Abstract
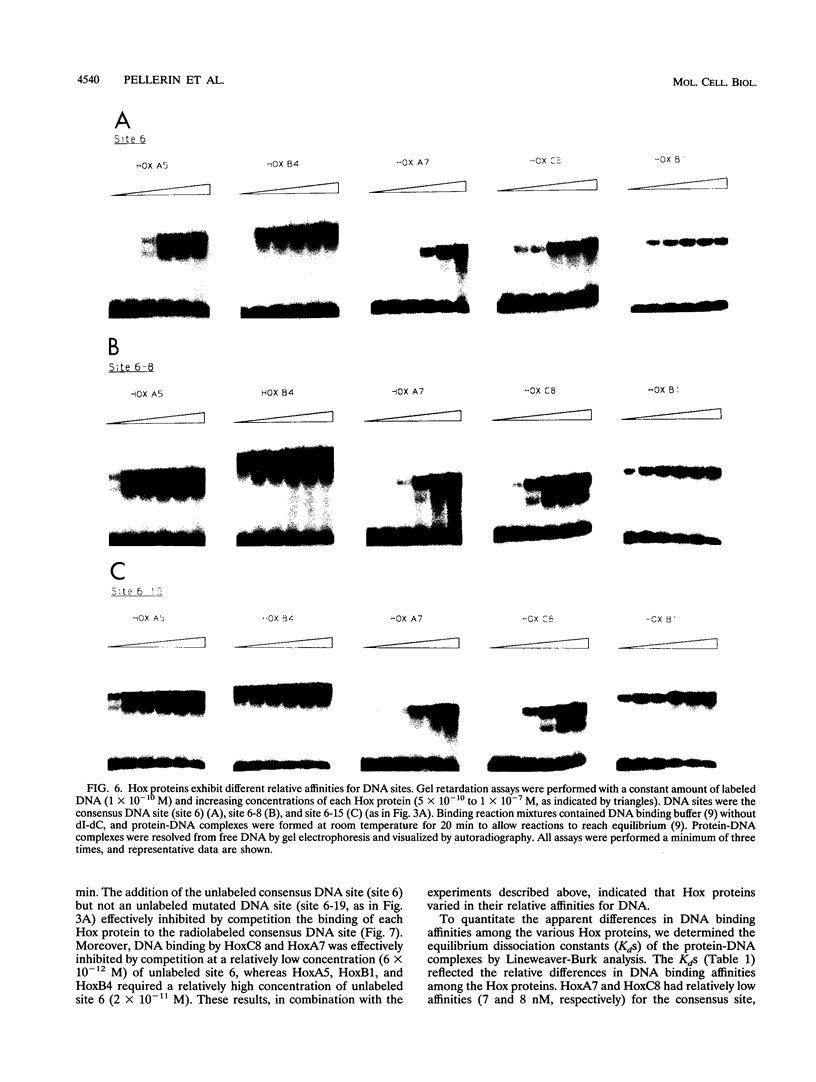
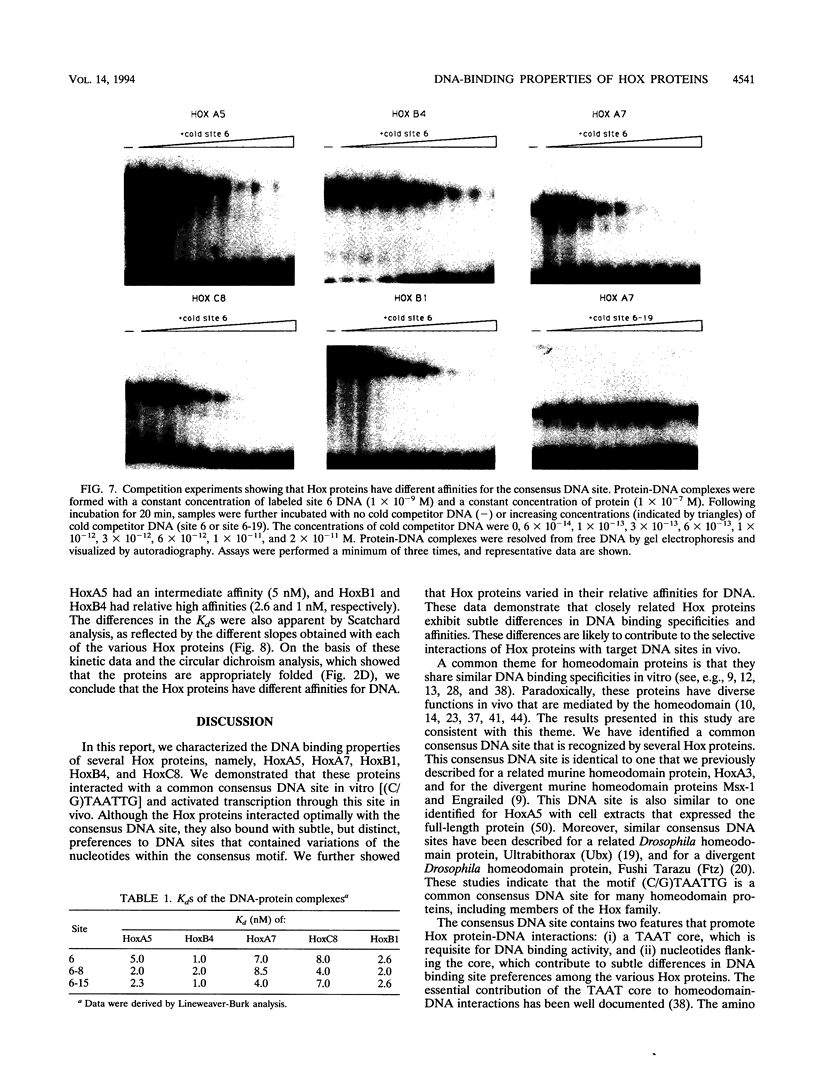
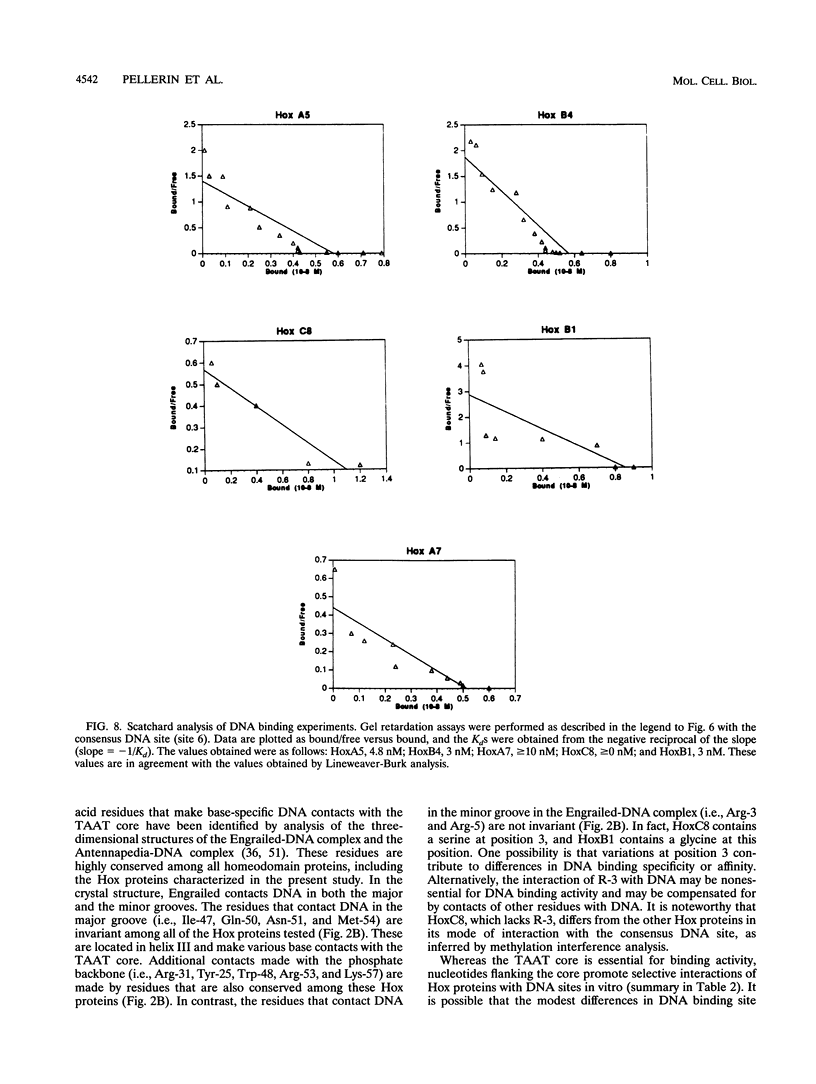
The hox genes, members of a family of essential developmental regulators, have the intriguing property that their expression in the developing murine embryo is colinear with their chromosomal organization. Members of the hox gene family share a conserved DNA binding domain, termed the homeodomain, which mediates interactions of Hox proteins with DNA regulatory elements in the transcriptional control regions of target genes. In this study, we characterized the DNA binding properties of five representative members of the Hox family: HoxA5, HoxB4, HoxA7, HoxC8, and HoxB1. To facilitate a comparative analysis of their DNA binding properties, we produced the homeodomain regions of these Hox proteins in Escherichia coli and obtained highly purified polypeptides. We showed that these Hox proteins interact in vitro with a common consensus DNA site that contains the motif (C/G)TAATTG. We further showed that the Hox proteins recognize the consensus DNA site in vivo, as determined by their ability to activate transcription through this site in transient transfection assays. Although they interact optimally with the consensus DNA site, the Hox proteins exhibit subtle, but distinct, preferences for DNA sites that contain variations of the nucleotides within the consensus motif. In addition to their modest differences in DNA binding specificities, the Hox proteins also vary in their relative affinities for DNA. Intriguingly, their relative affinities correlate with the positions of their respective genes on the hox cluster. These findings suggest that subtle differences in DNA binding specificity combined with differences in DNA binding affinity constitute features of the "Hox code" that contribute to the selective functions of Hox proteins during murine embryogenesis.
