Abstract
Alcohol dependence and associated cognitive impairment appear to result from maladaptive neuroplasticity in response to chronic alcohol consumption, neuroinflammation and neurodegeneration. The inherent stability of behavioral alterations associated with the addicted state suggests that transcriptional and epigenetic mechanisms are operative. NF-κB transcription factors are regulators of synaptic plasticity and inflammation, and responsive to a variety of stimuli including alcohol. These factors are abundant in the brain where they have diverse functions that depend on the composition of the NF-κB complex and cellular context. In neuron cell bodies, NF-κB is constitutively active, and involved in neuronal injury and neuroprotection. However, at the synapse, NF-κB is present in a latent form and upon activation is transported to the cell nucleus. In glia, NF-κB is inducible and regulates inflammatory processes that exacerbate alcohol-induced neurodegeneration. Animal studies demonstrate that acute alcohol exposure transiently activates NF-κB, which induces neuroinflammatory responses and neurodegeneration. Postmortem studies of brains of human alcoholics suggest that repeated cycles of alcohol consumption and withdrawal cause adaptive changes in the NF-κB system that may permit the system to better tolerate excessive stimulation. This type of tolerance, ensuring a low degree of responsiveness to applied stimuli, apparently differs from that in the immune system, and may represent a compensatory response that protects brain cells against alcohol neurotoxicity. This view is supported by findings showing preferential downregulation of pro-apoptotic gene expression in the affected brain areas in human alcoholics. Although further verification is needed, we speculate that NF-κB-driven neuroinflammation and disruption to neuroplasticity play a significant role in regulating alcohol dependence and cognitive impairment.
Keywords: alcoholism, dependence, addiction, neuroinflammation, neuroplasticity, gene transcription, transcription factors, NF-κB, p50, human brain
1. Introduction
Although the mechanisms underlying alcoholism remain to be elucidated, the molecular hypothesis postulates that alcohol abuse and dependence result from alterations in gene expression underlying neuroadaptations to chronic alcohol consumption. Molecular adaptations in the nucleus accumbens (NAc), ventral tegmental area, amygdala and dorsolateral prefrontal cortex (dl-PFC) (Di Chiara et al., 2004; Fadda and Rossetti, 1998; Koob, 2003; Nestler, 2005) have been implicated in the behavioral changes such as craving and relapse. Alcohol abuse also causes deficits in perceptual-motor skills, visual-spatial functions, abstraction and problem solving (Bowden and McCarter, 1993; Parsons and Nixon, 1993; Ratti et al., 2002; Schmidt et al., 2005). These impairments may be related to alcohol-induced damage to the dl-PFC and hippocampus (Beatty et al., 1996; Crews et al., 2005; Pfefferbaum et al., 2001; Sullivan and Pfefferbaum, 2005). White matter and cell loss in the dl-PFC and orbitofrontal cortex (OFC), and the loss of hippocampal volume and shrinkage of hippocampal neurons are characteristic of the maladaptive response (Agartz et al., 1999; Aschner and Allen, 2000; Harper et al., 1985; Jensen and Pakkenberg, 1993; Kril et al., 1997; Kril and Harper, 1989; Miguel-Hidalgo et al., 2006).
Work over the past 20 years has provided evidence for a role of several transcription factors (TFs) in regulation of gene expression underlying substance addiction. Prominent examples include cAMP-response element-binding protein, the glucocorticoid receptor, ΔFosB (a Fos family protein), and NF-κB (Nuclear Factor κ-light-chain-enhancer of activated B cells) (Ang et al., 2001; Carlezon et al., 2005; Deroche-Gamonet et al., 2003; Green et al., 2006; Green et al., 2008; Mackler et al., 2000; Russo et al., 2009).
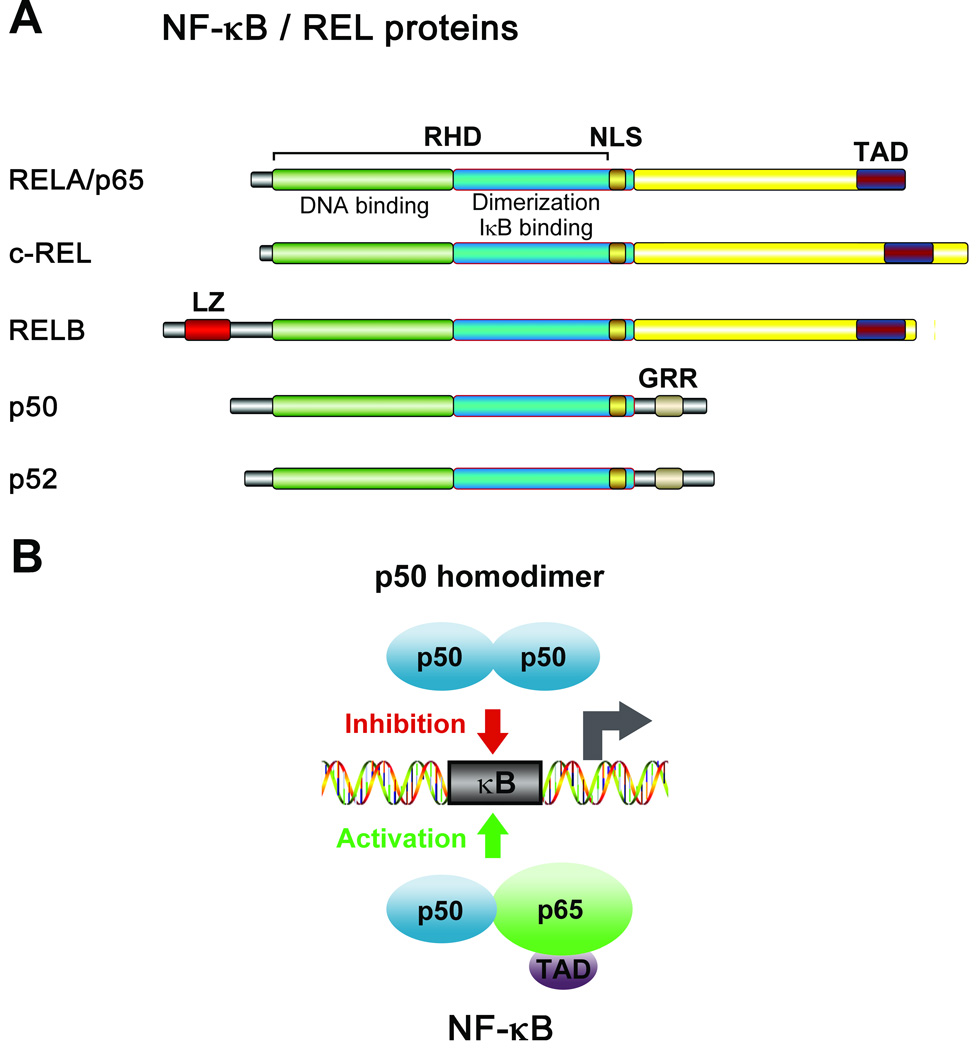
The NF-κB family of TFs (Fig. 1) is best studied for their critical role in the immune function and inflammation (Chen and Greene, 2004; Lin and Karin, 2007). In the brain, NF-κB regulates synaptic plasticity and memory, neuroinflammation, neuronal survival and death (Mattson, 2005; Meffert and Baltimore, 2005; Meffert et al., 2003; O'Neill and Kaltschmidt, 1997). Several findings have implicated NF-κB as mediator of neurotoxic or neuroplastic effects induced by addictive drugs. For example, the NF-κB system has been suggested to mediate the neurotoxic effects of amphetamine derivatives in striatum (Asanuma and Cadet, 1998), and levels of individual NF-κB subunits in the nucleus accumbens (NAc) were found to be altered by chronic cocaine exposure (Ang et al., 2001). A role for NF-κB-induced transcriptional changes has been proposed in the regulation of structural and behavioral plasticity to cocaine in the NAc (Russo et al., 2009). Cocaine-induced NF-κB-dependent gene transcription is speculated to mediate the rewarding effects of cocaine, since inhibiting NF-κB blocks its rewarding effects. Activation of NF-κB is accompanied by elevations in the number of dendritic spines on NAc neurons (Russo et al., 2009).
Fig. 1.
The NF-κB family is composed of five transcription factors, RelA (p65), c-Rel, RelB, p50 and p52 proteins (Chen and Greene, 2004). They are related through an N-terminal, 300 amino acid, DNA binding/dimerization domain, or the Rel homology domain (RHD). Through RHD these proteins form homo- and heterodimers that bind to DNA target elements known as κB sites in the promoter and enhancer gene regions. RelA (p65), c-Rel, RelB proteins are active in the form of heterodimers, while p50 and p52 may also bind to DNA as homodimers. RelA, c-Rel and RelB contain C-terminal transcription activation domain (TAD), which enable them to activate target gene expression. p50 and p52 do not have TAD; therefore, p50 and p52 homodimers repress transcription unless they are bound to a protein containing a TAD, such as RelA, c-Rel or RelB. NLS, a nuclear localization signal. GRR, a glycine rich region is involved in posttranslational processing of the respective precursor proteins. LZ, the leucine zipper is required for transactivation by RelB.
The NF-κB family of transcription factors may play a unique role in alcoholism due to their polyfunctionality, which is associated with their ability to respond to a variety of physiological and pathogenic extracellular signals including stimulation by growth factors, inflammatory intermediates, and glutamate excitotoxicity. In turn, the NF-κB-mediated response is highly contextual depending on the cell type in which this factor is expressed, the constellation of NF-κB/Rel homo- and heterodimers, and on co-factors involved in the epigenetic and/or transcriptional control of NF-κB function. This review focuses on the role of NF-κB in neuronal plasticity, neuroinflammation and neurodegeneration; all of which are relevant for alcohol dependence, abuse, and associated cognitive impairment. Special emphasis is given to the molecular analysis of the NF-κB system in postmortem brains of human alcoholics. This approach is especially important since no single transcription factor from the human CNS has been systematically studied in relation to an addictive disorder. Molecular findings from human autopsy samples support the hypothesis that the NF-κB system is downregulated in brain areas affected by alcohol consumption and withdrawal. Moreover, the downregulation of the NF-κB system appears to be an adaptive mechanism that protects the remaining neurons against alcohol neurotoxicity and neuroinflammation.
2. The NF-κB/Rel family of transcription factors in the brain
Transcription factors of the NF-κB/Rel family are inducible proteins that regulate the expression of genes involved in inflammation, immune responses, and cell survival (Kaltschmidt et al., 2005; Li and Verma, 2002; Mattson, 2005; Yamamoto and Gaynor, 2001). These factors are homo- or heterodimers of p65 (Rel A), p50 and other proteins of the NF-κB/Rel family (Fig. 1). The p65/p50 heterodimer (NF-κB) generally activates gene transcription, while the p50 homodimer represses it (Guan et al., 2005; Li et al., 1994). In most cell types, NF-κB is sequestered in the cytoplasm as part of a complex with inhibitor IκB proteins. Nuclear translocation of NF-κB is induced by multiple extracellular stimuli that trigger activation of an IκB kinase (IKK) complex, which phosphorylates the IκBs leading to their ubiquitination and proteasomal degradation. The released NF-κB migrates to the nucleus to act as a transcription factor. The IKK complex contains the two kinases IKKα and IKKβ and the regulatory subunit NEMO/IKKγ, and functions as integrator of signals regulating NF-κB activity. Transactivating capacity is also regulated in the cell nuclei through phosphorylation of p65 and p50 by IKKβ and other kinases, and p65 by lysine (37/218/221) methylation (Ea and Baltimore, 2009; Ghosh and Karin, 2002; Lu et al., 2010; Moynagh, 2005; Yamamoto and Gaynor, 2004).
