Abstract
Mechanical loading induces positive changes in the skeleton due to direct effects on bone cells, which may include regulation of transcription factors that support osteoblast differentiation and function. Flow effects on osteoblast transcription factors have generally been evaluated after short exposures. In this work, we assayed flow effects on osteogenic genes at early and late time points in a preosteoblast (CIMC-4) cell line and evaluated both steady and oscillatory flows. Four hours of steady unidirectional flow decreased the level of RANKL mRNA 53 ± 7% below that of nonflowed cells, but increases in Runx2 and osterix mRNA (44 ± 22% and 129 ± 12%, respectively) were significant only after 12–19 h of continuous flow. Late flow effects on RANKL and osterix were also induced by an intermittent flow–rest protocol (four cycles of 1 h on/1 h off + overnight rest). Four hours of oscillatory flow decreased RANKL mRNA at this early time point (63 ± 2%) but did not alter either osterix or Runx2. When oscillatory flow was delivered using the intermittent flow–rest protocol, Runx2 and osterix mRNA increased significantly (85 ± 19% and 161 ± 22%, respectively). Both β-catenin and ERK1/2, known to be involved in RANKL regulation, were rapidly activated by steady flow. Inhibition of flow-activated ERK1/2 prevented the increase in osterix mRNA but not Runx2; Runx2 phosphorylation was increased by flow, an effect which likely contributes to osterix induction. This work shows that both steady and oscillatory fluid flows can support enhancement of an osteogenic phenotype.
Keywords: Loading, Mechanical, Osteoblast, Shear, ERK1/2
Bone remodeling involves coordination of bone resorption and formation, with an imbalance between these two processes resulting in net bone loss or gain. The influence of mechanical loading on bone remodeling is well established. Skeletal loading through exercise has been shown to promote site-specific increases in bone volume and/or mineral density [1, 2]. Conversely, the lack of mechanical stimulation from disuse or exposure to microgravity results in bone loss [3–5]. Experimental animal models confirm that bone formation can be enhanced by controlled mechanical stimulation and is sensitive to multiple loading parameters, including magnitude, cycle number, and frequency [6–8]. Understanding how whole-bone loading is translated into cellular information, however, continues to be a challenge as physiological loading must be replicated in vitro to study mechanical effects on signaling pathways.
During weight-bearing activities, skeletal loading produces a complex array of tissue-level changes including matrix deformation, fluid flow through cannaliculi, interstitial fluid flow, and pressure changes in the intramedullary cavity and within the cortices. These expose cells resident within the tissue to numerous biophysical stimuli including fluid shear stress, substrate strain, and hydrostatic pressure [9–11]. In vitro studies have shown that mechanical loading enhances transcriptional regulation of genes associated with bone formation, including bone matrix proteins [12–14] and transcription factors that support osteoblast differentiation and function [15, 16]. In contrast, loading suppresses expression of the osteoclastogenesis support factor RANKL in bone cells [9, 17].
Interstitial fluid flow in bone is driven by dynamic externally applied mechanical loading, producing an oscillating pattern of fluid motion. Upon loading, fluid is forced out of regions of high compressive strain and then returns when the load is removed. Although an in vitro oscillatory model of fluid flow is more representative of fluid motion in bone, simpler in vitro models of unidirectional fluid flow, either steady or pulsating, have also been used. Studies that directly compared different flow models found that the effects of unidirectional and oscillatory fluid flows (OFF) were different for some aspects of the cellular response [18] but similar in other ways [19], and this comparison was influenced by the cell type studied.
In this work we evaluated early and late effects of steady flow and OFF on the regulation of RANKL, Runx2, and osterix, a set of genes shown previously to be subject to coordinated regulation by substrate deformation [16]. Using the preosteoblast CIMC-4 cell line, we explored how the duration of the steady flow period influenced alterations in steady-state mRNA levels and determined that fluid flow could be applied intermittently to promote changes in mRNA levels. Our system allowed us to relate flow-activated signaling events to specific genes.
Materials and Methods
Experimental Overview
To understand how the genes of interest were affected by fluid flow, we selected early (4 h) and late (19–22 h) time points for analysis based upon previously published studies [12, 13, 16, 17] and initially evaluated effects of continuous steady flow. Guided by studies showing that osteopontin exhibited a late response to OFF following an early flow exposure combined with an extended rest period [12, 20], we evaluated a flow–rest protocol (4 h flow + overnight rest) for both steady flow and OFF. Having found that the flow–rest protocol was insufficient to produce a late flow response in the genes of interest, we asked whether applying the 4-h flow period as a series of intermittent 1-h flow periods separated by 1-h periods without flow followed by overnight rest (i.e., intermittent flow + rest) would support a late flow response. To better understand the late response to steady flow, we also evaluated an intermediate (12 h) time point. Having defined that the late time point led to robust changes in all genes, we used this time point to evaluate the potential contributions of signaling pathways to the steady flow response. We were interested in evaluating the flow response in cells committed to the osteoblast lineage. Therefore, we used the preosteoblast CIMC-4 cell line, which has been previously used to study effects of cytokines and mechanical strain on osteoblast gene expression [16, 21, 22].
Reagents
Fetal bovine serum (FBS) was from Hyclone (Logan, UT). Culture medium, glutamine, antibiotics, reverse transcriptase, and Taq polymerase were from Invitrogen (Carlsbad, CA). Thapsigargin was from Sigma-Aldrich (St. Louis, MO). U0126 was from Promega (Madison, WI). The RNA isolation kit and DNase I were from Qiagen (Valencia, CA), and random primers were from Ambion (Austin, TX).
Cell Culture
The CIMC-4 cell line was prepared from mouse calvariae and expresses an interferon-γ (IFN-γ)-inducible, temperature-sensitive SV40 large T antigen transgene [21]. Cells were maintained in permissive conditions at 33°C in α-MEM containing 10% FBS and 100 U/ml IFN-γ. Medium was changed every 3–4 days, and passages above 24 were not used. Prior to experiments, cells were cultured for 1 week in nonpermissive conditions at 37°C in MEM containing 10% FBS, 1.25 mM glutamine, and 100 µg/ml penicillin/streptomycin as previously described [16, 22]. Cultures were treated with 10 nM 1,25(OH)D3 for 24–48 h prior to flow initiation, and 1,25(OH)2D3 was included in the medium throughout the flow experiment. HEPES (20 mM) was added to the culture medium for OFF experiments. The MEK inhibitor U0126 (10 µM) and thapsigargin (50 nM) were used to evaluate the potential role of specific signaling pathways in flow-mediated gene changes. Thapsigargin is an inhibitor of the endoplasmic reticular Ca2+-ATPase that causes calcium discharge and was used to empty the intracellular calcium stores. U0126 and thapsigargin were dissolved in DMSO to give a final concentration of DMSO in the culture medium of 0.1% and 0.01%, respectively, and DMSO was likewise added to the basal culture medium. Each pharmacological agent was given 1 h prior to flow initiation and was present in the culture medium throughout the experiment.
