Abstract
Background
In 2011, the Ministry of Health raised the CD4 threshold for antiretroviral therapy (ART) eligibility from <250 cells/µl and <350 cells/µl, but at the same time only 8.8% of facilities in Malawi with HIV services provided CD4 testing. We conducted a record review at 10 rural clinics in Thyolo District to assess the impact of introducing CD4 testing on identifying patients eligible for ART.
Methods
We abstracted CD4 counts of all ART-naïve, HIV-infected patients with WHO clinical stages 1 and 2 and an initial CD4 test between May 2008 and June 2009. At four clinics, we also abstracted CD4 counts of patients not initially eligible for ART who were retested before April 2010.
Results
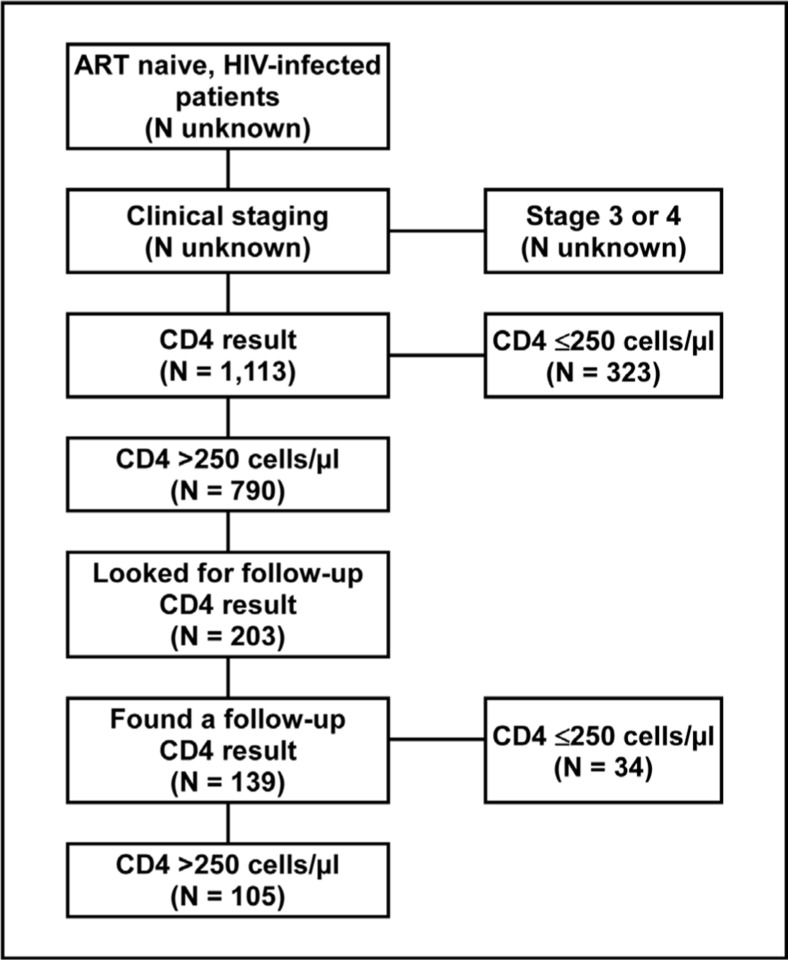
Of 1,113 patients tested, the initial CD4 was “≤250 cells/µl” and “≤350 cells/µl” in 534 (48.0%). Of 203 patients with follow-up results, the most recent CD4 was ≤250 cells/µl in 34 (24.5%), and ≤350 cells/µl in 64 (46.0%).
Conclusions
CD4 testing in rural clinics is feasible and identifies many patients eligible for ART who would not be identified without CD4 testing. CD4 testing needs to be scaled-up to identify patients eligible for ART. ART services need to be scaled-up concurrently to meet the resulting increased demand.