The NF-κB TFs had been also identified in the brain where their basal expression levels are higher compared to most peripheral tissues (Bakalkin et al., 1993). The p50/p65 heterodimeric variant of NF-κB is the major κB-binding complex detected in the adult rodent brain (Table 1) (Bakalkin et al., 1993; Kaltschmidt et al., 1993; Meffert et al., 2003; Schmidt-Ullrich et al., 1996) (Fig. 2). In contrast, three complexes, cRel/p65, p50/p65, and p50 homodimers are present in the developing rat brain (Bakalkin et al., 1993). In the adult brain, p65 and p50 NF-κB subunits are abundantly expressed in neurons. A substantial fraction of NF-κB (existing as p50/p65 heterodimers) is located in the cell nucleus and is constitutively active (Kaltschmidt et al., 2005; Kaltschmidt et al., 1993; Meffert and Baltimore, 2005). Constitutive NF-κB activity in glutamatergic neurons of the hippocampus and cerebral cortex (Table 1) (Kaltschmidt et al., 1997; Kaltschmidt et al., 2005; Kaltschmidt et al., 1993) can be suppressed by N-methyl-d-aspartate, and to a lesser extent AMPA, glutamate receptor antagonists, as well as L-type Ca2+ channel blockers (Lilienbaum and Israel, 2003; Meffert et al., 2003). Inducible NF-κB is detected in synapses, while glutamatergic stimulation activates retrograde transport of p65 protein from synapses to the cell nucleus (Kaltschmidt et al., 1993; Meberg et al., 1996; Meffert et al., 2003). Thus, NF-κB is involved in translation of short-lasting synaptic signals to persistent changes in gene expression (Meffert et al., 2003; Wellmann et al., 2001). The phosphorylated forms of IκBα and activated IKK were found within the axon initial segment, the site where action potentials are generated (Schultz et al., 2006), suggesting that NF-κB activation is associated with processing of neuronal information at this site.
Table 1.
NF-κB (p65/p50 heterodimer) and p50 homodimer in the murine and human brain. Activation and localization in neurons and glia.
| Murine brain | Human brain | References | ||
|---|---|---|---|---|
| Dominant form | NF-κB | p50 homodimer >> NF-κB |
(Bakalkin et al., 1993) (Kaltschmidt and Kaltschmidt, 2009) (Okvist et al., 2007) |
|
| Neurons | Glia | |||
| Cytosol | Latent NF-κB |
(Kaltschmidt and Kaltschmidt, 2009) | ||
| Nucleus | NF-κB: a) constitutively active b) transcriptionally silent |
a) (Kaltschmidt and Kaltschmidt, 2009) b) (Mao et al., 2009) |
||
| Synapses | Latent NF-κB | (Kaltschmidt and Kaltschmidt, 2009) | ||
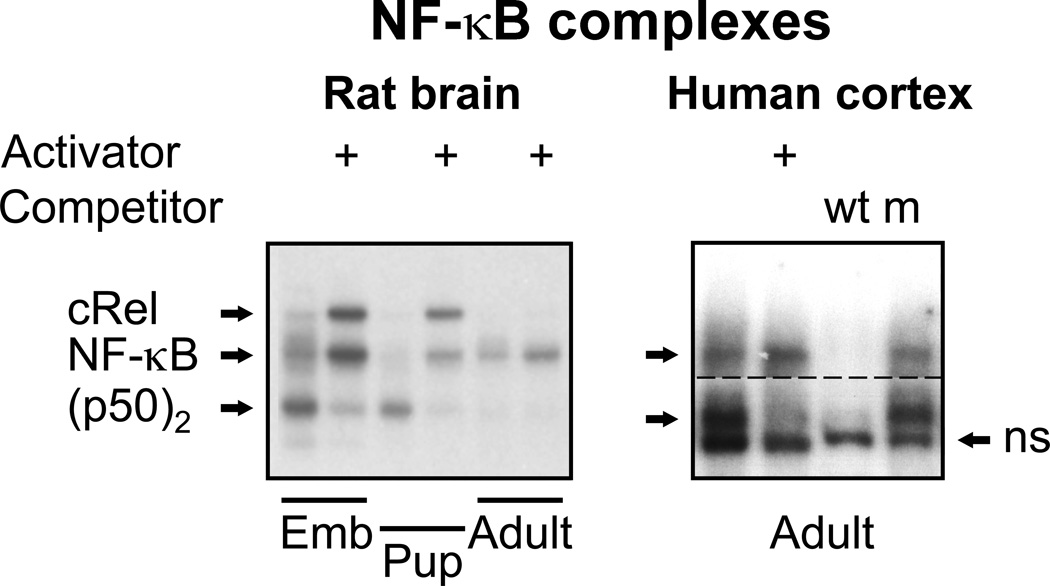
Fig. 2.
κB–binding factors identified by an electromobility shift assay (EMSA) in the brain of 17-day-old rat embryos (Emb), 5-day-old rat pups and adult rats (the left panel), and in the dl- PFC of human male subject (the right panel). The constitutively active κB-DNA binding activity (c) was determined by incubation of tissue extracts with labeled κB – HIV enhancer oligonucleotide. To measure total (t; constitutive plus latent) DNA-binding activity of the NF-κB factors, latent factors were activated by treatment with 0.6% deoxycholate that dissociated IκB inhibitory protein from complexes with NF-κB. The specificity of DNA-protein complexes was assessed by competition with wild type-κB (wt) or mutant-κB (m) oligonucleotides. Three complexes in the rat brain consisted of cRel/p65, NF-κB (p65/p50) and p50 homodimer, respectively. Two upper sequence-specific complexes in the human dl-PFC consisted of NF-κB (p65/p50) and p50 homodimer, respectively. The lower complex (ns) showed no DNA-binding sequence specificity and was likely formed by Ku protein. For the right upper image, film was exposured with a gel for longer time than for the lower one. Figure was reproduced from (Bakalkin et al., 1993) and (Okvist et al., 2007) with modifications and permission from the publishers.
The dominant view that NF-κB is constitutively active in neurons has been challenged by Barger and collaborators (Table 1) (for review, see (Mao et al., 2009)). Analysis of NF-κB and NF-κB-driven transcription in highly purified murine neuronal cultures prompted these investigators to conclude that neurons are incapable to activating the κB element with the traditional Rel-family subunits that comprise NF-κB. They alternatively propose that the activation of κB-mediated transcription in neurons involves SP TFs (SP3 and SP4), while the NF-κB-dependent κB activation that is detected within the brain occurs within glia. In vivo analysis of interactions of the NF-κB TFs with their target neuronal and glial genes using chromatin immunoprecipitation assay might help to resolve the contradiction between the two sets of experimental observations (Kaltschmidt et al., 2005; Kaltschmidt et al., 1993; Mao et al., 2009; Meffert and Baltimore, 2005).
NF-κB mediated alterations in gene transcription may be essential for synaptic plasticity underlying learning and memory formation (Grilli and Memo, 1999; Guerrini et al., 1995; Kaltschmidt et al., 2006; Kaltschmidt et al., 2005; Mattson, 2005; Meberg et al., 1996; Meffert and Baltimore, 2005; Meffert et al., 2003; O'Neill and Kaltschmidt, 1997; O'Riordan et al., 2006). Activation of this TF is apparently required for development of long-term potentiation (LTP) and long-term depression (LTD) (O'Riordan et al., 2006), fear conditioning (Yeh et al., 2004), and spatial memory (Meffert et al., 2003; O'Mahony et al., 2006). In a hierarchical transcriptional network, NF-κB regulates, through its effects on expression of PKA, phosphorylation of CREB that is essential for transduction of synaptic signals into the cell nucleus and eventually for learning and memory (Kaltschmidt et al., 2006). NF-κB has been also identified as regulator of neuronal morphology, including increases in dendritic arborization and axonal outgrowth that are thought to be important in learning and memory acquisition (Gutierrez et al., 2005).
A role in synaptic plasticity was demonstrated in experiments with several lines of genetically modified mice (reviewed in (Kaltschmidt and Kaltschmidt, 2009)). Mice deficient in tumor necrosis factor receptor superfamily member 1A (TNFRI; −/−) or p65 (−/−); and conditional knockouts with neuronal promoter-specific ablation of NF-κB predominantly in glutamatergic neurons in the basal forebrain (CamKII-tTA/tetOtnIκB-α) by expressing the super-repressor IκB-α, all demonstrated impairment in cognitive tests including the radial arm maze or water maze, as well as functional deficits in LTP or LTD (Fridmacher et al., 2003; Kaltschmidt et al., 2006). On the other hand, preferential expression of super-repressor IκB-α (Prion promoter-driven tTA/tetO super-repressor IκB-α) in inhibitory GABAergic interneurons resulted in enhanced spatial learning and memory (Kaltschmidt et al., 2006; O'Mahony et al., 2006). Thus, the change in balance in the excitatory versus inhibitory activity, respectively, of glutamatergic and GABAergic neurons, caused by the overexpression of the NF-κB super-repressor IκB-α in GABA neurons, improves spatial learning and memory.
A number of studies provide evidence that NF-κB activation protects neurons against amyloid β peptide toxicity (Barger et al., 1995) and excitotoxic or oxidative stress (Mattson, 2005; Sarnico et al., 2009). Overexpression of p65 protected cortical neurons against apoptotic cell death, while IκB super repressor or dominant inhibitory NF- κB - inducing kinase (NIK) reduced the survival of cortical neurons (Bhakar et al., 2002).
The role of NF-κB in glia (we use the term “glia” to collectively refer to astroglia and microglia in this review) has been mainly studied in relation to brain injury (for details, see reviews by (Block et al., 2007; Kaltschmidt and Kaltschmidt, 2009), which is not in the focus of this paper. Briefly, in glia of naïve animals NF-κB is present in a latent form (Table 1) (Bhakar et al., 2002; Schmidt-Ullrich et al., 1996) but may be activated under pathological conditions such as exposure to amyloid β peptide (Aβ) leading to the production of nitric oxide (Akama et al., 1998) or exposure to HIV-1 Tat (El-Hage et al., 2008). Responses to injury are triggered by endogenous ligands for Toll-like receptors (TLR) and TLR signaling is mediated through the NF-κB (Akira and Takeda, 2004). Inhibition of astroglial NF-κB signaling leads to reduced chemokine expression and leukocyte entry into the injured CNS (Brambilla et al., 2005; Khorooshi et al., 2008) indicating the regulatory effect of astrocytes on immune response (Farina et al., 2007). Microgliosis is a common phenomenon in neurodegenerative disorders and NF-κB plays a central role in microglia activation. Microglial activation is underscored by the release reactive oxygen species and the production of proinflammatory cytokines that cause secondary neurotoxicity. This activation involves NF-κB, which is a key transcriptional regulator of proinflammatory cytokines such as TNF-α, IL-1β, and interferon-γ (Block et al., 2007).