Steady Fluid Flow
Cells were seeded onto 100-mm-diameter dishes (6,000–12,000 cells/cm2) which had been coated with rat tail type I collagen (BD Biosciences, Bedford, MA). Cultures were exposed to unidirectional fluid flow using a modified cone-and-plate shear apparatus with a fixed 0.5° cone angle and a constant cone rotation of 200 rpm, corresponding to a shear stress of 8 dyn/cm2 [23].
Oscillatory Fluid Flow
Cells were seeded onto glass slides (6,000 cells/cm2) which had been coated with rat tail type I collagen. OFF was delivered by a Hamilton glass syringe in series with rigid-walled tubing and a parallel plate flow chamber (internal dimensions of 74 × 34 × 0.28 mm), as previously described [18]. The syringe was mounted in and driven by a mechanical loading device. The flow rate was selected to yield peak shear stresses of 1 Pa (10 dyn/cm2). The dynamic flow profile was sinusoidal at a frequency of 1 Hz. No-flow control slides were similarly loaded into flow chambers, but the loading device to drive fluid flow was not activated. Given the chamber size and the modest level of fluid movement within the chamber, medium and gas exchange was limited. As such, slides were held in the chambers for no more than 7 h, after which cells were either harvested or transferred to dishes for an extended rest period.
Real-Time RT-PCR
Total RNA was isolated and reverse-transcribed as previously described [22, 24]. Aliquots of cDNA were diluted fivefold to 5,000-fold to generate relative standard curves. RANKL, Runx2, osterix, Wisp1, and 18S primers were as previously described [22, 24]. Osteopontin primers were (5′–3′) tgcctgacccatctcagaa and attcatccgagtccacagaa. PCR products were normalized to 18S amplicons in the RT sample.
Western Blotting
Whole-cell lysates and cytoplasmic and nuclear fractionates were prepared as previously described [22, 24], and protein (5–20 µg) was separated on a polyacrylamide gel, then transferred to a PVDF membrane. Antibodies directed against phospho-ERK1/2 (Cell Signaling, Danvers, MA), active β-catenin (clone 8E7; Upstate, Temecula, CA), total ERK1/2, and actin (Santa Cruz Biotechnology, Santa Cruz, CA) were used. The antibody for active β-catenin was specific for the hypophosphorylated form of β-catenin [25]. Secondary antibody conjugated with horseradish peroxidase was detected by chemiluminescence.
Immunoprecipitation
Following flow application, cultures were lysed in immunoprecipitation buffer (150 mM NaCl, 50 mM Tris HCl, 1 mM EGTA, 0.24% sodium deoxycholate, 1% IGEPAL, pH 7.5). Aprotinin, leupeptin, and pepstatin were added fresh prior to lysis. Lysates (600 µg) were combined with a Runx2 goat polyclonal antibody (Santa Cruz Biotechnology) and incubated overnight at 4°C. Protein A/G+ agarose beads (Santa Cruz Biotechnology) were then added and incubated for 1 h at 4°C. Following rinses, beads were resuspended in 40 µl 2× loading buffer. Immunoprecipitation samples were analyzed by immunoblotting with antibodies directed against phosphorylated serine (clone 4A4, Upstate) and rabbit Runx2 (Santa Cruz Biotechnology).
Statistical Analysis
Results are expressed as the mean ± SEM. Significance was evaluated by one-way analysis of variance or Student’s t-test (Prism; GraphPad, San Diego, CA). All experiments were replicated at least once.
Results
Steady Fluid Flow Induces Changes in mRNA Levels Consistent with an Osteogenic Phenotype
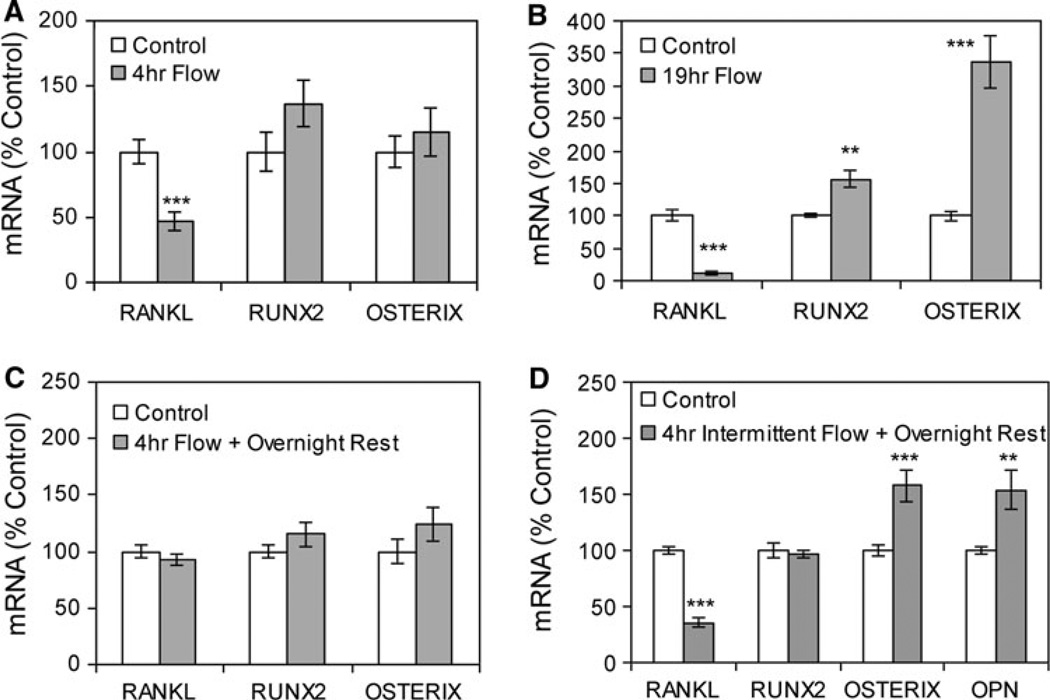
CIMC-4 preosteoblast cells were subjected to steady unidirectional fluid flow for 4 h, and steady-state mRNA levels were evaluated by real-time RT-PCR. RANKL mRNA was decreased 53 ± 7% in cultures exposed to flow compared to no-flow cultures, but no significant change in Runx2 or osterixm RNA levels occurred in response to flow at this time point (Fig. 1a). In contrast, 19 h of continuous flow led to a significant late response in all three genes. RANKL mRNA expression was decreased 89 ± 3%, Runx2 mRNA was increased 56 ± 13%, and osterixm RNA was increased 237 ± 41% in cultures subjected to flow compared to no-flow cultures (Fig. 1b).