Introduction
In Malawi, the adult HIV-prevalence was estimated to be approximately 12 percent in 2010, with an estimated 800,000 adults living with HIV.1 Since implementation of the national HIV programme in 2004, considerable progress has been made towards expanding access to antiretroviral therapy (ART) by means of a policy of decentralisation and increasing the number of sites that provide ART services. During the fourth quarter of 2011, 806 sites provided HIV testing and counselling (HTC) services, of which 241 (29.9%) were outside health facilities.2 By the end of December 2011, 323,628 patients were alive and on ART provided at 554 ART sites distributed throughout the country.2
From 2008 to 2011, the Malawi national guidelines recommended initiating ART among adolescents and adults with World Health Organization (WHO) clinical stage 3 or 4 HIV disease, and those with a CD4 count ≤250 cells/µl, regardless of clinical stage.3 In November 2009, WHO released revised guidelines recommending that ART be initiated in all HIVinfected adolescents and adults with stage 3 or 4 disease, or a CD4 count ≤350 cells/µl, regardless of clinical stage.4 In July 2011, the Malawi Ministry of Health released new guidelines raising the CD4 threshold for ART initiation to ≤350 cells/µl, in line with WHO recommendations.5
In Malawi, many HIV-infected persons, particularly those living in rural areas, have limited access to HIV services, including clinical staging and CD4 testing. At the end of 2011, only 58 of the 662 facilities with integrated HIV services (8.8%) provided CD4 testing.2 During the fourth quarter of 2011, 56 percent of ART initiations were among patients with clinical stage 1 or 2 disease, despite only 58 of 79 installed CD4 testing devices (73%) being functional.2
Since 2003, Médecins Sans Frontières (MSF) has been supporting the Thyolo District Health Office of the Ministry of Health (MOH) with scaling-up access to ART in Thyolo District in the Southern Region of Malawi. The Southern Region had an HIV prevalence of 20.5 percent in 2007, the most recent year for which figures are available, almost double that of the Central and Northern Regions.1 In Thyolo District, the expansion and decentralisation of HIV services to the primary care level, combined with task shifting, has resulted in increased uptake of HIV care with acceptable programme outcomes.6 Starting in 2008, CD4 testing was introduced in phases in ten clinics that provide basic primary health care services. As a result, the number of CD4 tests performed in Thyolo District increased by 38.2 percent between 2008 and 2011, from 12,386 tests in 2008 to 17,118 in 2011.
In order to assess the impact of CD4 testing on the number of patients found to be eligible for ART, we analysed the CD4 test results of ART-naïve patients with HIV infection attending clinics in Thyolo District. We also used CD4 test results to estimate the impact of raising the CD4 threshold for ART initiation from 250 cells/µl to 350 cells/µl on increasing the number of patients eligible for ART.
Methods
Setting
We reviewed CD4 test results from ten rural primary health care clinics in Thyolo District that provide CD4 testing to screen for ART eligibility. The clinics are located in remote parts of Thyolo District, 15 to 65 kilometres from the district health laboratory, and are managed by mobile nurses who provide clinical services, including HIV care (clinical staging, blood draws for CD4 testing, and ART initiation and refill), antenatal care and prevention of mother-to-child transmission (PMTCT), family planning, and chronic care on set days each month. The clinics are staffed by health surveillance assistants (HSAs) who provide HIV testing and counselling (HTC) and basic primary health care services on a daily basis, and patient support attendants (PSAs). Despite decentralisation of services, many patients still have to travel long distances to reach clinics. Poor roads sometimes make access to clinics and transport of specimens to the laboratory difficult, particularly during the rainy season.
Population
The study population comprised HIV-infected, ART-naïve patients who attended any of the ten clinics and required CD4 testing to assess their eligibility for ART. We included all ART-naïve HIV-infected persons, aged 15 years or older, who had an initial CD4 test to screen for ART eligibility between May 2008 and June 2009 at any of the ten clinics offering CD4 testing. Patients with clinical stage 3 or 4 disease, and children under the age of 15 years, were excluded from the analysis.
Procedures
A nurse performed clinical staging and drew blood for CD4 testing on designated days. HIV-infected persons with clinical stage 3 or 4 disease were referred to the nearest ART initiation site without having a CD4 test. Those with clinical stage 1 or 2 disease, had blood drawn for CD4 testing. Blood samples were transported to the laboratory at Thyolo District Hospital by motorbike or in vehicles that visited the clinic on the day of specimen collection. CD4 testing was done in the hospital laboratory using a Partec CyFlow® counter. Patients were asked to return to the health post for their CD4 test result four weeks after the initial visit. Those with CD4 counts ≤250 cells/µl were referred to the nearest ART initiation site, and those with a CD4 count >250 cells/µl were given an appointment to return for retesting. CD4 testing was repeated after 3 months if the CD4 count was between 251 and 350 cells/µl; after 6 months if the CD4 count was between 351 and 500 cells/µl; and after 12 months if the CD4 count was >500 cells/µl.