3. Alcohol addiction and neurotoxicity
3.1. Animal studies
Acute or short-term ethanol administration has been demonstrated to activate the NF-κB system in the brain, and this in turn triggers the expression of TNF-α, as well as other proinflammatory cytokines and NF-κB-regulated genes (Crews et al., 2006b; Rulten et al., 2006; Ward et al., 1996; Zou and Crews, 2006, 2010). In these experiments, rats received a single intraperitoneal (i.p.) 2 g/kg ethanol dose (Ward et al., 1996), or intragastric administration of ethanol, 8–12 g/kg/day, 3 times per day for 4 days (Crews et al., 2006b; Zou and Crews, 2006, 2010); or mice were given a single i.p. 2.5 g/kg ethanol dose (Rulten et al., 2006). Analysis of whole mouse brain transcriptomes by DNA microarrays consistently identify the NF-κB pathway as involved in the acute response to ethanol (Rulten et al., 2006). While both NF-κB function and RelA expression were upregulated shortly after ethanol administration (Rulten et al., 2006), the increase appears to be transient since it is not sustained following sub-chronic ethanol treatment (a 9% ethanol-containing liquid diet for 15 days) (Mittal et al., 1999).
Although cellular and molecular mechanisms by which alcohol causes brain damage are not fully understood, numerous studies suggest a role of inflammatory processes (Altura et al., 2002; Blanco et al., 2005; Davis and Syapin, 2004; Lee et al., 2004; Martinez et al., 1992; Minambres et al., 2006; Nelson et al., 1989; Valles et al., 2004). At the transcription level, inflammation is primarily regulated by NF-κB and related factors in a variety of cells and tissues. The hallmark of neuroinflammation is the activation of glia and the production of cytokines and inflammatory mediators that can trigger neuronal damage (Minghetti, 2005; Ubogu et al., 2006). Sub-chronic alcohol administration upregulates inflammatory mediators in the rat brain, which includes key neuroinflammatory signaling processes in astrocytes (Altura et al., 2002; Blanco et al., 2005). These processes are initiated by signaling through TLR4/type I IL-1R, which plays roles in the innate host defense against microbial infections, inflammation and injury. This is followed by activation of NF-κB and the upregulation of inducible NO synthase and cyclooxygenase-2 expression. In glial cells, TLR4 is critical for an ethanol-induced inflammatory response (Alfonso-Loeches et al., 2010). Inactivation of TLR4 by siRNA or gene deletion abolished ethanol-induces activation of NF-κB and microtubule-associated protein kinase pathways, and the production of inflammatory mediators in astrocytes. Whereas ethanol exposure upregulates the levels of microglial (CD11b) and astrocyte (glial fibrillary acidic protein) markers, inducible COX-2, interleukin (IL)-1β, IL-6 and TNF-α in the cerebral cortex of wild-type mice, TLR4 ablation protects against ethanol-induced glial activation, induction of inflammatory mediators, and apoptosis. These findings support of the concept that a TLR4-driven NF-κB-mediated response underlies the neuroinflammation, brain injury, and neurodegeneration caused by ethanol exposure.
Increases in NF-κB DNA binding activity induced by ethanol treatment correlate with the increased expression of proinflammatory genes in hippocampal-entorhinal cortex slice cultures (Zou and Crews, 2010). Blockade of NF-κB activation by p65 siRNA or the antioxidant butylated hydroxytoluene, reduces the induction of proinflammatory TNF-α, MCP-1, IL-1β, protease TACE, tissue plasminogen activator (tPA) and inducible nitric oxide synthase by ethanol. In an in vivo pharmacological study, butylated hydroxytoluene treatment prevented both damage to corticolimbic brain areas and reductions in neurogenesis induced by binge-like ethanol administration (Crews et al., 2006a; Crews et al., 2006b). Ethanol treatment also increased the DNA binding activity of NF-κB, COX2 expression, and microglial activation, while antioxidant administration caused the opposite effects in animals. This correlative evidence supports the hypothesis that binge ethanol-induced brain damage involves a neuroinflammatory mechanism that is under control of NF-κB regulated transcription.
Besides brain-derived proinflammatory factors, neuroinflammation can be promoted by serum-derived factors, such as TNF-α and other cytokines, originating from increases in systemic and hepatic inflammation caused by heavy alcohol drinking (Crews et al., 2006b; Wang et al., 2010). Serum cytokines may enter the brain where they activate microglia and astroglia. TNF-α acting through the NF-κB system can inhibit glutamate transporters. Consequently, the excess glutamate, which may attain excitotoxic levels, reportedly contributes to increased drinking and neurodegeneration (Crews et al., 2006b). Altogether, these observations suggest that alcohol effects on immune and inflammatory responses in the brain are mediated through NF-κB, and NF-κB-controlled genes.
In contrast to adult animals, NF-κB may be neuroprotective in the developing brain (Bonthius et al., 2009). Alcohol-induced damage to the developing brain leads to fetal alcohol syndrome; however, mature neurons while maturation of neurons results in acquisition of resistance to ethanol. This process apparently involves the protective NO-cGMP-cyclic GMP-dependent protein kinase pathway G (NO-cGMP-PKG). NF-κB was found as a downstream effector through which the NO-cGMP-PKG pathway signals its neuroprotective effects against alcohol in immature cerebellar granule neurons in culture. Thus, NF-κB – regulated gene transcription may be required for the acquisition of alcohol resistance by maturing neurons.
A role for the NF-κB system in the regulation of ethanol consumption is underscored by meta-analysis of the transcriptome of experimental animals (Mulligan et al., 2006). A comparison of alcohol-preferring and non-preferring mice showed a marked overrepresentation of gene networks associated with NF-κB activation in alcohol-preferring mice suggesting that NF-κB regulates a variety of transcripts involved in establishing a high level of voluntary alcohol drinking.
3.2. Human studies
3.2.1. Association of NFKB1 / p50 with alcoholism
Human genetic and postmortem molecular studies highlight the importance of NF-κB in alcohol dependence and toxicity (Edenberg et al., 2008; Flatscher-Bader et al., 2005; Okvist et al., 2007). A seminal finding is that polymorphisms in the nuclear factor of κ-light polypeptide gene enhancer in B-cells 1/the p50 protein precursor gene (NFKB1) are highly correlated with an increased risk for alcoholism in a family-based association study (Edenberg et al., 2008). NFKB1 is located on chromosome 4q, which is linked to alcohol dependence, and the association is much stronger in individuals with an early onset of alcoholism. The findings prompt speculation that there may be other important genetic variants that affect expression of NFKB1 and NF-κB/p50-regulated genes and influence alcohol dependence and toxicity in humans.
3.2.2. NF-κB in the brain of human alcoholics
Alcohol abuse and dependence are associated with widespread changes in gene expression in several brain areas including the dl-PFC and striatum in humans (Alexander-Kaufman et al., 2006; Flatscher-Bader et al., 2005; Lewohl et al., 2000; Liu et al., 2004). The differentially expressed genes are implicated in immune responsiveness, cell survival, inflammation, and signal transduction. Pronounced differences have been found in genes involved in myelination, apoptosis and neurogenesis.
Re-programming gene expression in alcoholics is hypothesized to involve TFs of the NF-κB family. This hypothesis was addressed by analyzing postmortem specimens from the dl-PFC, a region that is involved in alcohol dependence and cognitive processes, and displays functional and histopathological deficits in human alcoholics. The motor cortex (MC), which is not engaged in these processes and less affected by alcohol (Kril et al., 1997; Kril and Harper, 1989), was analyzed as a control area (Okvist et al., 2007). The principal findings are that the DNA-binding of NF-κB and p50 homodimer, as well as RELA (p65) expression, are downregulated in the dl-PFC of alcoholics (Fig. 3) (Okvist et al., 2007). Importantly, the p50 homodimer is the dominant κB-binding factor in the human brain while NF-κB predominates over other members of this family in the adult rodent brain (Fig. 2). The stoichiometry between RELA, NFKB1, and IKKβ mRNA and between respective p65, p50, and IKKβ proteins is also altered. No significant differences between controls and alcoholics were evident in the MC suggesting that NF-κB-mediated adaptive mechanisms differ between brain areas. Downregulation of NF-κB DNA-binding in alcoholics may result from the decrease in RELA expression or from alterations in stoichiometry in p65, p50 and IKKβ proteins (Fig. 3). In addition, NF-κB DNA-binding and transactivation functions in alcoholics may be attenuated through alterations in posttranslational modifications of NF-κB proteins. These alterations may include phosphorylation at several sites by the IKK complex, or reversible lysine methylation (37/218/221) (Ea and Baltimore, 2009; Ghosh and Karin, 2002; Lu et al., 2010; Yamamoto and Gaynor, 2004; Zhong et al., 2002). While p50 homodimer-DNA binding was downregulated in alcoholics, no changes in p50 protein levels were evident suggesting the effects of ethanol may be mediated by post-translational modifications (Fig. 3). p50 phosphorylation by protein kinase A and other kinases regulates binding of the p50 homodimer to DNA, which results in transcriptional repression of NF-κB dependent genes (Guan et al., 2005; Li et al., 1994).
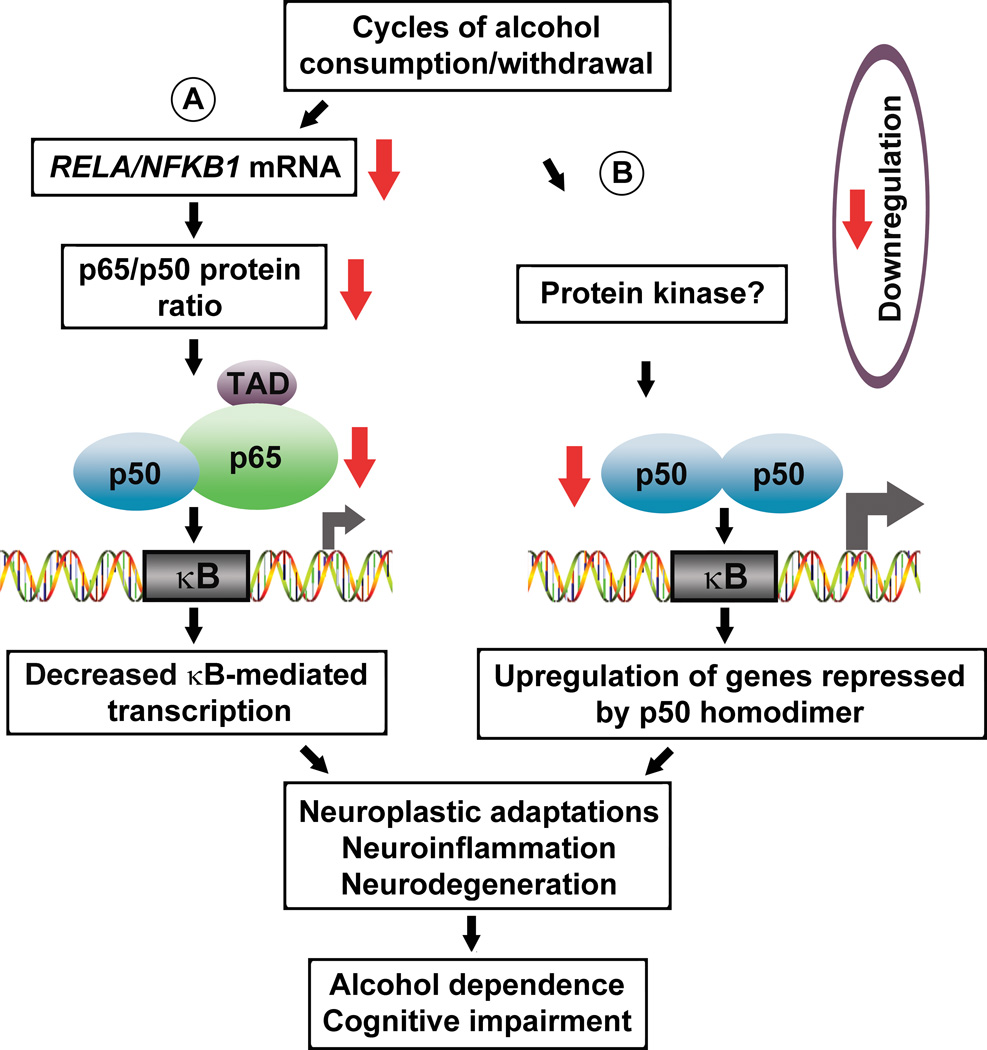
Fig. 3.