Fig. 1.
Steady fluid flow induces changes in mRNA levels consistent with an osteogenic phenotype. a Steady fluid flow was applied for 4 h. Expression of RANKL, Runx2, and osterix, corrected for 18S mRNA, was analyzed by real-time RT-PCR. Data were normalized to the mRNA level measured in control cultures. ***Significant difference from no-flow control (P < 0.001, n = 3 experiments). b Steady fluid flow was applied for 19 h, and cultures were analyzed as above (**P < 0.01, n = 3 experiments). c Steady fluid flow was applied for 4 h, followed by an overnight rest period, with mRNA levels analyzed 19 h after flow initiation as in b. Data were compiled from four experiments. d Four hours of steady fluid flow was applied intermittently as 1-h flow treatments separated by 1-h rest periods, with mRNA levels analyzed at 19 h after flow initiation and including osteopontin (OPN) mRNA. Data were compiled from three experiments
No change in steady-state mRNA levels was measured when flow was applied for 4 h followed by an overnight rest period prior to RNA collection (Fig. 1c). However, when 4 h of steady fluid flow was applied intermittently as four 1-h treatments separated by 1-h rest periods, with gene expression analyzed the next day, RANKL mRNA was decreased 65 ± 4% and osterix mRNA was increased 58 ± 14% compared to no-flow cultures (Fig. 1d). The level of Runx2 mRNA was unchanged. The level of osteopontin mRNA, shown previously to be responsive to fluid flow [12, 19], was significantly increased 54 ± 17%.
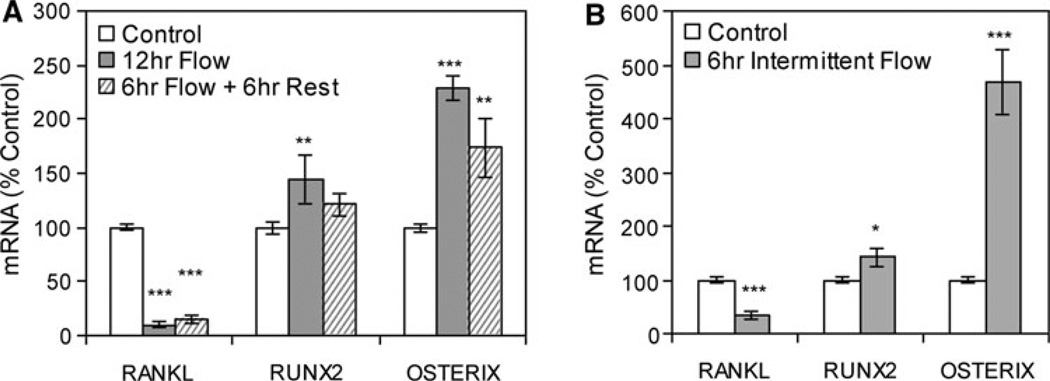
Steady-state mRNA levels were also measured at an intermediate 12-h time point. Continuous flow for 12 h led to a significant response in all genes (Fig. 2a): RANKL mRNA was decreased 90 ± 3%, Runx2 mRNA was increased 44 ± 22%, and osterix mRNA was increased 129 ± 12% in cultures subjected to flow compared to no-flow cultures, similar to the late response with flow applied for 19 h. Steady flow for 6 h followed by a 6-h rest period also induced significant changes in RANKL and osterix, but an effect on Runx2 was not more than a trend (Fig. 2a). Intermittent application of fluid flow was also evaluated. Here, steady flow was applied for 1 h followed by rest for 1 h (as in Fig. 1d) for a total of 12 h. In this case, all three genes responded to intermittent steady flow (Fig. 2b): RANKL mRNA was decreased 66 ± 7%, Runx2 mRNA was increased 42 ± 17%, and osterix mRNA was increased 369 ± 60% compared to no-flow cultures.
Fig. 2.
Steady fluid flow does not have to be continuous to induce changes in mRNA levels. a Designated mRNA was amplified by real-time RT-PCR. Cultures were subjected to continuous steady flow for 12 h or to flow for 6 h, followed by a 6-h rest period. Data were normalized to the mRNA level measured in control cells and compiled from three experiments. ***Significant difference from no-flow control, P < 0.001 (**P < 0.01). b Six hours of steady fluid flow was applied intermittently (1-h flow, then 1-h rest and repeat flow–rest), with analysis of mRNA as above (*P < 0.05, n = 3 experiments)
Oscillatory Fluid Flow Effects on mRNA Levels are Similar to Those of Steady Flow
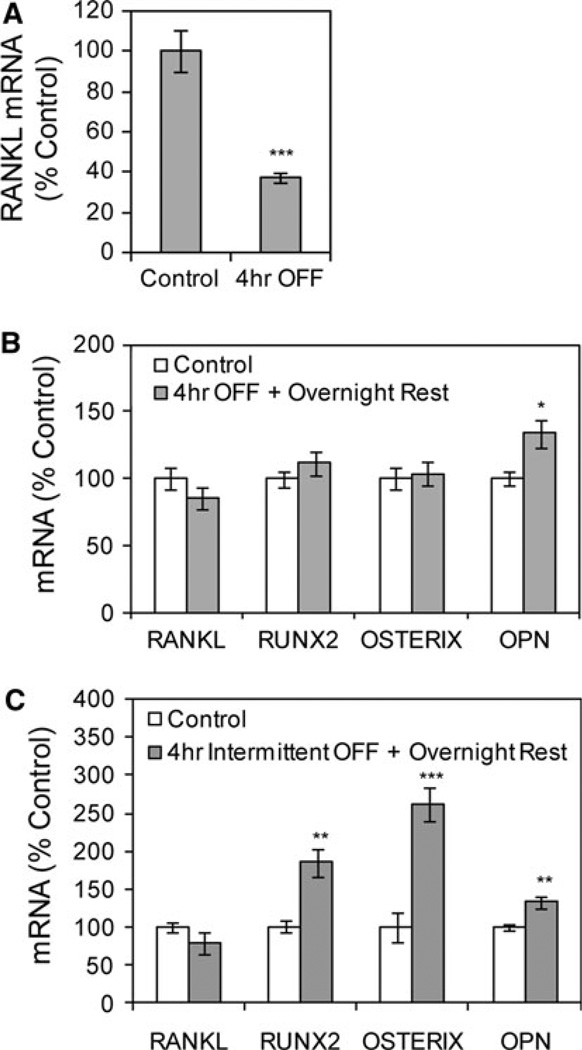
The response of bone cells to steady and oscillatory fluid flows has been shown to differ in some aspects of mechanical signaling [18, 19]. As shown in Figure 3a, 4 h of OFF decreased the level of RANKL mRNA 63 ± 2% compared to the level in control cultures, which were placed into flow chambers without subsequent fluid flow, consistent with a previous study [17]. Importantly, placement of slides into the closed flow chambers in the absence of OFF led to a significant 110 ± 30% increase (P < 0.01) in the level of RANKL mRNA compared to slides that remained in culture dishes at this 4-h time point. This increase in RANKL mRNA in the absence of flow suggests that containment of the slide within the flow chamber for 4 h subjects the cells to environmental stress, likely due to limited medium and gas exchange, and as such inclusion of slides in the flow chamber without flow activation is a critical control for the OFF experiments.