Full text
PDF

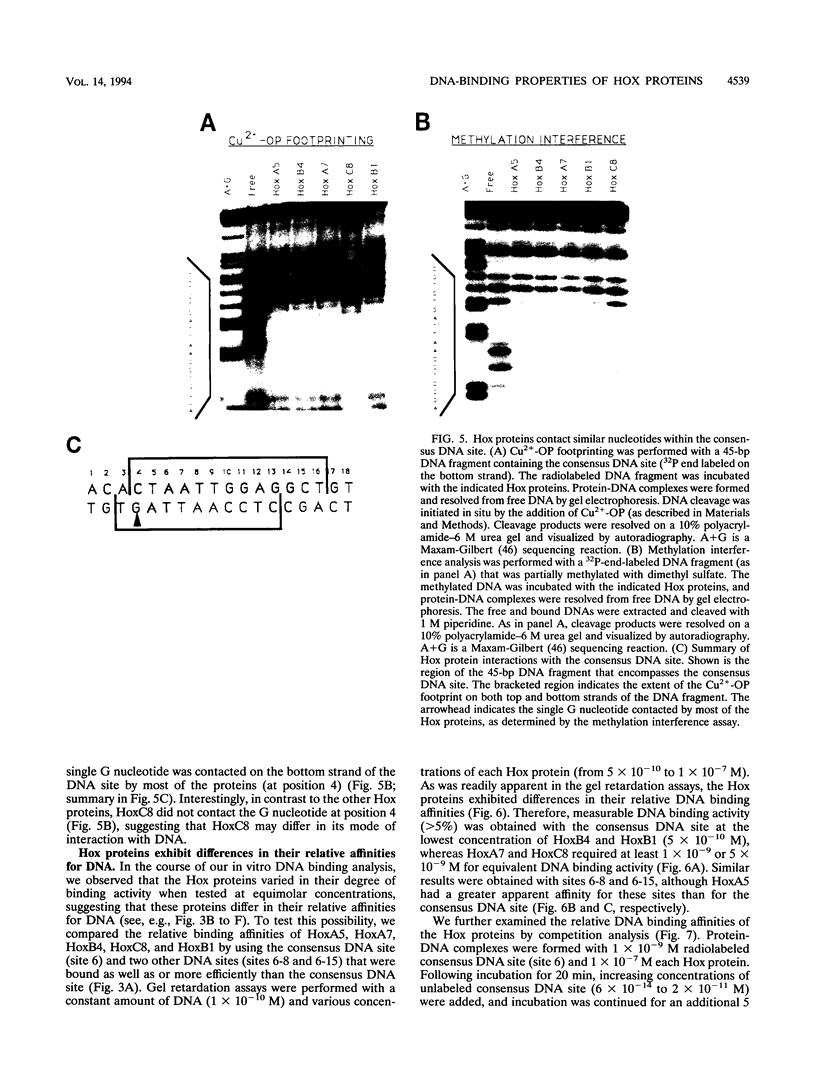



Images in this article
Selected References
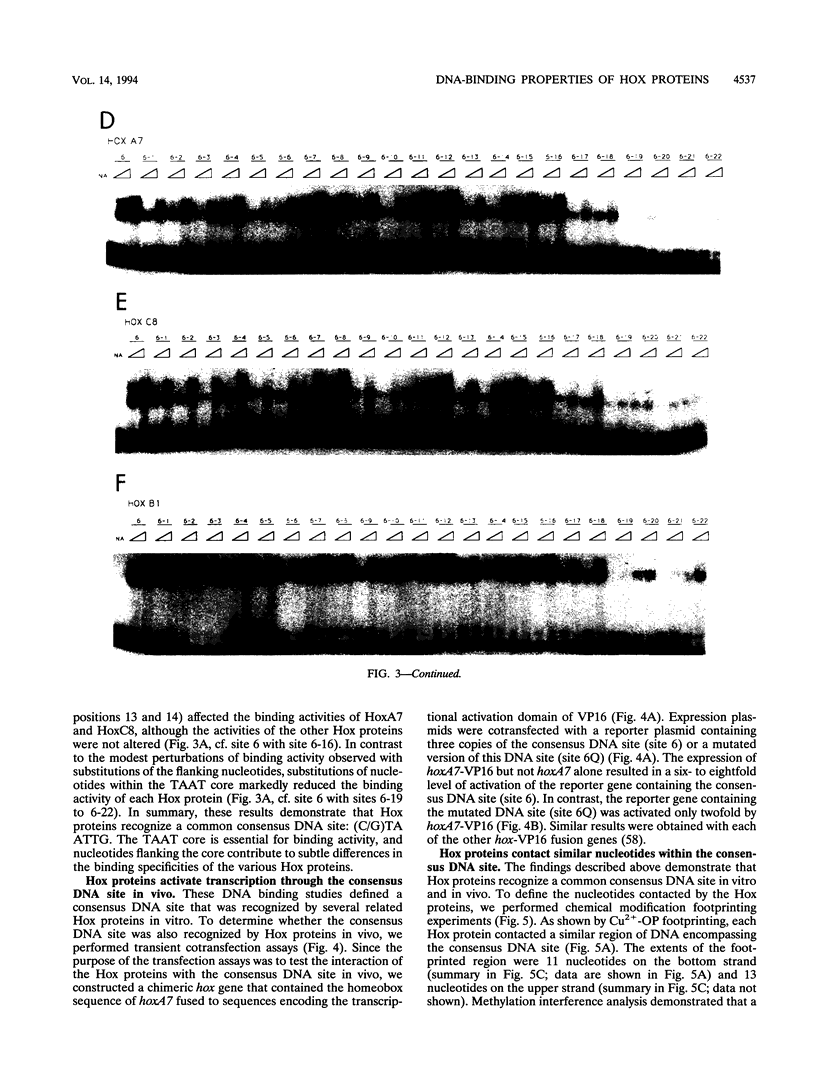