Model for molecular adaptations in NF-κB and p50 homodimer induced by cycles of alcohol consumption and withdrawal in the dl-PFC of human alcoholics (Okvist et al., 2007). (A) Chronic alcohol intake inhibits RELA expression and induces alterations in stoichiometry of RELA/NFKB1 mRNAs and p65/p50 proteins, resulting in downregulation of NF-κB DNA-binding. In cells with dominant NF-κB this adaptation results in inhibition of NF-κB-dependent transcription. (B) Alcohol may act upstream of an unidentified protein kinase that modulates DNA-binding of the p50 homodimer leading to hypophosphorylation of p50 and downregulation of p50 homodimer DNA-binding. The p50 homodimer is dominant κB – binding factor in human brain. In control individuals, the p50 homodimer inhibits gene transcription acting through κB - binding sites because it prevails over NF-κB. In alcoholics, downregulation of the p50 homodimer eliminates transcriptional repression leading to upregulation of basal or κB-mediated transcription. Dysregulation of the NF-κB/p50 homodimer-dependent gene transcription contributes to neuroplastic adaptations, neuroinflammation and neurodegeneration. Figure was reproduced from (Okvist et al., 2007) with modifications and permission from the publisher.
Alcohol withdrawal results in neuronal excitation (Fadda and Rossetti, 1998; Grant and Lovinger, 1995; Grant et al., 1990; Mittal et al., 1999) through activation of glutamate receptors and calcium influx, which in turn activate NF-κB (Grilli and Memo, 1999; Guerrini et al., 1995; Kaltschmidt et al., 2005; Mattson, 2005; Meberg et al., 1996; Meffert and Baltimore, 2005; Meffert et al., 2003; O'Neill and Kaltschmidt, 1997). Cycles of alcohol intoxication and withdrawal initially activate NF-κB; however, when repeated chronically for years, may lead to adaptive changes in the NF-κB system such that the system better tolerates excessive stimulation. One of the adaptive processes (Fig. 3) is the downregulation of the DNA-binding activity of the p50 homodimer, a dominant κB-binding factor (Fig. 2). This may result in a derepression of genes that possess κB-elements in their promoters. These types of adaptive changes, which limit the amount of responsiveness to chronic stimulation (due to low NF-κB activity) while stabilizing basal κB-mediated transcription (due to low p50 homodimer activity), could potentially be exploited as compensatory response that protects brain cells against alcohol neurotoxicity.
In the brain, this adaptation may differ mechanistically from that observed in the immune system (Grundstrom et al., 2004; Hajishengallis and Genco, 2004), which is based on the over-expression of the inhibitory p50 subunit (Biswas and Lopez-Collazo, 2009). The immune system has ability to i) downregulate excessive inflammatory reactions that can damage tissues, and to ii) inhibit excessive immune responses involving T cells (Biswas and Lopez-Collazo, 2009; Grundstrom et al., 2004; Hajishengallis and Genco, 2004; Irla et al., 2010). Prior exposure of monocytic cells to LPS results in a transient repression proinflammatory cytokine responsiveness to a subsequent LPS challenge that is termed “endotoxin tolerance”. T cell tolerance is characterized by a cessation in cell proliferation and diminished expression of Th1 cytokines. The NF-κB system is involved in these processes. In particular, the upregulation of the p50 homodimer, which represses NF-κB-dependent transcription, appears to regulate both endotoxin tolerance and T cell tolerance.
Animal studies demonstrate that acute ethanol administration increases NF-κB DNA-binding activity, while three weeks of ethanol treatment downregulates NF-κB (Rulten et al., 2006; Ward et al., 1996). These data and human postmortem observations are in an apparent contradiction. To resolve it, we may propose that the phase of initial activation of the NF-κB system after acute ethanol administration is followed by the chronic phase when the system is downregulated. Data on acute effects of alcohol on NF-κB in human brain are not available. Even if several first episodes of ethanol intake could activate the NF-κB system, mechanisms of this activation and following repression in humans may be different from those in rodents due to differences in the constellation of NF-κB / c-Rel subunits in the brain.
The hypothesis on activation of the protective mechanisms and inhibition of cell death pathways is supported by human molecular findings. Thus, several components of intrinsic apoptotic pathway including activated caspase-3, the key pro-apoptotic protein, along with both pro-apoptotic PDCD8 (apoptosis-inducing factor, AIF) and BID, were found to be decreased in the dl-PFC of alcoholics (Johansson et al., 2009). No differences were found in the motor cortex. Consistently, the up-regulation of several neuroprotective genes was found in the dl-PFC in human alcoholics (Flatscher-Bader et al., 2005; Okvist et al., 2007). It has been demonstrated that activated caspase-3 and AIF play a critical role in the execution of both neuronal apoptotic and necrotic pathways (Niquet et al., 2003; Niquet et al., 2006; Young et al., 2005). Thus downregulation of these two proteins in the PFC of alcoholics may prevent or delay cell death that may occur by apoptosis or necrosis. Adaptations in regulated cell death pathways could limit the extent of alcohol-induced brain damage, thus protecting cognitive and other dl-PFC functions in alcoholics. Such adaptations may be developed after initial activation of cell death pathways resulting in gross loss of gray and white matter during the first years of heavy alcohol drinking. Albeit this hypothesis should be considered with a caution as based on correlative molecular data. Thus, the possibility that molecular changes identified may contribute to the shift from the apoptotic to necrotic pathway of cell death in the dl-PFC cannot be ruled out.
3.2.3. NF-κB / p50 homodimer – mediated regulation of genes differentially expressed in alcoholics
The ability to negatively regulate NF-κB target genes is essential for maintaining cellular homeostasis. Not surprisingly, the sustained activation of NF-κB is associated with several inflammatory diseases (Guan et al., 2005; Rao et al., 2010; Smale, 2010; Yamamoto and Gaynor, 2001). The p50 homodimer is important in maintaining homeostasis through its ability to suppress the transactivation activities of NF-κB by occupying the same κB sites and recruiting corepressor complexes containing histone deacetylases (HDAC) (Hoberg et al., 2006). Following stimulation, NF-κB displaces DNA-bound p50 / HDAC.
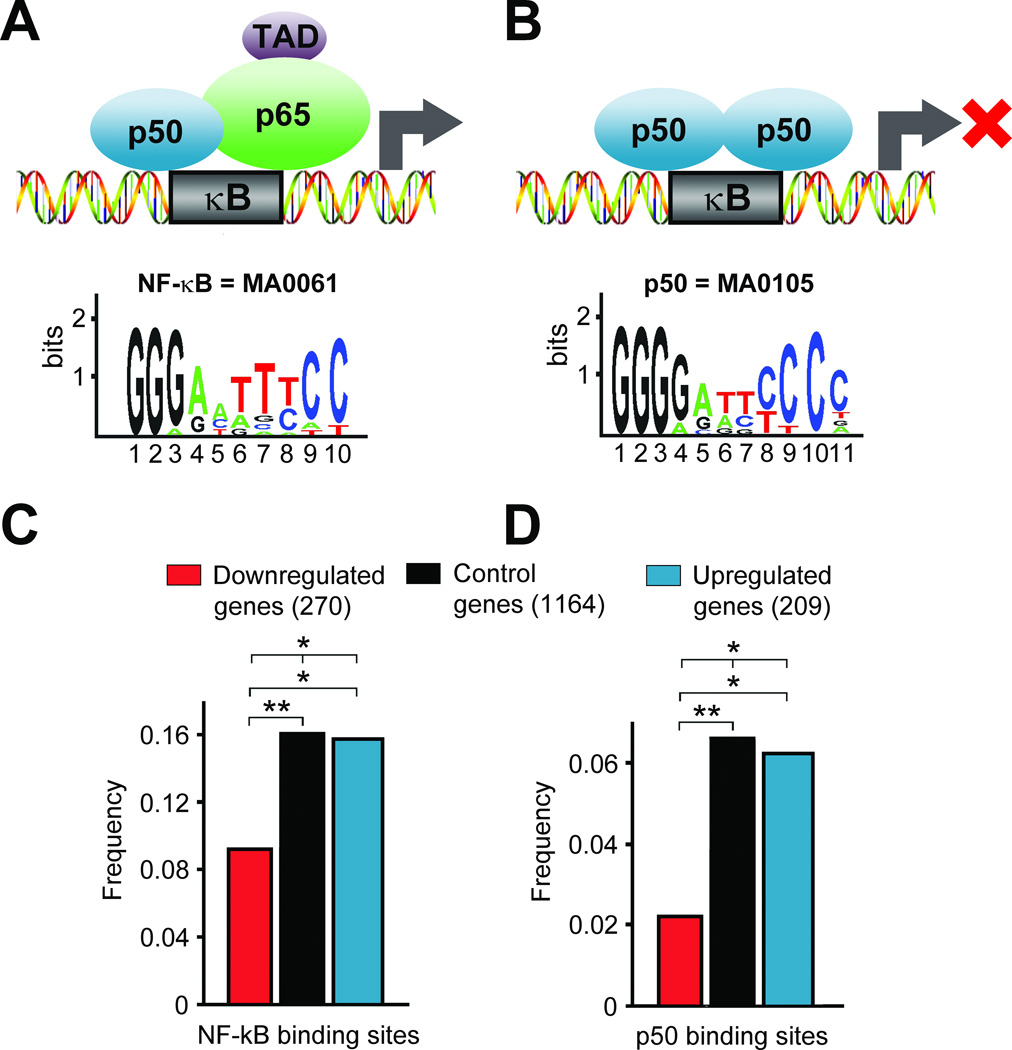
Downregulation of DNA-binding of the p50 homodimer and NF-κB in alcoholics may result in changes in expression of genes regulated through κB elements. This hypothesis was tested using a computational approach that compared the number of genes containing κB elements between sets of genes downregulated and upregulated in the dl-PFC of alcoholics (Liu et al., 2006). p50 binds to κB sites as a homodimer and therefore has a slightly higher affinity for symmetric κB elements compared to NF-κB (Chen-Park et al., 2002; Chen and Greene, 2004), which is reflected in the composition of the κB matrix of the “p50 homodimer”-subtype present in the JASPAR database (Fig. 4). Analysis of genes differentially expressed in the dl-PFC of alcoholics demonstrated that 58 genes contain one or more putative κB binding sites for the p50 homodimer and NF-κB (the “NF-κB”-subtype), and 19 genes contain at least one putative “p50 homodimer”-subtype κB binding site (Okvist et al., 2007). Computational analysis showed significant differences in the frequency of genes with each “NF-κB”- or “p50 homodimer”- subtype of κB elements between the up- and down regulated genes and a control set of genes. In particular, the frequency of each binding site subtype in the downregulated genes was lower compared to the upregulated genes and control genes (Fig. 4) (Okvist et al., 2007). Thus, genes with κB elements were generally upregulated in the dl-PFC in alcoholics. The upregulated genes may be derepressed due to decreased activity of the p50 homodimer, which inhibits transcription and is the dominant κB binding factor in human brain.