Fig. 3.
Oscillatory fluid flow (OFF) effects on mRNA levels are similar to those of steady flow. a OFF was applied for 4 h. RANKL mRNA, corrected for 18S mRNA, was analyzed by real-time RT-PCR. Data were normalized to the mRNA level measured in control cultures placed into flow chambers without subsequent fluid flow. ***Significant difference from no-flow control (P < 0.001, n = 3 experiments). b OFF was applied for 4 h, followed by an overnight rest period, with mRNA levels analyzed 22 h after flow initiation (*P < 0.05, n = 3 experiments). OPN, osteopontin. c Four hours of OFF was applied intermittently as 1-h flow treatments separated by 1-h rest periods, with mRNA levels analyzed at 22 h after flow initiation (**P < 0.01, n = 2 experiments)
Repeating the flow–rest protocols used with steady flow, effects of OFF were evaluated at the late time point when steady flow caused significant changes in mRNA levels. First, OFF was applied for 4 h, after which cultures were removed from flow chambers for an overnight rest period before analysis. No changes were detected in RANKL, Runx2, or osterix mRNA levels in response to this flow protocol (Fig. 3b), consistent with the lack of response to 4 h of steady flow (see Fig. 1c). However, osteopontin mRNA was significantly increased 34 ± 10% (P < 0.01), as expected.
When OFF was delivered as four 1-h treatments separated by 1-h rest periods, with steady-state mRNA levels analyzed the next day, levels of RUNX2, osterix, and osteopontin mRNA were significantly increased (85 ± 19%, 161 ± 22%, and 33 ± 8%, respectively) (Fig. 3c). The level of RANKL mRNA was unchanged, in contrast to the late effect for steady flow (see Fig. 1d).
Fluid Flow Regulation of Osterix Requires Multiple Signaling Events
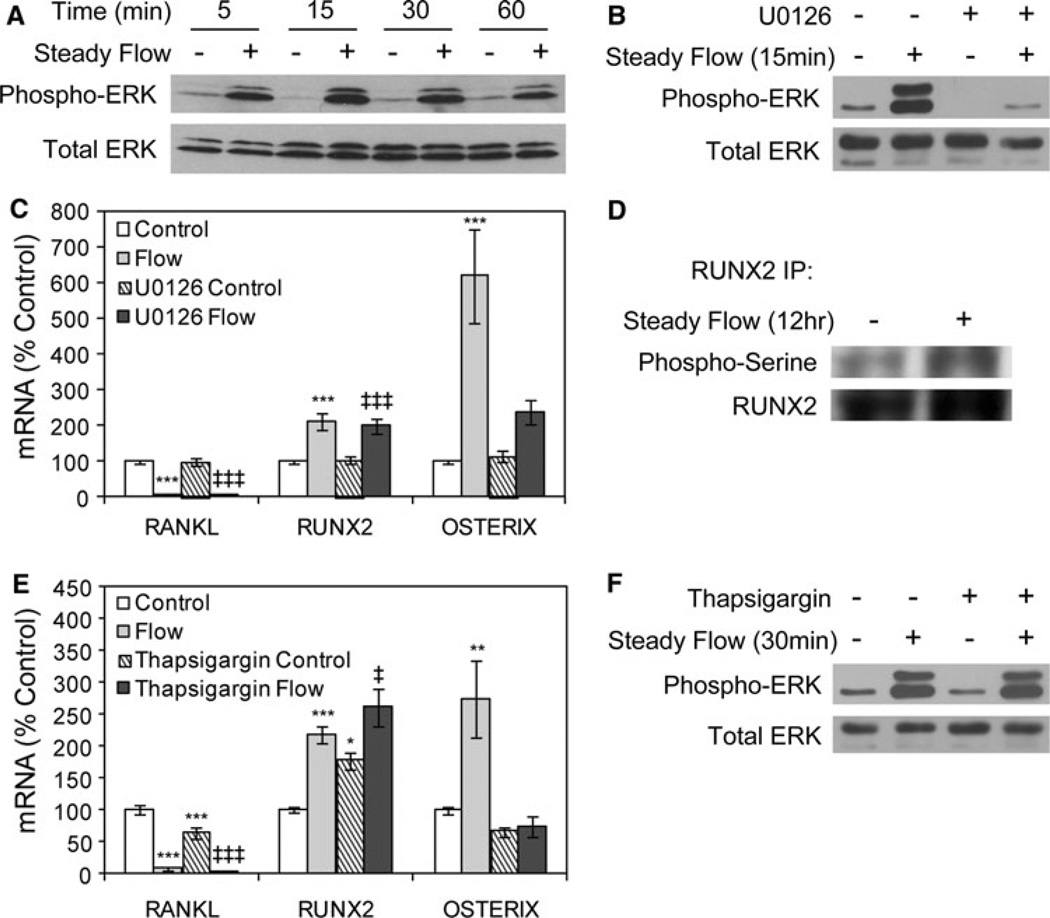
In vitro mechanical loading of bone cells involves activation of ERK1/2 MAPK [12, 26, 27]. ERK1/2 was activated in CIMC-4 cultures exposed to steady fluid flow. ERK1/2 phosphorylation increased after 5–60 min of flow, with a peak response at 15 min (Fig. 4a). By 4 h of flow, ERK1/2 phosphorylation returned to baseline (data not shown).
Fig. 4.