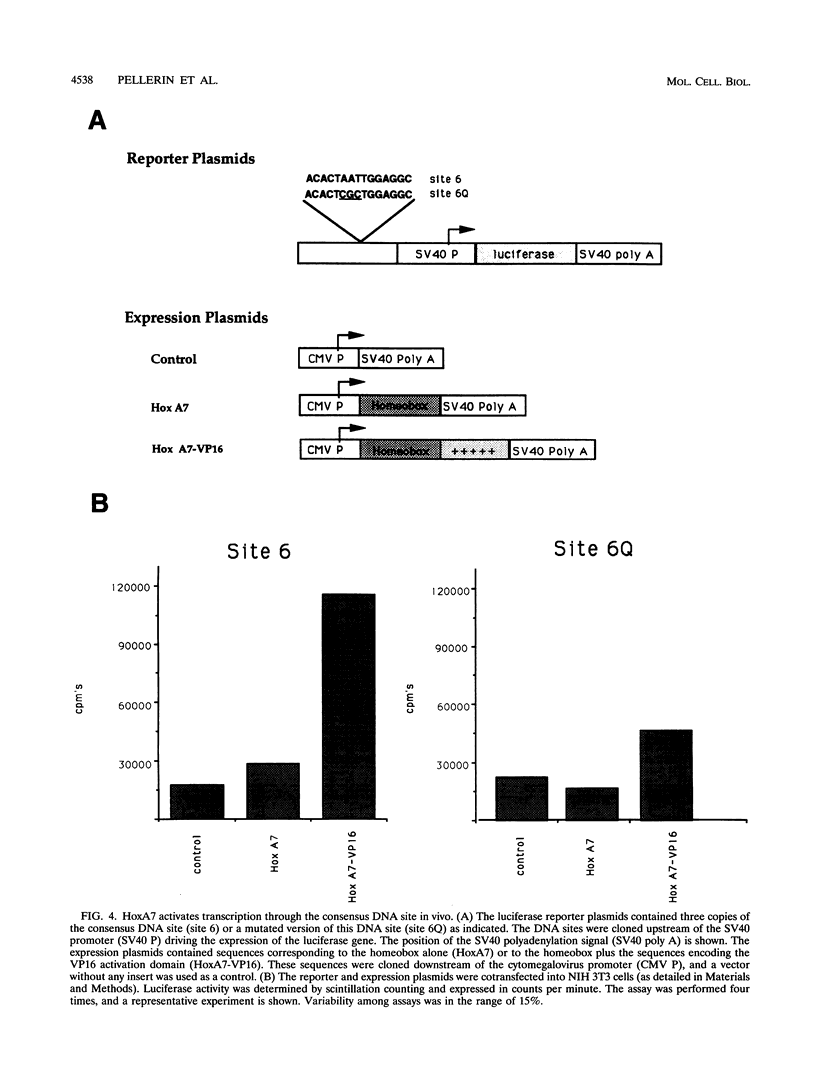
These references are in PubMed. This may not be the complete list of references from this article.