Data collection and analysis
We conducted a retrospective review of clinic records. We abstracted the baseline CD4 test results, age, sex, and the initial clinical stage, of all patients who had a first CD4 test during the study period. At four of the ten clinics, we also abstracted the most recent CD4 test results of patients with an initial CD4 count >250 cells/µl (who were not eligible for ART in terms of the eligibility criteria in effect at the time), who returned to the clinic for repeat CD4 testing to reassess their ART eligibility. The data were entered into a Microsoft Excel spreadsheet, and analyzed using Stata version 11 (StataCorp, College Station, Texas, USA). The Wilcoxon rank-sum test was used to assess whether baseline CD4 and follow-up CD4 differed significantly according to baseline clinical stage.
Ethical considerations
This study was approved by Dr. Likaka, the District Health Officer (DHO) of Thyolo District, and met the criteria for an exemption from ethical review7 and the requirement to obtain informed consent.8
Results
A total of 1,113 patients were included in the analysis. Their age ranged from 15 to 76 years with a median of 35 years. The majority of patients (77.9%) were female. Two hundred and ten (18.9%) patients had stage 1 disease; 830 (74.6%) had stage 2 disease; and 73 (6.6%) did not have their clinical stage recorded. The median initial CD4 count was 360 cells/µl (Table 1). Among those with a follow-up CD4 test result available, the median follow-up CD4 count was 364 cells/µl. Patients' initial clinical stage was significantly associated with their initial CD4 count (p = 0.0003), but not with their most recent follow-up CD4 count (p =0.8678). Of the 1,113 patients tested, 323 (29.0%) had an initial CD4 count 250 cells/µl, and were eligible for ART in terms of the guidelines in effect at the time. An additional 211 (19.0%) had a CD4 count between 250 cells/µl and 350 cells/µl, making 534 (48.0%) of those tested eligible according to the current guidelines. Of 203 patients considered in the follow-up analysis, 139 (68.5%) had one or more follow-up CD4 test results. The median interval between the initial CD4 test and the most recent test was 13 months (range: 6 weeks to 28.7 months). Of the 139 patients retested, 34 (24.5%) had a follow-up CD4 count less than ≤250 cells/µl and were eligible for ART in terms of the guidelines in effect at the time. An additional 30 (21.6%), had a CD4 count between 250 cells/µl and 350 cells/µl, making 64 (46.0%) of those retested eligible according to the current guidelines.
Table 1.
Initial and folow up interval acording to the initial WHO Clinical survey
| WHO clinical stage at baseline |
Median | Range |
| Baseline CD4 (cells/µl) | ||
| Stage 1(n=210) | 413 | 23–1254 |
| Stage 2 (n=830) | 347 | 10–1848 |
| Not specified (n=73) | 397 | 70–1264 |
| All (n=1113) | 360 | 10–1848 |
| Follow-up CD4 (cells/µl) | ||
| Stage 1(n=22) | 394 | 214–691 |
| Stage 2 (n=89) | 364 | 28–1471 |
| Not specified (n=28) | 362 | 123–1204 |
| All(n=139) | 364 | 28–1471 |
| Interval between baseline CD4 and most recent CD4 (months) | ||
| Stage 1(n=22) | 12.0 | 3.0–15.5 |
| Stage2(n=89) | 13.8 | 1.4–28.7 |
| Not specified (n=28) | 11.3 | 3.6–17.8 |
| All(n=139) | 13.0 | 1.4–28.7 |
Discussion
Our findings demonstrate that CD4 testing is feasible in rural settings with limited resources, under routine programme conditions. Our findings confirm that CD4 testing of HIV-infected patients with clinical stage 1 or 2 disease is critical in order to determine ART eligibility, and that in the absence of CD4 testing, a substantial portion of those eligible will not be offered ART. Our findings also confirm the value of follow-up CD4 testing for early identification of patients who become eligible for ART after the initial CD4 test.
