleep complaints across the three months by relapse status to test the hypothesis that sleep disturbances over the first three months of cessation treatment would differ between women who did and did not maintain smoking abstinence by 3 months TQD, while controlling for medication group. The BDI sleep item did not significantly change over time (p=.50) and there was no smoking status by time interaction (p=.45). The degree of drowsiness (p<.0001) and insomnia (p<.0004) changed significantly over time (See Figures 1a and 1b), however, only drowsiness showed a significant smoking status by time interaction (p <.02). Women who had relapsed by 3 months reported more drowsiness than did those who maintained abstinence particularly during weeks 7-12 after the TQD. To further evaluate if change in drowsiness over time was related to relapse, we ran a Cox proportional hazards model on time to relapse with drowsiness as a time dependent covariate. Drowsiness was not a significant time dependent covariate (χ2=0.01, p=0.99). Lastly, for sleep quality, we observed a nonsignificant decrease in all participants 1 month post-TQD with some improvement by three months (F=2.84, p=.06). There was no group by time interaction for sleep quality (p = . 57), although women who remained abstinent by 3 months (N = 94) reported (albeit nonsignficant) better sleep quality at 3 months post-quit than women who relapsed.
Figure 1.
Figure 1a. Percentage of women who reported mild to severe drowsiness by relapse group. We used a cut-off of 30+ to indicate mild to severe drowsiness. A greater percentage of women who relapsed by 3 months reported at least mild drowsiness beginning 7 weeks post-quit.
Figure 1b. Percentage of women who reported mild to severe insomnia by relapse group. We used a cut-off of 30+ to indicate mild to severe insomnia. The number of women reporting at least mild insomnia increased 4-6 weeks post quit then subsequently decreased at 3 months.
We next tested the hypothesis that sleep complaints prior to treatment would be associated with smoking cessation outcome by 3 months while controlling for treatment group and depressive symptoms. Sleep disturbance (the single BDI question) (p = .54), symptoms of insomnia (p = .52), sleep quality composite score (p =.42) and drowsiness (p = .14) were not related to cessation outcome.
Finally, we tested the hypothesis that sleep complaints among women who remained abstinent at 1 month post quit would be associated with smoking cessation outcomes at 3 months while controlling for baseline sleep complaints, treatment group and baseline depressive symptoms. At 1 month, 44.1 % (142/322) of women, were abstinent. Among this subsample who were abstinent, we did not find an association between the sleep disturbance measured by the single BDI question (p = .45), symptoms of insomnia (p = .51) or drowsiness (p = .88) or sleep quality (p = .15) and later abstinence at 3 months after TQD.
3. Discussion
We found that among a cohort of adult women undergoing a smoking cessation treatment self-reported sleep varies across time. Symptoms of drowsiness, insomnia, and sleep quality change in a non-linear manner across the first 3 months after the quit date. However, there were few differences in sleep between women who did and did not successfully quit. Among those who relapsed, only drowsiness differed by group. A greater percentage of women who relapsed by 3 months reported mild to severe levels of drowsiness than women who remained abstinent from week 7 through 12 post-quit. However, sleep disturbance, quality or symptoms of insomnia did not differ over three months differed between women who remained abstinent and those who did not. In addition, we expected that sleep complaints at baseline or 1 month after a TQD would be associated with outcome at 3 months TQD. Contrary to our hypotheses, however, we found no association between sleep complaints at baseline or 1-month TQD and outcome at 3 months TQD.
Our findings, provide modest support for other reports that drowsiness may not only be a symptom of nicotine withdrawal, but also a factor in smoking relapse (Hamidovic and de Wit H., 2009). Women who show improvements in daytime sleepiness may have more success at remaining abstinent, as proposed by Hamidovic and colleagues who found that among 14 healthy adults daytime sleepiness increased the risk of smoking relapse following a sleep deprivation protocol (Hamidovic and de Wit H., 2009). We observed that a greater percentage of women who relapsed reported mild to severe drowsiness from week 7 -12 TQD than women who remained abstinent. More information is required to support or refute this hypothesis.