Fig. 4.
κB elements in genes differentially expressed in alcoholics (Okvist et al., 2007). Matrices from the JASPAR database were used to identify “NF-κB” (A) and “p50 homodimer” (B) subtypes of κB binding sites. Both NF-κB (p65/p50 heterodimer) and p50 homodimer bind to the “NF-κB” subtype of κB elements with high affinity and specificity, whereas p50 homodimer has higher affinity for more symmetric “p50 homodimer”-subtype of κB binding sites (Chen-Park et al., 2002). The matrix logos provide visual representation profiles of nucleotide conservation in κB elements. The maximal conservation amounts are 2 bits for each position. Higher conservation indicates increased biological importance for a base. C, D. Frequencies of occurrence of the “NF-κB”- (C) and “p50 homodimer”- (D) subtypes of κB binding sites in 270 and 209 genes downregulated and upregulated in alcoholics, respectively, and in 1164 control genes studied for comparison (Okvist et al., 2007). Both the group of differentially expressed genes (270 and 209 genes), and the group of control 1164 genes randomly selected from the set, which did not include genes differentially expressed in alcoholics, were taken from (Liu et al., 2006). Significance of differences between upregulated, downregulated and control genes in the frequency of occurrence of genes with κB elements was evaluated with Chi-square test for pair-wise comparison; ** P<0.01, * P < 0.05. Figure was reproduced from (Okvist et al., 2007) with modifications and permission from the publisher.
Expression of several NF-κB-driven genes, for example the murine chemokine keratinocyte chemoattractant (KC) may require stabilization of the constitutively unstable mRNA (Hartupee et al., 2008). To date, dependence of the levels of short-lived mRNAs in brain on postmortem interval, RNA integrity number, and brain pH has not been systematically addressed. Because these mRNAs may rapidly degrade during postmortem period, NF-κB dependent genes identified by the genome-wide scan may be limited to abundant and/or “stable” mRNAs that still produce a reliable microarray signal, and, consequently, a number of NF-κB dependent genes may be underestimated in the computational analysis (Okvist et al., 2007).
Downregulation of p50 homodimers may allow the recruitment of NF-κB if this TF is present within the cell in a transcription competent form and in amounts sufficient to activate transcription from κB elements. Alternatively, the removal of co-repressor complexes targeted to κB sites by the p50 homodimer could result in derepression of basal gene transcription that involves other transcription factors besides NF-κB. Indeed, NF-κB cooperates with SP1, nuclear receptors, or AP-1 for binding to both overlapping or non-overlapping target DNA elements (Hirano et al., 1998). It was demonstrated that the interaction of SP1 with κB elements keeps basal expression of NF-κB-dependent genes at the elevated levels; whereas, the p50 homodimer displaces SP1 from these sites and thereby inhibits basal SP1-driven expression (Hirano et al., 1998). In contrast, the downregulation of NF-κB in alcoholics may lead to decreased transcription of genes that are normally activated by NF-κB.
In the human brain, κB-driven transcription may be regulated not only by competitive binding between transcriptionally active NF-κB and repressive p50 homodimers, but by the modulation of inhibitory and transactivation potential of the p50 homodimer, a dominant κB-binding complex (Fig. 2). This modulation may be performed through recruitment of several IκB proteins including BCL-3, IκBζ and IκBNS, which have been identified as pleiotropic p50 cofactors (reviewed in (Ghosh and Hayden, 2008)). In contrast to other IκB proteins, BCL-3 has a transactivation domain, and in complex with a p50 homodimer may facilitate or inhibit κB-mediated transcription depending on its posttranscriptional modifications. The formation of transcriptionally active BCL-3 – p50-p50 complexes requires phosphorylation or ubiquitylation of BCL-3 protein. BCL-3 may also stabilize repressive p50 homodimers thereby preventing the binding of transcriptionally active NF-κB. Similarly to BCL-3, IκBζ associates with the p50 homodimer, and selectively inhibits or activates κB-driven transcription. A role of these cofactors in the human brain has not yet been studied.
Bioinformatics searches for NF-κB/p50 homodimer target genes identified nine potential NF-κB/p50 targets, and seven genes associated with the NF-κB/p50 pathway within a set of genes that were differentially expressed in alcoholics (Okvist et al., 2007). The upregulated genes included the death-associated protein 6 (DAXX), which is involved in life-or-death processes and in spatial learning. DAXX acts as an anti-apoptotic regulator through its ability to repress gene transcription activated by NF-κB / RelB (Michaelson and Leder, 2003; Puto and Reed, 2008). Daxx may control epigenetic silencing of RelB by DNA methylation through recruitment of DNA methyl transferase 1 to RelB DNA-target sites resulting in synergistic repression (Puto and Reed, 2008). Several other genes relevant for alcohol dependence and neurotoxicity have also been identified as NF-κB targets. They include K-ras, a regulator of alcohol intake discovered in genome-wide gene expression analysis (Repunte-Canonigo et al., 2010), and the opioid receptor and opioid peptide precursor genes including proenkephalin and prodynorphin, which play a role in addictive and cognitive processes (Bakalkin et al., 1994; Chen et al., 2006; Rattner et al., 1991).
3.2.4. NF-κB - ΔFosB interplay in addicted brain
It has been proposed that NF-κB may crosstalk with other TFs that regulate addictive behavior. In animal studies, upregulation of ΔFosB, a product of the Fosb gene, is induced by chronic administration of virtually all drugs of abuse and alcohol. The elevated levels persist for several weeks and ΔFosB reportedly can trigger the sustained expression of a variety of genes associated with substance addiction. Accordingly, ΔFosB has been hypothesized to have a direct role of in the maintenance of addiction (Ehrlich et al., 2002; Hiroi et al., 1997; Hope et al., 1994; Nye and Nestler, 1996; Pich et al., 1997). NF-κB was identified by microarray screening as a ΔFosB target in the NAc after chronic cocaine treatment using ΔFosB-overexpressing mice (Ang et al., 2001). By analogy, ΔFosB may potentially regulate NF-κB in human alcoholics. However, emerging evidence suggests that this is unlikely: ΔFosB is produced at very low levels in human brain and, more importantly, ΔFosB does not show any changes in the levels of expression in the brains of human alcoholics (Watanabe et al., 2009). Thus, ΔFosB may not be involved in the maintenance of the addictive state, at least not in alcohol dependence in humans.
4. Conclusions
A remarkable number of the short-term responses and long-term adaptive changes to alcohol are regulated by the NF-κB family of TFs suggesting that they are pivotal in mediating the response of the brain to ethanol. Not only do NF-κB and p50 homodimers respond to acute and chronic ethanol exposure including activation by glutamate, cytokines and oxidative stress, but these TFs regulate synaptic plasticity, neuronal survival and neuroinflammation - the neurobehavioral substrate(s) underlying alcohol addiction (Grilli and Memo, 1999; Guerrini et al., 1995; Kaltschmidt and Kaltschmidt, 2009; Kaltschmidt et al., 2006; Kaltschmidt et al., 2005; Li and Verma, 2002; Meffert et al., 2003; Moynagh, 2005; O'Riordan et al., 2006). Induction of oxidative stress by alcohol, and alcoholism-associated alterations in the expression of inflammatory, cell survival and myelin genes (Alexander-Kaufman et al., 2006; Farris et al., 2010; Flatscher-Bader et al., 2005; Lewohl et al., 2000; Liu et al., 2004) regulated by NF-κB (Huang et al., 2002; Kaltschmidt et al., 2005; Li and Verma, 2002; Mattson, 2005; Nickols et al., 2003) corroborate this notion.
The importance of NF-κB in regulating the response of the brain to alcohol is supported by animal studies demonstrating that acute ethanol administration activates NF-κB and NF-κB-regulated transcription of TNF-α, proinflammatory cytokines, and other genes in the brain (Crews et al., 2006a; Crews et al., 2006b; Rulten et al., 2006; Ward et al., 1996; Zou and Crews, 2006, 2010). The NF-κB - mediated response may be critical for neuroinflammation and neurodegeneration induced by ethanol exposure in rodents. Cellular and molecular mechanisms involved in these ethanol-dependent NF-κB-driven processes are now under investigation in several laboratories. In contrast, a role of the NF-κB system in the regulation of alcohol consumption and in the cognitive control of alcohol seeking and drinking has attracted minimal attention. The mouse genetic models utilized to study the role of NF-κB in learning and memory (reviewed in (Kaltschmidt and Kaltschmidt, 2009; Meffert and Baltimore, 2005), have not yet been used in the study of alcoholism.
NF-κB is a prevailing form of κB binding protein in the adult rodent brain, while in adult human brains the inhibitory p50 homodimer strongly predominates over NF-κB (Fig. 2). The distinction suggests that the regulation of κB-mediated transcription and neurodegeneration may differ between humans and rodents. This assumption imposes limitations on the extent to which information gained on the role of the NF-κB / p50-mediated transcription in rodent models can be generalized to human neuropsychiatric disorders such as alcohol dependence and associated cognitive impairment. Specifically, these constraints may limit the applicability of animal gene expression data for construction of human molecular models of both alcohol dependence, and transcriptional tolerance to repeated effects of ethanol. It would be essential to compare the role of the κB-mediated transcription in alcohol-induced neurodegeneration and neuroinflammation in human and rodent brain cells.
Human genetic analysis demonstrated an association of polymorphisms in the NFKB1 (p50) gene with alcohol dependence (Edenberg et al., 2008), while molecular analysis of human brain identified a downregulation of the NF-κB/p50 homodimer system in the dl-PFC, an area affected by alcohol exposure in human brain (Okvist et al., 2007). These adaptations in NF-κB/p50 may underlie a shift to a new “steady-state” or “allostatic level” (Koob, 2003), caused by persistent alterations in expression of target genes relevant to alcohol-dependent state. Multiple mechanisms may assure the stability of these alterations. Hypothetically, these could involve (i) the feedback loop between NF-κB and IκB (Kearns et al., 2006); (ii) feed-back and feed-forward NF-κB/p50 interactions with other transcription factors such as DAXX (Michaelson and Leder, 2003; Puto and Reed, 2008), glucocorticoid receptors (Hirano et al., 1998; Rosenfeld et al., 2006), SP1 (Hirano et al., 1998; Teferedegne et al., 2006), or CREB (Kaltschmidt et al., 2006); and (iii) epigenetic regulations (Natoli, 2009; Rao et al., 2010). Allostasis in the NF-κB system may ensure tolerance to excessive ethanol stimulation and that protects brain cells against chronic, repeated exposure to ethanol. Downregulation of the inhibitory p50 homodimer is likely to contribute to the enhanced expression of genes regulated through κB elements in alcoholics. NF-κB and p50 homodimers are apparently principal but not sole mediators of cellular and behavioral effects of ethanol. They may act in cooperation with other transcription factors including CREB (Kaltschmidt et al., 2006) thus constituting gene regulatory networks that determine the course of both dependence to alcohol and associated impairment of cognitive functions (Farris et al., 2010) through controlling neuroplastic changes, neuroinflammation and neurodegeneration.