Other studies have also found that a substantial proportion of ART-naïve persons with clinical stage 1 and 2 disease have low CD4 counts. A study carried out at a demographic surveillance site in Malawi, found that assessing ART eligibility based on clinical staging alone missed two-thirds of those eligible for ART based on clinical staging and CD4 count combined.9 A study conducted in Uganda found that the sensitivity of WHO clinical staging to determine. ART eligibility was 53.5 percent at a CD4 threshold of 250 cells/µl, and 49.1 percent at a threshold of 350 cells/µl.10 A study conducted at four sites in Ghana found that 29.5 percent of patients with clinical stages 1 and 2 disease had a CD4 count 200 cells/µl.11
The female preponderance of patients in our study reflects the general female preponderance among people having HTC in Malawi: During the last quarter of 2011, 67 percent of people tested for HIV were female, of whom 48% were pregnant.2
In settings where CD4 testing is not available, people tend to start ART at an advanced stage of disease. Late ART initiation is associated with poorer health outcomes. A previous study conducted in Thyolo District found that there was a trend towards increased mortality within 6 months of ART initiation with decreasing CD4 count at time of ART initiation.12 A review of 18 cohort studies found that among HIV-infected persons with clinical stage 1 or 2 disease and a CD4 count of ≤450 cells/µl, the risk of developing AIDS-defining clinical conditions and the risk of death increased progressively the lower the CD4 count at time of ART initiation.13 A recent review found that mortality within the first year after initiating ART was generally higher in sub-Saharan African countries than in south-east Asian, Caribbean and Latin American countries, primarily due to advanced symptomatic HIV disease at the time of ART initiation.14 Another study found that, among patients with CD4 counts in the 200 to 500 cells/µl range, the risk of developing an AIDS-defining condition was greater before than after ART initiation.15 Currently, the availability of CD4 testing in Malawi is limited. In order to achieve 100 percent coverage among those eligible for ART, the limited availability of CD4 testing, especially in remote areas, needs to be addressed. In order to improve access to HIV clinical services, such services need to be decentralised further, and capacity to do clinical staging and CD4 testing needs to be expanded.
The cost of CD4 testing is the most important constraint: A CD4 test costs US $15 (about 3,800 Kwacha at current exchange rates). Other major barriers to making CD4 testing more widely available include a shortage of functional CD4 testing devices; limited human resources; and a poor transport infrastructure, particularly in rural areas. The task of drawing venous blood increases the burden of work for nurses, and laboratories need to have adequate capacity to add CD4 testing to the range of tests available, without compromising the availability of existing services.
These barriers could be addressed by employing and training additional laboratory staff to run and maintain CD4 testing devices with the back-up of a maintenance plan; task-shifting of blood draws from nurses to a lower cadre workers, such as health surveillance assistants (with appropriate training and supervision); improving transport of samples to laboratories by greater use of motorbikes; and using technology such as SMS to improve the speed with which CD4 results are reported to clinics.
In recent years, several POC CD4 testing devices have been become commercially available, and have been shown to produce results on finger-prick blood specimens with a similar level of accuracy to standard laboratory CD4 assays that use venous blood.16–19 In areas without ready access to a laboratory, the use of point-of-care (POC) CD4 testing is worth considering as it eliminates many barriers to CD4 testing. Primary care nurses can be trained to do POC CD4 testing and to produce results comparable to POC CD4 tests done by laboratory technicians.17 POC CD4 testing enables patients to be given their CD4 test result on the day of testing, thus eliminating the need to return to the clinic for CD4 test results and minimising pre-ART loss-to-follow-up. POC CD4 measuring devices are expensive, however, require maintenance, and should only be considered for health facilities with a large number of patients needing CD4 testing. We have not introduced POC CD4 testing at the clinics included in our study, primarily because of the limited number of patients per day needing CD4 testing.
Our study has some limitations: We do not have information on the number of patients with stage 3 and 4 disease who were referred to ART initiation site without CD4 testing, and are thus unable to estimate the impact of CD4 testing on increasing the overall number of people initiating an ART, or the impact of raising the CD4 threshold on the overall number of additional people eligible for ART. We were also unable to confirm whether patients returned for their CD4 test results, and whether those who were referred to an ART initiation site were initiated on ART. CD4 testing to screen for ART eligibility is only of value if those found to be eligible are initiated on ART. In the assessment of follow-up CD4 test results, we collected only the most recent follow-up CD4 test result, and are thus unable to assess whether patients returned for CD4 testing at the time points recommended in the national guidelines.