Fluid flow regulation of osterix requires multiple signaling events. a Immunoblots of total cellular proteins from CIMC-4 cultures exposed to steady flow for 5–60 min. b Cultures were treated with the MEK inhibitor U0126 (10 µM) for 1 h prior to flow exposure for 15 min; then, total cellular proteins were evaluated for ERK1/2 phosphorylation. c Cultures were treated with U0126 as in b; then, steady flow was applied for 19 h. mRNA levels were analyzed by real-time RT-PCR. Data were normalized to the mRNA level measured in basal control cells and compiled from three experiments. ***Significant difference from basal control, P < 0.001. ‡‡‡Significant difference from U0126 control, P < 0.001. d Immunoprecipitation of Runx2 was done on whole-cell lysates after steady flow was applied for 12 h. Shown are immunoblots for phosphorylated serine and Runx2. e Cultures were treated with thapsigargin to empty intracellular calcium stores. Thapsigargin (50 nM) was added to cultures 1 h prior to steady fluid flow for 19 h. Data were normalized to the mRNA level measured in basal control cells and compiled from three experiments. ***Significant difference from basal control, P < 0.001 (**P < 0.01, *P < 0.05). ‡‡‡Significant difference from thapsigargin control, P < 0.001 (‡P < 0.05). f Cultures were treated with thapsigargin as in e; then, steady fluid flow was applied for 30 min, and total cellular proteins were evaluated for ERK1/2 phosphorylation
Cultures were exposed to flow in the presence of the MEK inhibitor U0126 to evaluate the requirement for ERK1/2 MAPK activation in the late response to steady flow. Treatment with U0126 (10 µM) blocked ERK1/2 phosphorylation in response to fluid flow (Fig. 4b). Inhibition of ERK1/2 did not prevent flow-mediated changes in RANKL or Runx2 mRNA levels (Fig. 4c). In contrast, the increase in osterix mRNA required flow-induced ERK1/2 activation.
That flow regulation of osterix, but not Runx2, was disrupted by treatment with U0126 was interesting as osterix expression is known to be downstream of Runx2 [28]. However, phosphorylation of Runx2 is an important regulator of its activity, and ERK1/2 targets specific serine residues in Runx2 [29]. As shown in Figure 4d, the amount of phosphorylated serine detected in immunoprecipitates of Runx2 was higher in cultures subjected to steady fluid flow compared to no-flow control cultures, indicating that enhancement of Runx2 phosphorylation by fluid flow contributes to upregulation of osterix expression.
Increased intracellular calcium is another early cellular response to fluid flow [18] and has been linked to some flow-regulated genes in osteoblasts [12, 30]. Cultures were treated with thapsigargin to inhibit release of intracellular calcium. Exposure to thapsigargin significantly decreased the level of basal RANKL mRNA and increased the basal level of Runx2 mRNA, while the basal level of osterix mRNA was unchanged (Fig. 4e). Flow-mediated changes in RANKL and Runx2 mRNA levels were not disrupted by thapsigargin. All cultures exposed to flow showed a decrease in RANKL mRNA and an increase in Runx2 mRNA compared to no-flow cultures. In contrast, thapsigargin blocked the flow-induced increase in osterix mRNA. ERK1/2 activation by steady fluid flow was not reduced in the presence of thapsigargin (Fig. 4f).
Steady Fluid Flow Activates β-Catenin
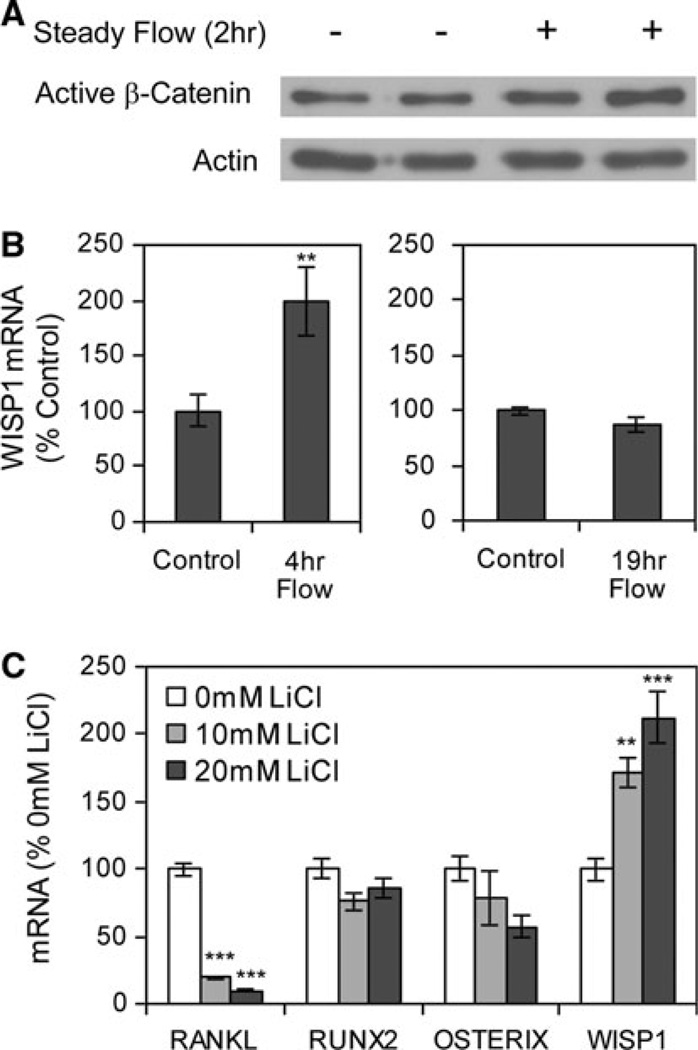
In CIMC-4 cultures, steady fluid flow increased the level of active (dephosphorylated) β-catenin in nuclear fractionates (Fig. 5a). Consistent with this result, the level of mRNA for Wisp1, a β-catenin target gene, was increased 99 ± 31% when steady flow was applied for 4 h (Fig. 5b). The response of Wisp1 to flow was transient. There was no difference in the level of Wisp1 mRNA between flow and control cultures after 19 h of continuous steady flow.
Fig. 5.
Steady fluid flow activates β-catenin. a The nuclear protein fractionate was analyzed for active β-catenin and actin (loading control) in cultures subjected to steady fluid flow for 2 h. b Wisp1 mRNA was amplified by real-time RT-PCR in cultures exposed to steady fluid flow for 4 h (left graph, compiled from three experiments) or for 19 h (right graph, compiled from three experiments). **Significant difference from unstrained control, P < 0.01. c The GSK3β inhibitor lithium chloride (LiCl) was applied for 19 h, and designated mRNA was amplified by real-time RT-PCR. ***Significant difference from 0 mM LiCl, P < 0.001 (n = 2 experiments)
Previous studies identified RANKL and Runx2 as target genes of β-catenin [31, 32]. Cultures were treated with lithium chloride, a GSK3β inhibitor that causes sustained β-catenin activation. Lithium chloride (20 mM) inhibited the level of RANKL mRNA but had no effect on Runx2 or osterix mRNA levels (Fig. 5c). Lithium chloride treatment also increased the level of Wisp1 mRNA. The data suggest that RANKL is negatively regulated by β-catenin in CIMC-4 cells.