- Abate C., Luk D., Gentz R., Rauscher F. J., 3rd, Curran T. Expression and purification of the leucine zipper and DNA-binding domains of Fos and Jun: both Fos and Jun contact DNA directly. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1032–1036. doi: 10.1073/pnas.87.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affolter M., Percival-Smith A., Müller M., Leupin W., Gehring W. J. DNA binding properties of the purified Antennapedia homeodomain. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4093–4097. doi: 10.1073/pnas.87.11.4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akam M. Hox and HOM: homologous gene clusters in insects and vertebrates. Cell. 1989 May 5;57(3):347–349. doi: 10.1016/0092-8674(89)90909-4. [DOI] [PubMed] [Google Scholar]
- Balling R., Mutter G., Gruss P., Kessel M. Craniofacial abnormalities induced by ectopic expression of the homeobox gene Hox-1.1 in transgenic mice. Cell. 1989 Jul 28;58(2):337–347. doi: 10.1016/0092-8674(89)90848-9. [DOI] [PubMed] [Google Scholar]
- Beachy P. A., Varkey J., Young K. E., von Kessler D. P., Sun B. I., Ekker S. C. Cooperative binding of an Ultrabithorax homeodomain protein to nearby and distant DNA sites. Mol Cell Biol. 1993 Nov;13(11):6941–6956. doi: 10.1128/mcb.13.11.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boncinelli E., Simeone A., Acampora D., Mavilio F. HOX gene activation by retinoic acid. Trends Genet. 1991 Oct;7(10):329–334. doi: 10.1016/0168-9525(91)90423-n. [DOI] [PubMed] [Google Scholar]
- Breier G., Dressler G. R., Gruss P. Primary structure and developmental expression pattern of Hox 3.1, a member of the murine Hox 3 homeobox gene cluster. EMBO J. 1988 May;7(5):1329–1336. doi: 10.1002/j.1460-2075.1988.tb02948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catron K. M., Iler N., Abate C. Nucleotides flanking a conserved TAAT core dictate the DNA binding specificity of three murine homeodomain proteins. Mol Cell Biol. 1993 Apr;13(4):2354–2365. doi: 10.1128/mcb.13.4.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. K., Mann R. S. The segment identity functions of Ultrabithorax are contained within its homeo domain and carboxy-terminal sequences. Genes Dev. 1993 May;7(5):796–811. doi: 10.1101/gad.7.5.796. [DOI] [PubMed] [Google Scholar]
- Chisaka O., Capecchi M. R. Regionally restricted developmental defects resulting from targeted disruption of the mouse homeobox gene hox-1.5. Nature. 1991 Apr 11;350(6318):473–479. doi: 10.1038/350473a0. [DOI] [PubMed] [Google Scholar]
- Corsetti M. T., Briata P., Sanseverino L., Daga A., Airoldi I., Simeone A., Palmisano G., Angelini C., Boncinelli E., Corte G. Differential DNA binding properties of three human homeodomain proteins. Nucleic Acids Res. 1992 Sep 11;20(17):4465–4472. doi: 10.1093/nar/20.17.4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplan C., Theis J., O'Farrell P. H. The sequence specificity of homeodomain-DNA interaction. Cell. 1988 Sep 23;54(7):1081–1090. doi: 10.1016/0092-8674(88)90123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessain S., Gross C. T., Kuziora M. A., McGinnis W. Antp-type homeodomains have distinct DNA binding specificities that correlate with their different regulatory functions in embryos. EMBO J. 1992 Mar;11(3):991–1002. doi: 10.1002/j.1460-2075.1992.tb05138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollé P., Izpisúa-Belmonte J. C., Falkenstein H., Renucci A., Duboule D. Coordinate expression of the murine Hox-5 complex homoeobox-containing genes during limb pattern formation. Nature. 1989 Dec 14;342(6251):767–772. doi: 10.1038/342767a0. [DOI] [PubMed] [Google Scholar]
- Driever W., Nüsslein-Volhard C. The bicoid protein determines position in the Drosophila embryo in a concentration-dependent manner. Cell. 1988 Jul 1;54(1):95–104. doi: 10.1016/0092-8674(88)90183-3. [DOI] [PubMed] [Google Scholar]