Nonetheless, our findings may be clinically relevant to the design of future smoking cessation programs in that they corroborate that sleep problems persist for many weeks following quitting (Jorenby et al. 1996), or may reappear with onset of relapse. Smoking reduces the cravings, subsequently improving sleep (Riemerth et al. 2009). Our data suggest that even among women who maintained abstinence through 3 months treatment sleep disturbance persisted. Thus, persistent sleep disturbance could impede success for those entering a smoking cessation program. Programs that emphasize healthy and satisfying sleep patterns could augment abstinence rates, particularly among women.
There are several limitations to these data. First, the study was not designed to answer the questions about sleep problems as predictors of relapse. Second, the questions used to determine sleep disturbance and sleep quality are not commonly used, and it is unclear if similar results would be found with other, more standard sleep measures. Lastly, high drop-out rates, resulting in our classifying those subjects as relapsed, is a common feature of smoking cessation programs and may have contributed to a significant loss of power and our inability to detect associations since we did not have longitudinal sleep data of those women.
In summary, our study documents that sleep disturbances persist over time in a percentage of women undergoing smoking cessation. Specifically, among women who relapsed by three months, drowsiness was reported more often than among those who remained abstinent. Furthermore, more women reported drowsiness as the treatment progressed. However, it is not clear whether the sleep disturbances examined are a preceding sign of relapse, a consequence of nicotine withdrawal or a result of some other unmeasured factor. Further evidence is needed to elucidate this relationship between sleep disturbance and smoking cessation outcome.
Acknowledgments
The authors would like to thank all the women that participated in this research. The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. This research was supported by grant R01 DA004174 (PI: Marcus). Dr. Okun's time is supported by R00 NR008013.
Abbreviations
- TQD
Targeted Quit Date
Footnotes
The authors have no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michele L. Okun, Email: okunml@upmc.edu, University of Pittsburgh School of Medicine, Department of Psychiatry, Western Psychiatric Institute and Clinic, 3811 O'Hara Street, E1120, Pittsburgh, PA 15213, T: 412 586-9434, F: 412 246-5300
Michele D. Levine, University of Pittsburgh School of Medicine, Western Psychiatric Institute and Clinic, 3811 O'Hara Street, Pittsburgh, PA 15213
Houck Patricia, University of Pittsburgh School of Medicine, Western Psychiatric Institute and Clinic, 3811 O'Hara Street, Pittsburgh, PA 15213
Kenneth A. Perkins, University of Pittsburgh School of Medicine, Western Psychiatric Institute and Clinic, 3811 O'Hara Street, Room 459, Pittsburgh, PA 15213
Marsha D. Marcus, University of Pittsburgh School of Medicine, Western Psychiatric Institute and Clinic, 3811 O'Hara Street, Pittsburgh, PA 15213
Reference List
- Aubin HJ. Tolerability and safety of sustained-release bupropion in the management of smoking cessation. Drugs. 2002;62(2):45–52. doi: 10.2165/00003495-200262002-00005. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bohadana A, Nilsson F, Rasmussen T, Martinet Y. Gender differences in quit rates following smoking cessation with combination nicotine therapy: influence of baseline smoking behavior. Nicotine and Tobacco Research. 2003;5:111–116. doi: 10.1080/1462220021000060482. [DOI] [PubMed] [Google Scholar]
- Boutou AK, Tsiata EA, Pataka A, Kontou PK, Pitsiou GG, Argyropoulou P. Smoking cessation in clinical practice: predictors of six-month continuous abstinence in a sample of Greek smokers. The Primary Care Respiratory Journal. 2008;17:32–38. doi: 10.3132/pcrj.2008.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM. The impact of tobacco dependence on sleep disorders. Insomnia. 2005:4–11. [Google Scholar]