Discussion
Loading of the skeleton causes multiple tissue-level changes including interstitial fluid flow, which is postulated to be a primary effector of anabolic mechanical effects in bone through direct cellular changes [33]. The response of bone cells to fluid flow may include regulation of transcription factors that support osteoblast differentiation and function. Flow effects on osteoblast transcription factors have typically been evaluated after short exposures [10, 15]. We here studied early and late effects of fluid flow on mRNA levels of Runx2 and osterix, transcription factors known to be necessary for early and late osteoprogenitor differentiation, respectively, as well as RANKL, the osteoclastogenesis support factor produced by bone cells which is known to rise with entry into the osteoprogenitor lineage and to decrease during maturation [34]. Runx2 and osterix mRNA were unchanged after 4 h of flow; significant increases in both Runx2 and osterix mRNA were detected as early as 12 h after flow initiation. Fluid flow also caused a rapid and, in the case of steady flow, a sustained inhibition of RANKL mRNA. This is the first evidence that fluid flow can induce late changes in transcription factors that support osteoprogenitor maturation.
While application of steady unidirectional fluid flow is thought to mimic blood flow and has been widely used to evaluate signaling in vascular cells [35, 36], bone scientists have advocated application of OFF to more closely mimic the physiologic condition within cannaliculi [18]. In vitro studies have shown that the response to unidirectional and oscillatory fluid flows differs for some aspects of mechanical signaling in bone cells, including calcium signaling and cytoskeletal reorganization [18, 19], but increases in osteopontin and COX2 levels are supported by both types of flow in osteoblasts [19]. In the work presented here, both steady and oscillatory fluid flows inhibited the level of RANKL mRNA at an early time point while enhancing Runx2 and osterix mRNA levels at a late time point. Differing from unidirectional flow, OFF was unable to suppress the late expression of RANKL.
Importantly, the response to flow was enhanced by incorporating a rest period between brief flow treatments. Altering the flow protocol, such that 4 h of flow was applied in 1-h treatments interspersed by 1-h rest periods, resulted in a significant change in the level of osterix mRNA by 19 h, whereas a single continuously applied 4-h flow was ineffective. By allowing the cell to experience intermittent rest periods, the mechanical signaling pathways activated by flow may “reset,” allowing reactivation at the next flow treatment. Insertion of rest periods has previously been shown to enhance in vivo bone formation [37, 38] as well as the in vitro flow response in bone cells [20].
Our data complement other evidence that flow activates ERK1/2 MAPK in osteoblasts [26, 27, 39]. ERK1/2 has been linked to flow regulation of osteopontin [12] and type I collagen [13], as well as cell proliferation [40]. Here, the MEK inhibitor U0126 prevented only the flow-induced increase in osterix mRNA but not changes in the levels of RANKL or Runx2 mRNA. The differential requirement of ERK1/2 for flow-induced changes in mRNA levels supports a multifold signaling response to mechanical input. Accordingly, calcium signaling, activated by fluid flow in bone cells [18], was also involved in flow induction of osterix. Disruption of intracellular calcium mobilization with thapsigargin specifically blocked the increase in osterix mRNA by steady flow but did not alter activation of ERK1/2. Osterix expression is known to be downstream of Runx2 [28]. The more robust effect of flow on osterix than on Runx2 likely reflects enhanced Runx2 activation by flow-induced phosphorylation. Indeed, ERK1/2 is known to phosphorylate Runx2 [29].
β-Catenin is another signaling pathway contributing to flow-induced gene regulation [41]. Our data support β-catenin as a target of mechanical loading in bone cells, also shown by others with fluid flow [42] and substrate stretch [22, 43]. As β-catenin is involved in regulation of both RANKL and Runx2 [31, 32], it suggests itself as an ERK1/2-independent signal. Indeed, we observed that activation of β-catenin via GSK3β inhibition caused a significant reduction in RANKL expression.
Skeletal loading exposes cells resident within bone tissue to multiple biophysical forces. Whether these forces elicit distinct, similar, or overlapping responses in bone tissue remains an open question. Prior in vitro comparisons of fluid shear stress and mechanical strain have suggested that osteoblasts respond differently to these two types of mechanical loading with respect to nitric oxide release [44], PGE2 release [44, 45], and osteopontin expression [46]. The transcriptional response to fluid flow described in this work is consistent with mechanical strain effects in CIMC-4 cultures, where prolonged exposure caused repression of RANKL mRNA as well as upregulation of Runx2 and osterix mRNA levels [16]. Interestingly, although both types of mechanical loading caused phosphorylation of ERK1/2 MAPK, a MEK inhibitor blocked strain-mediated changes in all three genes but only disrupted flow-mediated enhancement of osterix mRNA. Although this difference suggests a divergence in those signaling pathways mediating flow and strain effects, the similar gene response to fluid flow and mechanical strain indicates that osteoblasts recognize both types of loading as osteogenic.
In summary, both unidirectional and oscillatory fluid flows enhance levels of Runx2 and osterix mRNA when cells are exposed to continuous or repetitive applications of flow. Both types of flow also inhibit the level of RANKL mRNA, decreasing a stimulus for bone resorption. The coordinated regulation of these osteoblast transcription factors, along with RANKL, by fluid flow reflects progression toward a more osteogenic phenotype. Flow duration and inclusion of rest periods influence flow effects. Signaling pathways activated by fluid flow, including ERK1/2 MAPK, intracellular calcium mobilization, and β-catenin, integrate into a net response that is anticatabolic and proanabolic for bone cells.
Acknowledgement
This work was supported by National Institutes of Health grants AR42360 and AR52014 (to J.R.) and AR045989 (to C. R. J.).
Footnotes
The authors have stated that they have no conflict of interest.
Disclosures None.
Contributor Information
N. Case, Email: ncase@med.unc.edu, Department of Medicine, University of North Carolina, 125 MacNider Hall, 5030 Burnett-Womack, CB# 7005, Chapel Hill NC27599, USA.