- Duboule D., Dollé P. The structural and functional organization of the murine HOX gene family resembles that of Drosophila homeotic genes. EMBO J. 1989 May;8(5):1497–1505. doi: 10.1002/j.1460-2075.1989.tb03534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekker S. C., Young K. E., von Kessler D. P., Beachy P. A. Optimal DNA sequence recognition by the Ultrabithorax homeodomain of Drosophila. EMBO J. 1991 May;10(5):1179–1186. doi: 10.1002/j.1460-2075.1991.tb08058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekker S. C., von Kessler D. P., Beachy P. A. Differential DNA sequence recognition is a determinant of specificity in homeotic gene action. EMBO J. 1992 Nov;11(11):4059–4072. doi: 10.1002/j.1460-2075.1992.tb05499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florence B., Handrow R., Laughon A. DNA-binding specificity of the fushi tarazu homeodomain. Mol Cell Biol. 1991 Jul;11(7):3613–3623. doi: 10.1128/mcb.11.7.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman M. A., Boyle M., Martin G. R. Isolation of the mouse Hox-2.9 gene; analysis of embryonic expression suggests that positional information along the anterior-posterior axis is specified by mesoderm. Development. 1990 Oct;110(2):589–607. doi: 10.1242/dev.110.2.589. [DOI] [PubMed] [Google Scholar]
- Gehring W. J. Homeo boxes in the study of development. Science. 1987 Jun 5;236(4806):1245–1252. doi: 10.1126/science.2884726. [DOI] [PubMed] [Google Scholar]
- Gibson G., Schier A., LeMotte P., Gehring W. J. The specificities of Sex combs reduced and Antennapedia are defined by a distinct portion of each protein that includes the homeodomain. Cell. 1990 Sep 21;62(6):1087–1103. doi: 10.1016/0092-8674(90)90386-s. [DOI] [PubMed] [Google Scholar]
- Graham A., Papalopulu N., Krumlauf R. The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell. 1989 May 5;57(3):367–378. doi: 10.1016/0092-8674(89)90912-4. [DOI] [PubMed] [Google Scholar]
- Graham A., Papalopulu N., Lorimer J., McVey J. H., Tuddenham E. G., Krumlauf R. Characterization of a murine homeo box gene, Hox-2.6, related to the Drosophila Deformed gene. Genes Dev. 1988 Nov;2(11):1424–1438. doi: 10.1101/gad.2.11.1424. [DOI] [PubMed] [Google Scholar]
- Grueneberg D. A., Natesan S., Alexandre C., Gilman M. Z. Human and Drosophila homeodomain proteins that enhance the DNA-binding activity of serum response factor. Science. 1992 Aug 21;257(5073):1089–1095. doi: 10.1126/science.257.5073.1089. [DOI] [PubMed] [Google Scholar]
- Hoch M., Gerwin N., Taubert H., Jäckle H. Competition for overlapping sites in the regulatory region of the Drosophila gene Krüppel. Science. 1992 Apr 3;256(5053):94–97. doi: 10.1126/science.1348871. [DOI] [PubMed] [Google Scholar]
- Hoey T., Levine M. Divergent homeo box proteins recognize similar DNA sequences in Drosophila. Nature. 1988 Apr 28;332(6167):858–861. doi: 10.1038/332858a0. [DOI] [PubMed] [Google Scholar]
- Holland P. W., Hogan B. L. Expression of homeo box genes during mouse development: a review. Genes Dev. 1988 Jul;2(7):773–782. doi: 10.1101/gad.2.7.773. [DOI] [PubMed] [Google Scholar]
- Hunt P., Krumlauf R. Deciphering the Hox code: clues to patterning branchial regions of the head. Cell. 1991 Sep 20;66(6):1075–1078. doi: 10.1016/0092-8674(91)90029-x. [DOI] [PubMed] [Google Scholar]
- Ingham P. W., Martinez Arias A. Boundaries and fields in early embryos. Cell. 1992 Jan 24;68(2):221–235. doi: 10.1016/0092-8674(92)90467-q. [DOI] [PubMed] [Google Scholar]
- Ingham P. W. The molecular genetics of embryonic pattern formation in Drosophila. Nature. 1988 Sep 1;335(6185):25–34. doi: 10.1038/335025a0. [DOI] [PubMed] [Google Scholar]