Acknowledgments
This work was supported by a National Institute on Drug Abuse Independent Scientist Award (K02 DA027374) (KH) and grants from the Swedish Council for Working Life and Social Research (FAS), AFA Forsäkring and the Swedish Science Research Council to GB.
Footnotes
The authors declare no conflict of interest.
References
- Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Arch Gen Psychiatry. 1999;56:356–363. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- Akama KT, Albanese C, Pestell RG, Van Eldik LJ. Amyloid beta-peptide stimulates nitric oxide production in astrocytes through an NFkappaB-dependent mechanism. Proc Natl Acad Sci U S A. 1998;95:5795–5800. doi: 10.1073/pnas.95.10.5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Takeda K. Functions of toll-like receptors: lessons from KO mice. C R Biol. 2004;327:581–589. doi: 10.1016/j.crvi.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Alexander-Kaufman K, James G, Sheedy D, Harper C, Matsumoto I. Differential protein expression in the prefrontal white matter of human alcoholics: a proteomics study. Mol Psychiatry. 2006;11:56–65. doi: 10.1038/sj.mp.4001741. [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci. 2010;30:8285–8295. doi: 10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altura BM, Gebrewold A, Zhang A, Altura BT. Role of leukocytes in ethanol-induced microvascular injury in the rat brain in situ: potential role in alcohol brain pathology and stroke. Eur J Pharmacol. 2002;448:89–94. doi: 10.1016/s0014-2999(02)01899-x. [DOI] [PubMed] [Google Scholar]
- Ang E, Chen J, Zagouras P, Magna H, Holland J, Schaeffer E, Nestler EJ. Induction of nuclear factor-kappaB in nucleus accumbens by chronic cocaine administration. J Neurochem. 2001;79:221–224. doi: 10.1046/j.1471-4159.2001.00563.x. [DOI] [PubMed] [Google Scholar]
- Asanuma M, Cadet JL. Methamphetamine-induced increase in striatal NFkappaB DNA-binding activity is attenuated in superoxide dismutase transgenic mice. Brain Res Mol Brain Res. 1998;60:305–309. doi: 10.1016/s0169-328x(98)00188-0. [DOI] [PubMed] [Google Scholar]
- Aschner M, Allen JW. Astrocytes in methylmercury, ammonia, methionine sulfoximine and alcohol-induced neurotoxicity. Neurotoxicology. 2000;21:573–579. [PubMed] [Google Scholar]
- Bakalkin G, Yakovleva T, Terenius L. NF-kappa B-like factors in the murine brain. Developmentally-regulated and tissue-specific expression. Brain Res Mol Brain Res. 1993;20:137–146. doi: 10.1016/0169-328x(93)90119-a. [DOI] [PubMed] [Google Scholar]
- Bakalkin G, Yakovleva T, Terenius L. Prodynorphin gene expression relates to NF-kappa B factors. Brain Res Mol Brain Res. 1994;24:301–312. doi: 10.1016/0169-328x(94)90143-0. [DOI] [PubMed] [Google Scholar]
- Barger SW, Horster D, Furukawa K, Goodman Y, Krieglstein J, Mattson MP. Tumor necrosis factors alpha and beta protect neurons against amyloid beta-peptide toxicity: evidence for involvement of a kappa B-binding factor and attenuation of peroxide and Ca2+ accumulation. Proc Natl Acad Sci U S A. 1995;92:9328–9332. doi: 10.1073/pnas.92.20.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty WW, Hames KA, Blanco CR, Nixon SJ, Tivis LJ. Visuospatial perception, construction and memory in alcoholism. J Stud Alcohol. 1996;57:136–143. doi: 10.15288/jsa.1996.57.136. [DOI] [PubMed] [Google Scholar]
- Bhakar AL, Tannis LL, Zeindler C, Russo MP, Jobin C, Park DS, MacPherson S, Barker PA. Constitutive nuclear factor-kappa B activity is required for central neuron survival. J Neurosci. 2002;22:8466–8475. doi: 10.1523/JNEUROSCI.22-19-08466.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Blanco AM, Valles SL, Pascual M, Guerri C. Involvement of TLR4/type I IL-1 receptor signaling in the induction of inflammatory mediators and cell death induced by ethanol in cultured astrocytes. J Immunol. 2005;175:6893–6899. doi: 10.4049/jimmunol.175.10.6893. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, Luong T, Bonthius NE, Hostager BS, Karacay B. Nitric oxide utilizes NF-kappaB to signal its neuroprotective effect against alcohol toxicity. Neuropharmacology. 2009;56:716–731. doi: 10.1016/j.neuropharm.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Bowden SC, McCarter RJ. Spatial memory in alcohol-dependent subjects: using a push-button maze to test the principle of equiavailability. Brain Cogn. 1993;22:51–62. doi: 10.1006/brcg.1993.1024. [DOI] [PubMed] [Google Scholar]
- Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, Green EJ, Bethea JR. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med. 2005;202:145–156. doi: 10.1084/jem.20041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Chen-Park FE, Huang DB, Noro B, Thanos D, Ghosh G. The kappa B DNA sequence from the HIV long terminal repeat functions as an allosteric regulator of HIV transcription. J Biol Chem. 2002;277:24701–24708. doi: 10.1074/jbc.M200007200. [DOI] [PubMed] [Google Scholar]
- Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- Chen YL, Law PY, Loh HH. Nuclear factor kappaB signaling in opioid functions and receptor gene expression. J Neuroimmune Pharmacol. 2006;1:270–279. doi: 10.1007/s11481-006-9028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F, Nixon K, Kim D, Joseph J, Shukitt-Hale B, Qin L, Zou J. BHT blocks NF-kappaB activation and ethanol-induced brain damage. Alcohol Clin Exp Res. 2006a;30:1938–1949. doi: 10.1111/j.1530-0277.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, Qin L, Szabo G, Wheeler M, Zou J. Cytokines and alcohol. Alcohol Clin Exp Res. 2006b;30:720–730. doi: 10.1111/j.1530-0277.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- Crews FT, Buckley T, Dodd PR, Ende G, Foley N, Harper C, He J, Innes D, Loh el W, Pfefferbaum A, Zou J, Sullivan EV. Alcoholic neurobiology: changes in dependence and recovery. Alcohol Clin Exp Res. 2005;29:1504–1513. doi: 10.1097/01.alc.0000175013.50644.61. [DOI] [PubMed] [Google Scholar]
- Davis RL, Syapin PJ. Ethanol increases nuclear factor-kappa B activity in human astroglial cells. Neurosci Lett. 2004;371:128–132. doi: 10.1016/j.neulet.2004.08.051. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Sillaber I, Aouizerate B, Izawa R, Jaber M, Ghozland S, Kellendonk C, Le Moal M, Spanagel R, Schutz G, Tronche F, Piazza PV. The glucocorticoid receptor as a potential target to reduce cocaine abuse. J Neurosci. 2003;23:4785–4790. doi: 10.1523/JNEUROSCI.23-11-04785.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Ea CK, Baltimore D. Regulation of NF-kappaB activity through lysine monomethylation of p65. Proc Natl Acad Sci U S A. 2009;106:18972–18977. doi: 10.1073/pnas.0910439106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Xuei X, Wetherill LF, Bierut L, Bucholz K, Dick DM, Hesselbrock V, Kuperman S, Porjesz B, Schuckit MA, Tischfield JA, Almasy LA, Nurnberger JI, Jr, Foroud T. Association of NFKB1, which encodes a subunit of the transcription factor NF-kappaB, with alcohol dependence. Hum Mol Genet. 2008;17:963–970. doi: 10.1093/hmg/ddm368. [DOI] [PubMed] [Google Scholar]
- Ehrlich ME, Sommer J, Canas E, Unterwald EM. Periadolescent mice show enhanced DeltaFosB upregulation in response to cocaine and amphetamine. J Neurosci. 2002;22:9155–9159. doi: 10.1523/JNEUROSCI.22-21-09155.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Bruce-Keller AJ, Yakovleva T, Bazov I, Bakalkin G, Knapp PE, Hauser KF. Morphine exacerbates HIV-1 Tat-induced cytokine production in astrocytes through convergent effects on [Ca(2+)](i), NF-kappaB trafficking and transcription. PLoS One. 2008;3:e4093. doi: 10.1371/journal.pone.0004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadda F, Rossetti ZL. Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Prog Neurobiol. 1998;56:385–431. doi: 10.1016/s0301-0082(98)00032-x. [DOI] [PubMed] [Google Scholar]
- Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Farris SP, Wolen AR, Miles MF. Using expression genetics to study the neurobiology of ethanol and alcoholism. Int Rev Neurobiol. 2010;91:95–128. doi: 10.1016/S0074-7742(10)91004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatscher-Bader T, van der Brug M, Hwang JW, Gochee PA, Matsumoto I, Niwa S, Wilce PA. Alcohol-responsive genes in the frontal cortex and nucleus accumbens of human alcoholics. J Neurochem. 2005;93:359–370. doi: 10.1111/j.1471-4159.2004.03021.x. [DOI] [PubMed] [Google Scholar]
- Fridmacher V, Kaltschmidt B, Goudeau B, Ndiaye D, Rossi FM, Pfeiffer J, Kaltschmidt C, Israel A, Memet S. Forebrain-specific neuronal inhibition of nuclear factor-kappaB activity leads to loss of neuroprotection. J Neurosci. 2003;23:9403–9408. doi: 10.1523/JNEUROSCI.23-28-09403.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nat Rev Immunol. 2008;8:837–848. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- Grant KA, Lovinger DM. Cellular and behavioral neurobiology of alcohol: receptor-mediated neuronal processes. Clin Neurosci. 1995;3:155–164. [PubMed] [Google Scholar]
- Grant KA, Valverius P, Hudspith M, Tabakoff B. Ethanol withdrawal seizures and the NMDA receptor complex. Eur J Pharmacol. 1990;176:289–296. doi: 10.1016/0014-2999(90)90022-x. [DOI] [PubMed] [Google Scholar]
- Green TA, Alibhai IN, Hommel JD, DiLeone RJ, Kumar A, Theobald DE, Neve RL, Nestler EJ. Induction of inducible cAMP early repressor expression in nucleus accumbens by stress or amphetamine increases behavioral responses to emotional stimuli. J Neurosci. 2006;26:8235–8242. doi: 10.1523/JNEUROSCI.0880-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TA, Alibhai IN, Unterberg S, Neve RL, Ghose S, Tamminga CA, Nestler EJ. Induction of activating transcription factors (ATFs) ATF2, ATF3, and ATF4 in the nucleus accumbens and their regulation of emotional behavior. J Neurosci. 2008;28:2025–2032. doi: 10.1523/JNEUROSCI.5273-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli M, Memo M. Nuclear factor-kappaB/Rel proteins: a point of convergence of signalling pathways relevant in neuronal function and dysfunction. Biochem Pharmacol. 1999;57:1–7. doi: 10.1016/s0006-2952(98)00214-7. [DOI] [PubMed] [Google Scholar]