B. Sen, Department of Medicine, University of North Carolina, 125 MacNider Hall, 5030 Burnett-Womack, CB# 7005, Chapel Hill NC27599, USA
J. A. Thomas, Department of Medicine, University of North Carolina, 125 MacNider Hall, 5030 Burnett-Womack, CB# 7005, Chapel Hill NC27599, USA
M. Styner, Department of Medicine, University of North Carolina, 125 MacNider Hall, 5030 Burnett-Womack, CB# 7005, Chapel Hill NC27599, USA
Z. Xie, Department of Medicine, University of North Carolina, 125 MacNider Hall, 5030 Burnett-Womack, CB# 7005, Chapel Hill NC27599, USA
C. R. Jacobs, Department of Biomedical Engineering, Columbia University, 351 Engineering Terrace, Mail Code 8904, 1210 Amsterdam Avenue, New York, NY 10027, USA
J. Rubin, Department of Medicine, University of North Carolina, 125 MacNider Hall, 5030 Burnett-Womack, CB# 7005, Chapel Hill NC27599, USA
References
- 1.Haapasalo H, Kontulainen S, Sievanen H, Kannus P, Jarvinen M, Vuori I. Exercise-induced bone gain is due to enlargement in bone size without a change in volumetric bone density: a peripheral quantitative computed tomography study of the upper arms of male tennis players. Bone. 2000;27:351–357. doi: 10.1016/s8756-3282(00)00331-8. [DOI] [PubMed] [Google Scholar]
- 2.MacKelvie KJ, Khan KM, Petit MA, Janssen PA, McKay HA. A school-based exercise intervention elicits substantial bone health benefits: a 2-year randomized controlled trial in girls. Pediatrics. 2003;112:e447. doi: 10.1542/peds.112.6.e447. [DOI] [PubMed] [Google Scholar]
- 3.Smith SM, Wastney ME, Morukov BV, Larina IM, Nyquist LE, Abrams SA, Taran EN, Shih CY, Nillen JL, Davis-Street JE, Rice BL, Lane HW. Calcium metabolism before, during, and after a 3-mo spaceflight: kinetic and biochemical changes. Am J Physiol Regul Integr Comp Physiol. 1999;277:R1–R10. doi: 10.1152/ajpregu.1999.277.1.r1. [DOI] [PubMed] [Google Scholar]
- 4.Uebelhart D, Demiaux-Domenech B, Roth M, Chantraine A. Bone metabolism in spinal cord injured individuals and in others who have prolonged immobilisation. A review. Paraplegia. 1995;33:669–673. doi: 10.1038/sc.1995.140. [DOI] [PubMed] [Google Scholar]
- 5.Whedon G. Disuse osteoporosis: physiologic aspects. Calcif Tissue Int. 1984;36:S146–S150. doi: 10.1007/BF02406148. [DOI] [PubMed] [Google Scholar]
- 6.Rubin C, Lanyon L. Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am. 1984;66:397–402. [PubMed] [Google Scholar]
- 7.Rubin CT, McLeod KJ. Promotion of bony ingrowth by frequency-specific, low-amplitude mechanical strain. Clin Orthop Relat Res. 1994;298:165–174. [PubMed] [Google Scholar]
- 8.Qin YX, Rubin CT, McLeod KJ. Nonlinear dependence of loading intensity and cycle number in the maintenance of bone mass and morphology. J Orthop Res. 1998;16:482–489. doi: 10.1002/jor.1100160414. [DOI] [PubMed] [Google Scholar]
- 9.Rubin J, Murphy T, Nanes MS, Fan X. Mechanical strain inhibits expression of osteoclast differentiation factor by murine stromal cells. Am J Physiol Cell Physiol. 2000;278:C1126–C1132. doi: 10.1152/ajpcell.2000.278.6.C1126. [DOI] [PubMed] [Google Scholar]
- 10.Li YJ, Batra NN, You L, Meier SC, Coe IA, Yellowley CE, Jacobs CR. Oscillatory fluid flow affects human marrow stromal cell proliferation and differentiation. J Orthop Res. 2004;22:1283–1289. doi: 10.1016/j.orthres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Rubin J, Rubin C, Jacobs CR. Molecular pathways mediating mechanical signaling in bone. Gene. 2006;367:1–16. doi: 10.1016/j.gene.2005.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.You J, Reilly GC, Zhen X, Yellowley CE, Chen Q, Donahue HJ, Jacobs CR. Osteopontin gene regulation by oscillatory fluid flow via intracellular calcium mobilization and activation of mitogen-activated protein kinase in MC3T3–E1 osteoblasts. J Biol Chem. 2001;276:13365–13371. doi: 10.1074/jbc.M009846200. [DOI] [PubMed] [Google Scholar]
- 13.Wu CC, Li YS, Haga JH, Wang N, Lian IY, Su FC, Usami S, Chien S. Roles of MAP kinases in the regulation of bone matrix gene expressions in human osteoblasts by oscillatory fluid flow. J Cell Biochem. 2006;98:632–641. doi: 10.1002/jcb.20697. [DOI] [PubMed] [Google Scholar]
- 14.Harter L, Hruska K, Duncan R. Human osteoblast-like cells respond to mechanical strain with increased bone matrix protein production independent of hormonal regulation. Endocrinology. 1995;136:528–535. doi: 10.1210/endo.136.2.7530647. [DOI] [PubMed] [Google Scholar]
- 15.Arnsdorf EJ, Tummala P, Kwon RY, Jacobs CR. Mechanically induced osteogenic differentiation—the role of RhoA, ROCKII and cytoskeletal dynamics. J Cell Sci. 2009;122:546–553. doi: 10.1242/jcs.036293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan X, Rahnert JA, Murphy TC, Nanes MS, Greenfield EM, Rubin J. Response to mechanical strain in an immortalized pre-osteoblast cell is dependent on ERK1/2. J Cell Physiol. 2006;207:454–460. doi: 10.1002/jcp.20581. [DOI] [PubMed] [Google Scholar]
- 17.Kim CH, You L, Yellowley CE, Jacobs CR. Oscillatory fluid flow-induced shear stress decreases osteoclastogenesis through RANKL and OPG signaling. Bone. 2006;39:1043–1047. doi: 10.1016/j.bone.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs CR, Yellowley CE, Davis BR, Zhou Z, Cimbala JM, Donahue HJ. Differential effect of steady versus oscillating flow on bone cells. J Biomech. 1998;31:969–976. doi: 10.1016/s0021-9290(98)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponik SM, Triplett JW, Pavalko FM. Osteoblasts and osteocytes respond differently to oscillatory and unidirectional fluid flow profiles. J Cell Biochem. 2007;100:794–807. doi: 10.1002/jcb.21089. [DOI] [PubMed] [Google Scholar]
- 20.Batra NN, Li YJ, Yellowley CE, You L, Malone AM, Kim CH, Jacobs CR. Effects of short-term recovery periods on fluid-induced signaling in osteoblastic cells. J Biomech. 2005;38:1909–1917. doi: 10.1016/j.jbiomech.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Ragab AA, Nalepka JL, Bi Y, Greenfield EM. Cytokines synergistically induce osteoclast differentiation: support by immortalized or normal calvarial cells. Am J Physiol Cell Physiol. 2002;283:C679–C687. doi: 10.1152/ajpcell.00421.2001. [DOI] [PubMed] [Google Scholar]