- Kessel M., Gruss P. Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell. 1991 Oct 4;67(1):89–104. doi: 10.1016/0092-8674(91)90574-i. [DOI] [PubMed] [Google Scholar]
- Kessel M., Gruss P. Murine developmental control genes. Science. 1990 Jul 27;249(4967):374–379. doi: 10.1126/science.1974085. [DOI] [PubMed] [Google Scholar]
- Kessel M., Schulze F., Fibi M., Gruss P. Primary structure and nuclear localization of a murine homeodomain protein. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5306–5310. doi: 10.1073/pnas.84.15.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissinger C. R., Liu B. S., Martin-Blanco E., Kornberg T. B., Pabo C. O. Crystal structure of an engrailed homeodomain-DNA complex at 2.8 A resolution: a framework for understanding homeodomain-DNA interactions. Cell. 1990 Nov 2;63(3):579–590. doi: 10.1016/0092-8674(90)90453-l. [DOI] [PubMed] [Google Scholar]
- Kuziora M. A., McGinnis W. A homeodomain substitution changes the regulatory specificity of the deformed protein in Drosophila embryos. Cell. 1989 Nov 3;59(3):563–571. doi: 10.1016/0092-8674(89)90039-1. [DOI] [PubMed] [Google Scholar]
- Laughon A. DNA binding specificity of homeodomains. Biochemistry. 1991 Dec 3;30(48):11357–11367. doi: 10.1021/bi00112a001. [DOI] [PubMed] [Google Scholar]
- Le Mouellic H., Lallemand Y., Brûlet P. Homeosis in the mouse induced by a null mutation in the Hox-3.1 gene. Cell. 1992 Apr 17;69(2):251–264. doi: 10.1016/0092-8674(92)90406-3. [DOI] [PubMed] [Google Scholar]
- Levine M., Hoey T. Homeobox proteins as sequence-specific transcription factors. Cell. 1988 Nov 18;55(4):537–540. doi: 10.1016/0092-8674(88)90209-7. [DOI] [PubMed] [Google Scholar]
- Lin L., McGinnis W. Mapping functional specificity in the Dfd and Ubx homeo domains. Genes Dev. 1992 Jun;6(6):1071–1081. doi: 10.1101/gad.6.6.1071. [DOI] [PubMed] [Google Scholar]
- Lufkin T., Dierich A., LeMeur M., Mark M., Chambon P. Disruption of the Hox-1.6 homeobox gene results in defects in a region corresponding to its rostral domain of expression. Cell. 1991 Sep 20;66(6):1105–1119. doi: 10.1016/0092-8674(91)90034-v. [DOI] [PubMed] [Google Scholar]
- Lufkin T., Mark M., Hart C. P., Dollé P., LeMeur M., Chambon P. Homeotic transformation of the occipital bones of the skull by ectopic expression of a homeobox gene. Nature. 1992 Oct 29;359(6398):835–841. doi: 10.1038/359835a0. [DOI] [PubMed] [Google Scholar]
- Mann R. S., Hogness D. S. Functional dissection of Ultrabithorax proteins in D. melanogaster. Cell. 1990 Feb 23;60(4):597–610. doi: 10.1016/0092-8674(90)90663-y. [DOI] [PubMed] [Google Scholar]
- Marshall H., Nonchev S., Sham M. H., Muchamore I., Lumsden A., Krumlauf R. Retinoic acid alters hindbrain Hox code and induces transformation of rhombomeres 2/3 into a 4/5 identity. Nature. 1992 Dec 24;360(6406):737–741. doi: 10.1038/360737a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Garber R. L., Wirz J., Kuroiwa A., Gehring W. J. A homologous protein-coding sequence in Drosophila homeotic genes and its conservation in other metazoans. Cell. 1984 Jun;37(2):403–408. doi: 10.1016/0092-8674(84)90370-2. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Krumlauf R. Homeobox genes and axial patterning. Cell. 1992 Jan 24;68(2):283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- Mendel D. B., Khavari P. A., Conley P. B., Graves M. K., Hansen L. P., Admon A., Crabtree G. R. Characterization of a cofactor that regulates dimerization of a mammalian homeodomain protein. Science. 1991 Dec 20;254(5039):1762–1767. doi: 10.1126/science.1763325. [DOI] [PubMed] [Google Scholar]
- Odenwald W. F., Garbern J., Arnheiter H., Tournier-Lasserve E., Lazzarini R. A. The Hox-1.3 homeo box protein is a sequence-specific DNA-binding phosphoprotein. Genes Dev. 1989 Feb;3(2):158–172. doi: 10.1101/gad.3.2.158. [DOI] [PubMed] [Google Scholar]