- Grundstrom S, Anderson P, Scheipers P, Sundstedt A. Bcl-3 and NFkappaB p50-p50 homodimers act as transcriptional repressors in tolerant CD4+ T cells. J Biol Chem. 2004;279:8460–8468. doi: 10.1074/jbc.M312398200. [DOI] [PubMed] [Google Scholar]
- Guan H, Hou S, Ricciardi RP. DNA binding of repressor nuclear factorkappaB p50/p50 depends on phosphorylation of Ser337 by the protein kinase A catalytic subunit. J Biol Chem. 2005;280:9957–9962. doi: 10.1074/jbc.M412180200. [DOI] [PubMed] [Google Scholar]
- Guerrini L, Blasi F, Denis-Donini S. Synaptic activation of NF-kappa B by glutamate in cerebellar granule neurons in vitro. Proc Natl Acad Sci U S A. 1995;92:9077–9081. doi: 10.1073/pnas.92.20.9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez H, Hale VA, Dolcet X, Davies A. NF-kappaB signalling regulates the growth of neural processes in the developing PNS and CNS. Development. 2005;132:1713–1726. doi: 10.1242/dev.01702. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Genco RJ. Downregulation of the DNA-binding activity of nuclear factor-kappaB p65 subunit in Porphyromonas gingivalis fimbria-induced tolerance. Infect Immun. 2004;72:1188–1191. doi: 10.1128/IAI.72.2.1188-1191.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CG, Daly J, Kril J. Brain water in chronic alcoholics: a necropsy study. Lancet. 1985;2:327. doi: 10.1016/s0140-6736(85)90368-x. [DOI] [PubMed] [Google Scholar]
- Hirano F, Tanaka H, Hirano Y, Hiramoto M, Handa H, Makino I, Scheidereit C. Functional interference of Sp1 and NF-kappaB through the same DNA binding site. Mol Cell Biol. 1998;18:1266–1274. doi: 10.1128/mcb.18.3.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi N, Brown JR, Haile CN, Ye H, Greenberg ME, Nestler EJ. FosB mutant mice: loss of chronic cocaine induction of Fos-related proteins and heightened sensitivity to cocaine's psychomotor and rewarding effects. Proc Natl Acad Sci U S A. 1997;94:10397–10402. doi: 10.1073/pnas.94.19.10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoberg JE, Popko AE, Ramsey CS, Mayo MW. IkappaB kinase alphamediated derepression of SMRT potentiates acetylation of RelA/p65 by p300. Mol Cell Biol. 2006;26:457–471. doi: 10.1128/MCB.26.2.457-471.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope BT, Nye HE, Kelz MB, Self DW, Iadarola MJ, Nakabeppu Y, Duman RS, Nestler EJ. Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron. 1994;13:1235–1244. doi: 10.1016/0896-6273(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Huang CJ, Nazarian R, Lee J, Zhao PM, Espinosa-Jeffrey A, de Vellis J. Tumor necrosis factor modulates transcription of myelin basic protein gene through nuclear factor kappa B in a human oligodendroglioma cell line. Int J Dev Neurosci. 2002;20:289–296. doi: 10.1016/s0736-5748(02)00022-9. [DOI] [PubMed] [Google Scholar]
- Irla M, Hollander G, Reith W. Control of central self-tolerance induction by autoreactive CD4+ thymocytes. Trends Immunol. 2010;31:71–79. doi: 10.1016/j.it.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Jensen GB, Pakkenberg B. Do alcoholics drink their neurons away? Lancet. 1993;342:1201–1204. doi: 10.1016/0140-6736(93)92185-v. [DOI] [PubMed] [Google Scholar]
- Johansson S, Ekstrom TJ, Marinova Z, Okvist A, Sheedy D, Garrick T, Harper C, Kuzmin A, Yakovleva T, Bakalkin G. Dysregulation of cell death machinery in the prefrontal cortex of human alcoholics. Int J Neuropsychopharmacol. 2009;12:109–115. doi: 10.1017/S1461145708009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt B, Kaltschmidt C. NF-kappaB in the nervous system. Cold Spring Harb Perspect Biol. 2009;1:a001271. doi: 10.1101/cshperspect.a001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt B, Ndiaye D, Korte M, Pothion S, Arbibe L, Prullage M, Pfeiffer J, Lindecke A, Staiger V, Israel A, Kaltschmidt C, Memet S. NF-kappaB regulates spatial memory formation and synaptic plasticity through protein kinase A/CREB signaling. Mol Cell Biol. 2006;26:2936–2946. doi: 10.1128/MCB.26.8.2936-2946.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt B, Uherek M, Volk B, Baeuerle PA, Kaltschmidt C. Transcription factor NF-kappaB is activated in primary neurons by amyloid beta peptides and in neurons surrounding early plaques from patients with Alzheimer disease. Proc Natl Acad Sci U S A. 1997;94:2642–2647. doi: 10.1073/pnas.94.6.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt B, Widera D, Kaltschmidt C. Signaling via NF-kappaB in the nervous system. Biochim Biophys Acta. 2005;1745:287–299. doi: 10.1016/j.bbamcr.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt C, Kaltschmidt B, Baeuerle PA. Brain synapses contain inducible forms of the transcription factor NF-kappa B. Mech Dev. 1993;43:135–147. doi: 10.1016/0925-4773(93)90031-r. [DOI] [PubMed] [Google Scholar]
- Kearns JD, Basak S, Werner SL, Huang CS, Hoffmann A. IkappaBepsilon provides negative feedback to control NF-kappaB oscillations, signaling dynamics, and inflammatory gene expression. J Cell Biol. 2006;173:659–664. doi: 10.1083/jcb.200510155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorooshi R, Babcock AA, Owens T. NF-kappaB-driven STAT2 and CCL2 expression in astrocytes in response to brain injury. J Immunol. 2008;181:7284–7291. doi: 10.4049/jimmunol.181.10.7284. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–998. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Harper CG. Neuronal counts from four cortical regions of alcoholic brains. Acta Neuropathol. 1989;79:200–204. doi: 10.1007/BF00294379. [DOI] [PubMed] [Google Scholar]
- Lee H, Jeong J, Son E, Mosa A, Cho GJ, Choi WS, Ha JH, Kim IK, Lee MG, Kim CY, Suk K. Ethanol selectively modulates inflammatory activation signaling of brain microglia. J Neuroimmunol. 2004;156:88–95. doi: 10.1016/j.jneuroim.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Lewohl JM, Wang L, Miles MF, Zhang L, Dodd PR, Harris RA. Gene expression in human alcoholism: microarray analysis of frontal cortex. Alcohol Clin Exp Res. 2000;24:1873–1882. [PubMed] [Google Scholar]
- Li CC, Dai RM, Chen E, Longo DL. Phosphorylation of NF-KB1-p50 is involved in NF-kappa B activation and stable DNA binding. J Biol Chem. 1994;269:30089–30092. [PubMed] [Google Scholar]
- Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- Lilienbaum A, Israel A. From calcium to NF-kappa B signaling pathways in neurons. Mol Cell Biol. 2003;23:2680–2698. doi: 10.1128/MCB.23.8.2680-2698.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lewohl JM, Dodd PR, Randall PK, Harris RA, Mayfield RD. Gene expression profiling of individual cases reveals consistent transcriptional changes in alcoholic human brain. J Neurochem. 2004;90:1050–1058. doi: 10.1111/j.1471-4159.2004.02570.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Lewohl JM, Harris RA, Iyer VR, Dodd PR, Randall PK, Mayfield RD. Patterns of gene expression in the frontal cortex discriminate alcoholic from nonalcoholic individuals. Neuropsychopharmacology. 2006;31:1574–1582. doi: 10.1038/sj.npp.1300947. [DOI] [PubMed] [Google Scholar]
- Lu T, Jackson MW, Wang B, Yang M, Chance MR, Miyagi M, Gudkov AV, Stark GR. Regulation of NF-kappaB by NSD1/FBXL11-dependent reversible lysine methylation of p65. Proc Natl Acad Sci U S A. 2010;107:46–51. doi: 10.1073/pnas.0912493107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackler SA, Korutla L, Cha XY, Koebbe MJ, Fournier KM, Bowers MS, Kalivas PW. NAC-1 is a brain POZ/BTB protein that can prevent cocaine-induced sensitization in the rat. J Neurosci. 2000;20:6210–6217. doi: 10.1523/JNEUROSCI.20-16-06210.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao XR, Moerman-Herzog AM, Chen Y, Barger SW. Unique aspects of transcriptional regulation in neurons--nuances in NFkappaB and Sp1-related factors. J Neuroinflammation. 2009;6:16. doi: 10.1186/1742-2094-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez F, Abril ER, Earnest DL, Watson RR. Ethanol and cytokine secretion. Alcohol. 1992;9:455–458. doi: 10.1016/0741-8329(92)90080-t. [DOI] [PubMed] [Google Scholar]
- Mattson MP. NF-kappaB in the survival and plasticity of neurons. Neurochem Res. 2005;30:883–893. doi: 10.1007/s11064-005-6961-x. [DOI] [PubMed] [Google Scholar]
- Meberg PJ, Kinney WR, Valcourt EG, Routtenberg A. Gene expression of the transcription factor NF-kappa B in hippocampus: regulation by synaptic activity. Brain Res Mol Brain Res. 1996;38:179–190. doi: 10.1016/0169-328x(95)00229-l. [DOI] [PubMed] [Google Scholar]
- Meffert MK, Baltimore D. Physiological functions for brain NF-kappaB. Trends Neurosci. 2005;28:37–43. doi: 10.1016/j.tins.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D. NFkappa B functions in synaptic signaling and behavior. Nat Neurosci. 2003;6:1072–1078. doi: 10.1038/nn1110. [DOI] [PubMed] [Google Scholar]
- Michaelson JS, Leder P. RNAi reveals anti-apoptotic and transcriptionally repressive activities of DAXX. J Cell Sci. 2003;116:345–352. doi: 10.1242/jcs.00234. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Overholser JC, Meltzer HY, Stockmeier CA, Rajkowska G. Reduced glial and neuronal packing density in the orbitofrontal cortex in alcohol dependence and its relationship with suicide and duration of alcohol dependence. Alcohol Clin Exp Res. 2006;30:1845–1855. doi: 10.1111/j.1530-0277.2006.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minambres R, Guasch RM, Perez-Arago A, Guerri C. The RhoA/ROCKI/ MLC pathway is involved in the ethanol-induced apoptosis by anoikis in astrocytes. J Cell Sci. 2006;119:271–282. doi: 10.1242/jcs.02723. [DOI] [PubMed] [Google Scholar]
- Minghetti L. Role of inflammation in neurodegenerative diseases. Curr Opin Neurol. 2005;18:315–321. doi: 10.1097/01.wco.0000169752.54191.97. [DOI] [PubMed] [Google Scholar]
- Mittal N, Nathan JB, Pandey SC. Neuroadaptational changes in DNA binding of stimulatory protein-1 and nuclear factor-kB gene transcription factors during ethanol dependence. Eur J Pharmacol. 1999;386:113–119. doi: 10.1016/s0014-2999(99)00734-7. [DOI] [PubMed] [Google Scholar]
- Moynagh PN. The NF-kappaB pathway. J Cell Sci. 2005;118:4589–4592. doi: 10.1242/jcs.02579. [DOI] [PubMed] [Google Scholar]