In order to achieve the full benefit of CD4 testing, it is necessary to ensure that all those who are eligible for ART have access to ART. The expansion of CD4 testing should not outpace the ability of the health system to provide ART to those who are eligible. Although the number of people initiated on ART has steadily increased in recent years, ART coverage is still lower than targeted.1 As a consequence of the implementation of new national ART guidelines which raise the CD4 threshold for ART initiation to 350 cells/µl, a large number of people who were previously ineligible for ART, have become eligible. At health posts in Thyolo District, increasing the CD4 threshold from 250 cells/µl to 350 cells/µl has increased the proportion of people with clinical stage 1 and 2 disease eligible for ART by about two thirds, placing an additional strain on the health care system. There is thus an urgent need to expand ART services as well as CD4 testing services.
Figure 1.
Flow Diagram Showing the Number of Patient Records Included in the Record Review
Table 2.
CD4 Counts acording to WHO Clinical Stage at the Time of the Initial CD4 Test
| Initial CD4 result (10 clinics) | Follow-up CD4 result (4 clinics) | |||||||
| CD4 count | Stage 1 | Stage 2 | Not specified | All | Stage 1 | Stage 2 | Not specified |
All |
| (cells/µl) | (n = 210) | (n = 830) | (n = 73) | (n = 1113) | (n = 22) | (n = 89) | (n=28) | (n = 139) |
| <350 | 86(41.0%) | 422(50.3%) | 26(35.6%) | 534(48.0%) | 9(40.9%) | 42(47.2%) | 13(56.4%) | 64(46.0%) |
| <250 | 48(22.9%) | 260(31.3%) | 15(20.6%) | 323(29.0%) | 4(18.2%) | 25(28.1%) | 5(17.9%) | 34(24.5%) |
| 251–350 | 38(1B.1%) | 162(19.5%) | 11 (15.1%) | 211 (19.0%) | 5(22.7%) | 17(19.1%) | 8(28.6%) | 30(21.6%) |
| >350 | 124 (59.1%) | 408 (49.2%) | 47 (64.4%) | 579 (52.0%) | 13 (59.1%) | 47 (52.8%) | 15 (53.6%) | 75 (54.0%) |
| 351–500 | 45(21.4%) | 200(24.1%) | 22(30.1%) | 267(24.0%) | 9(40.9%) | 16(18.0%) | 3(10.7%) | 28(20.1%) |
| >500 | 79(37.6%) | 208(25.1%) | 25(34.3%) | 312(28.0%) | 4(18.2%) | 31(34.8%) | 12(42.9%) | 47(33.8%) |
References
- 1.Government of Malawi, author. Malawi HIV and AIDS Country Monitoring and Evaluation Report: 2008–2009. UNGASS Country Progress Report. Lilongwe: Government of Malawi; 2010. [Google Scholar]
- 2.Malawi Ministry of Health, author. Malawi Integrated HIV Programme Report (October – December, 2011) Lilongwe: Ministry of Health; 2012. [6 June 2012]. Available from: http://www.hivunitmohmw.org/uploads/Main/Quarterly_HIV_Programme_Report_2011_Q4.pdf. [Google Scholar]
- 3.MOH, author. Guidelines for the Use of Antiretroviral Therapy in Malawi. 3rd Edition. Lilongwe: MOH; 2008. [Google Scholar]
- 4.WHO, author. Antiretroviral therapy for HIV infection in adults and adolescents: Recommendations for a public health approach. Geneva: WHO; 2010. 2010 revision. [PubMed] [Google Scholar]
- 5.MOH, author. Clinical Management of HIV Infection in Children and Adults. First edition. Lilongwe: Ministry of Health; 2011. [Google Scholar]
- 6.Bemelmans M, Van Den Akker T, Ford N, Philips M, Zachariah R, Harries A, Schouten E, Hermann K, Mwagomba B, Massaquoi M. Providing universal access to antiretroviral therapy in Thyolo, Malawi through task shifting and decentralization of HIV/AIDS care. Trop Med Int Health. 2010;15:1413–1420. doi: 10.1111/j.1365-3156.2010.02649.x. [DOI] [PubMed] [Google Scholar]