- Otting G., Qian Y. Q., Billeter M., Müller M., Affolter M., Gehring W. J., Wüthrich K. Protein--DNA contacts in the structure of a homeodomain--DNA complex determined by nuclear magnetic resonance spectroscopy in solution. EMBO J. 1990 Oct;9(10):3085–3092. doi: 10.1002/j.1460-2075.1990.tb07505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan S., Gashler A., Siegel M. G., Chang L. C., Joseph L. J., Shows T. B., Le Beau M. M., Sukhatme V. P. EGR3, a novel member of the Egr family of genes encoding immediate-early transcription factors. Oncogene. 1991 Jun;6(6):917–928. [PubMed] [Google Scholar]
- Percival-Smith A., Müller M., Affolter M., Gehring W. J. The interaction with DNA of wild-type and mutant fushi tarazu homeodomains. EMBO J. 1990 Dec;9(12):3967–3974. doi: 10.1002/j.1460-2075.1990.tb07617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Solis R., Zheng H., Whiting J., Krumlauf R., Bradley A. Hoxb-4 (Hox-2.6) mutant mice show homeotic transformation of a cervical vertebra and defects in the closure of the sternal rudiments. Cell. 1993 Apr 23;73(2):279–294. doi: 10.1016/0092-8674(93)90229-j. [DOI] [PubMed] [Google Scholar]
- Sadowski I., Ma J., Triezenberg S., Ptashne M. GAL4-VP16 is an unusually potent transcriptional activator. Nature. 1988 Oct 6;335(6190):563–564. doi: 10.1038/335563a0. [DOI] [PubMed] [Google Scholar]
- Scott M. P., Tamkun J. W., Hartzell G. W., 3rd The structure and function of the homeodomain. Biochim Biophys Acta. 1989 Jul 28;989(1):25–48. doi: 10.1016/0304-419x(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Scott M. P. Vertebrate homeobox gene nomenclature. Cell. 1992 Nov 13;71(4):551–553. doi: 10.1016/0092-8674(92)90588-4. [DOI] [PubMed] [Google Scholar]
- Shang Z., Ebright Y. W., Iler N., Pendergrast P. S., Echelard Y., McMahon A. P., Ebright R. H., Abate C. DNA affinity cleaving analysis of homeodomain-DNA interaction: identification of homeodomain consensus sites in genomic DNA. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):118–122. doi: 10.1073/pnas.91.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigman D. S., Kuwabara M. D., Chen C. H., Bruice T. W. Nuclease activity of 1,10-phenanthroline-copper in study of protein-DNA interactions. Methods Enzymol. 1991;208:414–433. doi: 10.1016/0076-6879(91)08022-a. [DOI] [PubMed] [Google Scholar]
- Spaete R. R., Mocarski E. S. Regulation of cytomegalovirus gene expression: alpha and beta promoters are trans activated by viral functions in permissive human fibroblasts. J Virol. 1985 Oct;56(1):135–143. doi: 10.1128/jvi.56.1.135-143.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward R. Relocalization of the dorsal protein from the cytoplasm to the nucleus correlates with its function. Cell. 1989 Dec 22;59(6):1179–1188. doi: 10.1016/0092-8674(89)90773-3. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bhatt S., Cook M., Boncinelli E., Krumlauf R. Segmental expression of Hox-2 homoeobox-containing genes in the developing mouse hindbrain. Nature. 1989 Oct 5;341(6241):405–409. doi: 10.1038/341405a0. [DOI] [PubMed] [Google Scholar]
- Wissmann A., Hillen W. DNA contacts probed by modification protection and interference studies. Methods Enzymol. 1991;208:365–379. doi: 10.1016/0076-6879(91)08020-i. [DOI] [PubMed] [Google Scholar]
- Xue D., Tu Y., Chalfie M. Cooperative interactions between the Caenorhabditis elegans homeoproteins UNC-86 and MEC-3. Science. 1993 Sep 3;261(5126):1324–1328. doi: 10.1126/science.8103239. [DOI] [PubMed] [Google Scholar]
- Zeng W., Andrew D. J., Mathies L. D., Horner M. A., Scott M. P. Ectopic expression and function of the Antp and Scr homeotic genes: the N terminus of the homeodomain is critical to functional specificity. Development. 1993 Jun;118(2):339–352. doi: 10.1242/dev.118.2.339. [DOI] [PubMed] [Google Scholar]