- Mulligan MK, Ponomarev I, Hitzemann RJ, Belknap JK, Tabakoff B, Harris RA, Crabbe JC, Blednov YA, Grahame NJ, Phillips TJ, Finn DA, Hoffman PL, Iyer VR, Koob GF, Bergeson SE. Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. Proc Natl Acad Sci U S A. 2006;103:6368–6373. doi: 10.1073/pnas.0510188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natoli G. Control of NF-kappaB-dependent transcriptional responses by chromatin organization. Cold Spring Harb Perspect Biol. 2009;1:a000224. doi: 10.1101/cshperspect.a000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S, Bagby GJ, Bainton BG, Summer WR. The effects of acute and chronic alcoholism on tumor necrosis factor and the inflammatory response. J Infect Dis. 1989;160:422–429. doi: 10.1093/infdis/160.3.422. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Nickols JC, Valentine W, Kanwal S, Carter BD. Activation of the transcription factor NF-kappaB in Schwann cells is required for peripheral myelin formation. Nat Neurosci. 2003;6:161–167. doi: 10.1038/nn995. [DOI] [PubMed] [Google Scholar]
- Niquet J, Baldwin RA, Allen SG, Fujikawa DG, Wasterlain CG. Hypoxic neuronal necrosis: protein synthesis-independent activation of a cell death program. Proc Natl Acad Sci U S A. 2003;100:2825–2830. doi: 10.1073/pnas.0530113100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niquet J, Seo DW, Wasterlain CG. Mitochondrial pathways of neuronal necrosis. Biochem Soc Trans. 2006;34:1347–1351. doi: 10.1042/BST0341347. [DOI] [PubMed] [Google Scholar]
- Nye HE, Nestler EJ. Induction of chronic Fos-related antigens in rat brain by chronic morphine administration. Mol Pharmacol. 1996;49:636–645. [PubMed] [Google Scholar]
- O'Mahony A, Raber J, Montano M, Foehr E, Han V, Lu SM, Kwon H, LeFevour A, Chakraborty-Sett S, Greene WC. NF-kappaB/Rel regulates inhibitory and excitatory neuronal function and synaptic plasticity. Mol Cell Biol. 2006;26:7283–7298. doi: 10.1128/MCB.00510-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill LA, Kaltschmidt C. NF-kappa B: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997;20:252–258. doi: 10.1016/s0166-2236(96)01035-1. [DOI] [PubMed] [Google Scholar]
- O'Riordan KJ, Huang IC, Pizzi M, Spano P, Boroni F, Egli R, Desai P, Fitch O, Malone L, Ahn HJ, Liou HC, Sweatt JD, Levenson JM. Regulation of nuclear factor kappaB in the hippocampus by group I metabotropic glutamate receptors. J Neurosci. 2006;26:4870–4879. doi: 10.1523/JNEUROSCI.4527-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okvist A, Johansson S, Kuzmin A, Bazov I, Merino-Martinez R, Ponomarev I, Mayfield RD, Harris RA, Sheedy D, Garrick T, Harper C, Hurd YL, Terenius L, Ekstrom TJ, Bakalkin G, Yakovleva T. Neuroadaptations in human chronic alcoholics: dysregulation of the NF-kappaB system. PLoS One. 2007;2:e930. doi: 10.1371/journal.pone.0000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons OA, Nixon SJ. Neurobehavioral sequelae of alcoholism. Neurol Clin. 1993;11:205–218. [PubMed] [Google Scholar]
- Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, Sullivan EV. Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study. Neuroimage. 2001;14:7–20. doi: 10.1006/nimg.2001.0785. [DOI] [PubMed] [Google Scholar]
- Pich EM, Pagliusi SR, Tessari M, Talabot-Ayer D, Hooft van Huijsduijnen R, Chiamulera C. Common neural substrates for the addictive properties of nicotine and cocaine. Science. 1997;275:83–86. doi: 10.1126/science.275.5296.83. [DOI] [PubMed] [Google Scholar]
- Puto LA, Reed JC. Daxx represses RelB target promoters via DNA methyltransferase recruitment and DNA hypermethylation. Genes Dev. 2008;22:998–1010. doi: 10.1101/gad.1632208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao P, Hayden MS, Long M, Scott ML, West AP, Zhang D, Oeckinghaus A, Lynch C, Hoffmann A, Baltimore D, Ghosh S. IkappaBbeta acts to inhibit and activate gene expression during the inflammatory response. Nature. 2010;466:1115–1119. doi: 10.1038/nature09283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratti MT, Bo P, Giardini A, Soragna D. Chronic alcoholism and the frontal lobe: which executive functions are imparied? Acta Neurol Scand. 2002;105:276–281. doi: 10.1034/j.1600-0404.2002.0o315.x. [DOI] [PubMed] [Google Scholar]
- Rattner A, Korner M, Rosen H, Baeuerle PA, Citri Y. Nuclear factor kappa B activates proenkephalin transcription in T lymphocytes. Mol Cell Biol. 1991;11:1017–1022. doi: 10.1128/mcb.11.2.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repunte-Canonigo V, van der Stap LD, Chen J, Sabino V, Wagner U, Zorrilla EP, Schumann G, Roberts AJ, Sanna PP. Genome-wide gene expression analysis identifies K-ras as a regulator of alcohol intake. Brain Res. 2010;1339:1–10. doi: 10.1016/j.brainres.2010.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld MG, Lunyak VV, Glass CK. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 2006;20:1405–1428. doi: 10.1101/gad.1424806. [DOI] [PubMed] [Google Scholar]
- Rulten SL, Ripley TL, Hunt CL, Stephens DN, Mayne LV. Sp1 and NFkappaB pathways are regulated in brain in response to acute and chronic ethanol. Genes Brain Behav. 2006;5:257–273. doi: 10.1111/j.1601-183X.2005.00157.x. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Wilkinson MB, Mazei-Robison MS, Dietz DM, Maze I, Krishnan V, Renthal W, Graham A, Birnbaum SG, Green TA, Robison B, Lesselyong A, Perrotti LI, Bolanos CA, Kumar A, Clark MS, Neumaier JF, Neve RL, Bhakar AL, Barker PA, Nestler EJ. Nuclear factor kappa B signaling regulates neuronal morphology and cocaine reward. J Neurosci. 2009;29:3529–3537. doi: 10.1523/JNEUROSCI.6173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnico I, Lanzillotta A, Benarese M, Alghisi M, Baiguera C, Battistin L, Spano P, Pizzi M. NF-kappaB dimers in the regulation of neuronal survival. Int Rev Neurobiol. 2009;85:351–362. doi: 10.1016/S0074-7742(09)85024-1. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R, Memet S, Lilienbaum A, Feuillard J, Raphael M, Israel A. NF-kappaB activity in transgenic mice: developmental regulation and tissue specificity. Development. 1996;122:2117–2128. doi: 10.1242/dev.122.7.2117. [DOI] [PubMed] [Google Scholar]
- Schmidt KS, Gallo JL, Ferri C, Giovannetti T, Sestito N, Libon DJ, Schmidt PS. The neuropsychological profile of alcohol-related dementia suggests cortical and subcortical pathology. Dement Geriatr Cogn Disord. 2005;20:286–291. doi: 10.1159/000088306. [DOI] [PubMed] [Google Scholar]
- Schultz C, Konig HG, Del Turco D, Politi C, Eckert GP, Ghebremedhin E, Prehn JH, Kogel D, Deller T. Coincident enrichment of phosphorylated IkappaBalpha, activated IKK, and phosphorylated p65 in the axon initial segment of neurons. Mol Cell Neurosci. 2006;33:68–80. doi: 10.1016/j.mcn.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Smale ST. Selective transcription in response to an inflammatory stimulus. Cell. 2010;140:833–844. doi: 10.1016/j.cell.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Teferedegne B, Green MR, Guo Z, Boss JM. Mechanism of action of a distal NF-kappaB-dependent enhancer. Mol Cell Biol. 2006;26:5759–5770. doi: 10.1128/MCB.00271-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubogu EE, Cossoy MB, Ransohoff RM. The expression and function of chemokines involved in CNS inflammation. Trends Pharmacol Sci. 2006;27:48–55. doi: 10.1016/j.tips.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Valles SL, Blanco AM, Pascual M, Guerri C. Chronic ethanol treatment enhances inflammatory mediators and cell death in the brain and in astrocytes. Brain Pathol. 2004;14:365–371. doi: 10.1111/j.1750-3639.2004.tb00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HJ, Zakhari S, Jung MK. Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World J Gastroenterol. 2010;16:1304–1313. doi: 10.3748/wjg.v16.i11.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RJ, Zhang Y, Crichton RR, Piret B, Piette J, de Witte P. Identification of the nuclear transcription factor NFkappaB in rat after in vivo ethanol administration. FEBS Lett. 1996;389:119–122. doi: 10.1016/0014-5793(96)00545-5. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Henriksson R, Ohnishi YN, Ohnishi YH, Harper C, Sheedy D, Garrick T, Nyberg F, Nestler EJ, Bakalkin G, Yakovleva T. FOSB proteins in the orbitofrontal and dorsolateral prefrontal cortices of human alcoholics. Addict Biol. 2009;14:294–297. doi: 10.1111/j.1369-1600.2009.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellmann H, Kaltschmidt B, Kaltschmidt C. Retrograde transport of transcription factor NF-kappa B in living neurons. J Biol Chem. 2001;276:11821–11829. doi: 10.1074/jbc.M009253200. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Gaynor RB. IkappaB kinases: key regulators of the NF-kappaB pathway. Trends Biochem Sci. 2004;29:72–79. doi: 10.1016/j.tibs.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Yeh SH, Lin CH, Gean PW. Acetylation of nuclear factor-kappaB in rat amygdala improves long-term but not short-term retention of fear memory. Mol Pharmacol. 2004;65:1286–1292. doi: 10.1124/mol.65.5.1286. [DOI] [PubMed] [Google Scholar]
- Young C, Roth KA, Klocke BJ, West T, Holtzman DM, Labruyere J, Qin YQ, Dikranian K, Olney JW. Role of caspase-3 in ethanol-induced developmental neurodegeneration. Neurobiol Dis. 2005;20:608–614. doi: 10.1016/j.nbd.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Zhong H, May MJ, Jimi E, Ghosh S. The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol Cell. 2002;9:625–636. doi: 10.1016/s1097-2765(02)00477-x. [DOI] [PubMed] [Google Scholar]
- Zou J, Crews F. CREB and NF-kappaB transcription factors regulate sensitivity to excitotoxic and oxidative stress induced neuronal cell death. Cell Mol Neurobiol. 2006;26:385–405. doi: 10.1007/s10571-006-9045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Crews F. Induction of innate immune gene expression cascades in brain slice cultures by ethanol: key role of NF-kappaB and proinflammatory cytokines. Alcohol Clin Exp Res. 2010;34:777–789. doi: 10.1111/j.1530-0277.2010.01150.x. [DOI] [PubMed] [Google Scholar]