- 22.Case N, Ma M, Sen B, Xie Z, Gross TS, Rubin J. Beta-catenin levels influence rapid mechanical responses in osteoblasts. J Biol Chem. 2008;283:29196–29205. doi: 10.1074/jbc.M801907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jo H, Song H, Mowbray A. Role of NADPH oxidases in disturbed flow- and BMP4-induced inflammation and atherosclerosis. Antioxid Redox Signal. 2006;8:1609–1619. doi: 10.1089/ars.2006.8.1609. [DOI] [PubMed] [Google Scholar]
- 24.Sen B, Xie Z, Case N, Ma M, Rubin C, Rubin J. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology. 2008;149:6065–6075. doi: 10.1210/en.2008-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H. Wnt signaling controls the phosphorylation status of beta-catenin. J Biol Chem. 2002;277:17901–17905. doi: 10.1074/jbc.M111635200. [DOI] [PubMed] [Google Scholar]
- 26.Jessop HL, Rawlinson SC, Pitsillides AA, Lanyon LE. Mechanical strain and fluid movement both activate extracellular regulated kinase (ERK) in osteoblast-like cells but via different signaling pathways. Bone. 2002;31:186–194. doi: 10.1016/s8756-3282(02)00797-4. [DOI] [PubMed] [Google Scholar]
- 27.Yang CM, Chien CS, Yao CC, Hsiao LD, Huang YC, Wu CB. Mechanical strain induces collagenase-3 (MMP-13) expression in MC3T3–E1 osteoblastic cells. J Biol Chem. 2004;279:22158–22165. doi: 10.1074/jbc.M401343200. [DOI] [PubMed] [Google Scholar]
- 28.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger–containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 29.Ge C, Xiao G, Jiang D, Yang Q, Hatch NE, Roca H, Franceschi RT. Identification and functional characterization of ERK/MAPK phosphorylation sites in the Runx2 transcription factor. J Biol Chem. 2009;284:32533–32543. doi: 10.1074/jbc.M109.040980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen NX, Ryder KD, Pavalko FM, Turner CH, Burr DB, Qiu J, Duncan RL. Ca2+ regulates fluid shear-induced cytoskeletal reorganization and gene expression in osteoblasts. Am J Physiol Cell Physiol. 2000;278:C989–C997. doi: 10.1152/ajpcell.2000.278.5.C989. [DOI] [PubMed] [Google Scholar]
- 31.Spencer GJ, Utting JC, Etheridge SL, Arnett TR, Genever PG. Wnt signalling in osteoblasts regulates expression of the receptor activator of NFkappaB ligand and inhibits osteoclastogenesis in vitro. J Cell Sci. 2006;119:1283–1296. doi: 10.1242/jcs.02883. [DOI] [PubMed] [Google Scholar]
- 32.Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS, Javed A, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280:33132–33140. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
- 33.Knothe Tate ML. “Whither flows the fluid in bone?” An osteocyte’s perspective. J Biomech. 2003;36:1409–1424. doi: 10.1016/s0021-9290(03)00123-4. [DOI] [PubMed] [Google Scholar]
- 34.Atkins GJ, Kostakis P, Pan B, Farrugia A, Gronthos S, Evdokiou A, Harrison K, Findlay DM, Zannettino AC. RANKL expression is related to the differentiation state of human osteoblasts. J Bone Miner Res. 2003;18:1088–1098. doi: 10.1359/jbmr.2003.18.6.1088. [DOI] [PubMed] [Google Scholar]
- 35.Go YM, Boo YC, Park H, Maland MC, Patel R, Pritchard KA, Jr, Fujio Y, Walsh K, Darley-Usmar V, Jo H. Protein kinase B/Akt activates c-Jun NH2-terminal kinase by increasing NO production in response to shear stress. J Appl Physiol. 2001;91:1574–1581. doi: 10.1152/jappl.2001.91.4.1574. [DOI] [PubMed] [Google Scholar]
- 36.Traub O, Berk BC. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol. 1998;18:677–685. doi: 10.1161/01.atv.18.5.677. [DOI] [PubMed] [Google Scholar]
- 37.Srinivasan S, Ausk BJ, Poliachik SL, Warner SE, Richardson TS, Gross TS. Rest-inserted + loading rapidly amplifies the response of bone to small increases in strain and load cycles. J Appl Physiol. 2007;102:1945–1952. doi: 10.1152/japplphysiol.00507.2006. [DOI] [PubMed] [Google Scholar]
- 38.Robling AG, Burr DB, Turner CH. Partitioning a daily mechanical stimulus into discrete loading bouts improves the osteogenic response to loading. J Bone Miner Res. 2000;15:1596–1602. doi: 10.1359/jbmr.2000.15.8.1596. [DOI] [PubMed] [Google Scholar]
- 39.Weyts FA, Li YS, van Leeuwen J, Weinans H, Chien S. ERK activation and alphavbeta3 integrin signaling through Shc recruitment in response to mechanical stimulation in human osteoblasts. J Cell Biochem. 2002;87:85–92. doi: 10.1002/jcb.10278. [DOI] [PubMed] [Google Scholar]
- 40.Jiang GL, White CR, Stevens HY, Frangos JA. Temporal gradients in shear stimulate osteoblastic proliferation via ERK1/2 and retinoblastoma protein. Am J Physiol Endocrinol Metab. 2002;283:E383–E389. doi: 10.1152/ajpendo.00547.2001. [DOI] [PubMed] [Google Scholar]
- 41.Arnsdorf EJ, Tummala P, Jacobs CR. Non-canonical Wnt signaling and N-cadherin related beta-catenin signaling play a role in mechanically induced osteogenic cell fate. PLoS One. 2009;4:e5388. doi: 10.1371/journal.pone.0005388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Norvell SM, Alvarez M, Bidwell JP, Pavalko FM. Fluid shear stress induces beta-catenin signaling in osteoblasts. Calcif Tissue Int. 2004;75:396–404. doi: 10.1007/s00223-004-0213-y. [DOI] [PubMed] [Google Scholar]
- 43.Sen B, Styner M, Xie Z, Case N, Rubin CT, Rubin J. Mechanical loading regulates NFATC1 and β-catenin signaling through a GSK3β control node. J Biol Chem. 2009;284:34607–34617. doi: 10.1074/jbc.M109.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smalt R, Mitchell F, Howard R, Chambers T. Induction of NO and prostaglandin E2 in osteoblasts by wall-shear stress but not mechanical strain. Am J Physiol Endocrinol Metab. 1997;273:E751–E758. doi: 10.1152/ajpendo.1997.273.4.E751. [DOI] [PubMed] [Google Scholar]
- 45.McGarry JG, Klein-Nulend J, Mullender MG, Prendergast PJ. A comparison of strain and fluid shear stress in stimulating bone cell responses—a computational and experimental study. FASEB J. 2005;19:482–484. doi: 10.1096/fj.04-2210fje. [DOI] [PubMed] [Google Scholar]
- 46.You J, Yellowley CE, Donahue HJ, Zhang Y, Chen Q, Jacobs CR. Substrate deformation levels associated with routine physical activity are less stimulatory to bone cells relative to loading-induced oscillatory fluid flow. J Biomech Eng. 2000;122:387–393. doi: 10.1115/1.1287161. [DOI] [PubMed] [Google Scholar]