- Colrain IM, Trinder J, Swan GE. The impact of smoking cessation on objective and subjective markers of sleep: review, synthesis, and recommendations. Nicotine and Tobacco Research. 2004;6:913–925. doi: 10.1080/14622200412331324938. [DOI] [PubMed] [Google Scholar]
- Driver HS. Sleep in women. Journal of Psychosomatic Research. 1996;40:227–230. doi: 10.1016/0022-3999(96)00030-x. [DOI] [PubMed] [Google Scholar]
- Ebbert JO, Croghan IT, Sood A, Schroeder DR, Hays JT, Hurt RD. Varenicline and bupropion sustained-release combination therapy for smoking cessation. Nicotine and Tobacco Research. 2009;11:234–239. doi: 10.1093/ntr/ntn031. [DOI] [PubMed] [Google Scholar]
- Gourlay SG, Forbes A, Marriner T, McNeil JJ. Predictors and timing of adverse experiences during trandsdermal nicotine therapy. Drug Safety. 1999;20:545–555. doi: 10.2165/00002018-199920060-00007. [DOI] [PubMed] [Google Scholar]
- Groeger JA, Zijlstra FR, Dijk DJ. Sleep quantity, sleep difficulties and their perceived consequences in a representative sample of some 2000 British adults. Journal of Sleep Research. 2004;13:359–371. doi: 10.1111/j.1365-2869.2004.00418.x. [DOI] [PubMed] [Google Scholar]
- Hamidovic A, de Wit H. Sleep deprivation increases cigarette smoking. Pharmacology Biochemistry & Behavior. 2009;93:263–269. doi: 10.1016/j.pbb.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorenby DE, Hatsukami DK, Smith SS, Fiore MC, Allen S, Jensen J, Baker TB. Characterization of tobacco withdrawal symptoms: transdermal nicotine reduces hunger and weight gain. Psychopharmacology (Berlin) 1996;128:130–138. doi: 10.1007/s002130050118. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, Smith SS, Muramoto ML, Daughton DM, Doan K, Fiore MC, Baker TB. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. New England Journal of Medicine. 1999;340:685–691. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- Levine MD, Perkins KA, Kalarchian MA, Cheng Y, Houck PR, Slane JD, Marcus MD. Bupropion and cognitive behavioral therapy for weight-concerned women smokers. Archives of Internal Medicine. 2010;170:543–550. doi: 10.1001/archinternmed.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Reviews. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine and Tobacco Research. 2008;10:1245–1250. doi: 10.1080/14622200802097506. [DOI] [PubMed] [Google Scholar]
- Rapp K, Buechele G, Weiland SK. Sleep duration and smoking cessation in student nurses. Addictive Behaviors. 2007;32:1505–1510. doi: 10.1016/j.addbeh.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Riemerth A, Kunze U, Groman E. Nocturnal sleep-disturbing nicotine craving and accomplishment with a smoking cessation program. Wiener Medizinische Wochenschrift. 2009;159:47–52. doi: 10.1007/s10354-008-0640-x. [DOI] [PubMed] [Google Scholar]
- Welsch SK, Stevens SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin smoking withdrawal scale. Experimental and Clinical Psychopharmacology. 1999;7:354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- West R. Bupropion SR for smoking cessation. Expert Opinion on Pharmacotherapy. 2003;4:533–540. doi: 10.1517/14656566.4.4.533. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Fiore MC, Baker TB, Young TB. Tobacco withdrawal and nicotine replacement influence objective measures of sleep. Journal of Consulting and Clinical Psychology. 1995;63:658–667. doi: 10.1037//0022-006x.63.4.658. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Fiore MC, Young TB, McClure JB, de Moor CA, Baker TB. Gender differences in response to nicotine replacement therapy: objective and subjective indexes of tobacco withdrawal. Experimental and Clinical Psychopharmacology. 1999a;7:135–144. doi: 10.1037//1064-1297.7.2.135. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Kenford SL, Smith SS, Fiore MC, Jorenby DE, Baker TB. Gender differences in smoking cessation. Journal of Consulting and Clinical Psychology. 1999b;67:555–562. doi: 10.1037//0022-006x.67.4.555. [DOI] [PubMed] [Google Scholar]
- Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29:85–93. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]