- 7.Schopper D, Upshur R, Matthys F, Singh JA, Bandewar SS, Ahmad A, van Dongen E. Research ethics review in humanitarian contexts: The experience of the independent ethics review board of Médecins Sans Frontières. PLoS Med. 2009;6(7):e1000115. doi: 10.1371/journal.pmed.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doyal L. Journals should not publish research to which patients have not given fully informed consent — with three exceptions. Br Med J. 1997;314:1107–1111. doi: 10.1136/bmj.314.7087.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawn SD, Harries AD, Wood R. Strategies to reduce early morbidity and mortality in adults receiving antiretroviral therapy in resource-limited settings. Curr Opin HIV AIDS. 2010;5:18–26. doi: 10.1097/COH.0b013e328333850f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torpey K, Lartey M, Amenyah R, Addo NA, Obeng-Baah J, Rahman Y, Suzuki C, Mukadi YD, Colebunders R. Initiating antiretroviral treatment in a resource-constrained setting: does clinical staging effectively identify patients in need? Int J STD AIDS. 2009;20:395–398. doi: 10.1258/ijsa.2008.008333. [DOI] [PubMed] [Google Scholar]
- 11.When To Start Consortium. Sterne JA, May M, Costagliola D, de Wolf F, Phillips AN, Harris R, Funk MJ, Geskus RB, Gill J, Dabis F, Miró JM, Justice AC, Ledergerber B, Fätkenheuer G, Hogg RS, Monforte AD, Saag M, Smith C, Staszewski S, Egger M, Cole SR. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–1363. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGrath N, Kranzer K, Saul J, Crampin AC, Malema S, Kachiwanda L, Zaba B, Jahn A, Fine PE, Glynn JR. Estimating the need for antiretroviral treatment and an assessment of a simplified HIV/AIDS case definition in rural Malawi. AIDS. 2007;21(Suppl 6):S105–S113. doi: 10.1097/01.aids.0000299417.69432.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baveewo S, Ssali F, Karamagi C, Kalyango JN, Hahn JA, Ekoru K, Mugyenyi P, Katabira E. Validation of World Health Organisation HIV/AIDS clinical staging in predicting initiation of antiretroviral therapy and clinical predictors of low CD4 cell count in Uganda. PLoS One. 2011;6:e19089. doi: 10.1371/journal.pone.0019089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guiguet M, Porter K, Phillips A, Costagliola D, Babiker A. Clinical progression rates by CD4 cell category before and after the initiation of combination antiretroviral therapy (cART) Open AIDS J. 2008;2:3–9. doi: 10.2174/1874613600802010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zachariah R, Fitzgerald M, Massaquoi M, Pasulani O, Arnould L, Makombe S, Harries AD. Risk factors for high early mortality in patients on antiretroviral treatment in a rural district of Malawi. AIDS. 2006;20:2355–2360. doi: 10.1097/QAD.0b013e32801086b0. [DOI] [PubMed] [Google Scholar]
- 16.Mtapuri-Zinyowera S, Chideme M, Mangwanya D, Mugurungi O, Gudukeya S, Hatzold K, Mangwiro A, Bhattacharya G, Lehe J, Peter T. Evaluation of the PIMA point-of-care CD4 analyzer in VCT clinics in Zimbabwe. J Acquir Immune Defic Syndr. 2010;55:1–7. doi: 10.1097/QAI.0b013e3181e93071. [DOI] [PubMed] [Google Scholar]
- 17.Jani IV, Sitoe NE, Chongo PL, Alfai ER, Quevedo JI, Tobaiwa O, Lehe JD, Peter TF. Accurate CD4 T-cell enumeration and antiretroviral drug toxicity monitoring in primary healthcare clinics using point-of-care testing. AIDS. 2011;25:807–812. doi: 10.1097/QAD.0b013e328344f424. [DOI] [PubMed] [Google Scholar]
- 18.Diaw PA, Daneau G, Coly AA, Ndiaye BP, Wade D, Camara M, Mboup S, Kestens L, Dieye TN. Multisite evaluation of a point-of-care instrument for CD4(+) T-cell enumeration using venous and finger-prick blood: the Pima CD4. J Acquir Immune Defic Syndr. 2011;58:e103–e111. doi: 10.1097/QAI.0b013e318235b378. [DOI] [PubMed] [Google Scholar]
- 19.Glencross DK, Coetzee LM, Faal M, Masango M, Stevens WS, Venter WF, Osih R. Performance evaluation of the point-of-care CD4 analyser Pima using capillary blood sampling in field tests in South Africa. J Int AIDS Soc. 2012;15:3. doi: 10.1186/1758-2652-15-3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]