Abstract
In the title molecule, C10H8O2, all non-H atoms are essentailly coplanar (r.m.s. deviation = 0.0192 Å), indicating an effective conjugation of the carbonyl group, the benzene ring and the lone pair of the propynyloxy O atom. In the crystal, π–π stacking interactions [centroid–centroid distance = 3.5585 (15) Å] connect molecules into inversion dimers which are linked by Csp—H⋯O=C hydrogen bonds, forming a ladder-like structure.
Related literature
For related structures of 4-(prop-2-yn-1-yloxy)benzenes, see: Berscheid et al. (1992 ▶); Mohr et al. (2003 ▶); Nieger et al. (2004 ▶); Ranjith et al. (2010 ▶); Zhang et al. (2011 ▶).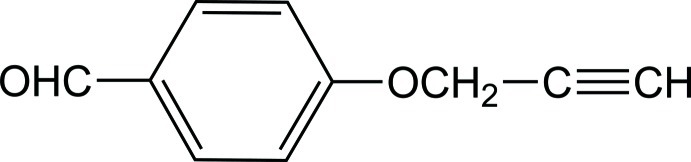
Experimental
Crystal data
C10H8O2
M r = 160.17
Monoclinic,
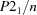
a = 7.906 (3) Å
b = 7.385 (2) Å
c = 14.036 (5) Å
β = 102.025 (5)°
V = 801.5 (5) Å3
Z = 4
Mo Kα radiation
μ = 0.09 mm−1
T = 93 K
0.20 × 0.10 × 0.10 mm
Data collection
Rigaku Saturn724+ diffractometer
Absorption correction: numerical (NUMABS; Rigaku, 1999 ▶) T min = 0.984, T max = 0.991
6309 measured reflections
1832 independent reflections
1669 reflections with F 2 > 2σ(F 2)
R int = 0.044
Refinement
R[F 2 > 2σ(F 2)] = 0.037
wR(F 2) = 0.101
S = 1.06
1831 reflections
141 parameters
H-atom parameters constrained
Δρmax = 0.32 e Å−3
Δρmin = −0.17 e Å−3
Data collection: CrystalClear (Rigaku, 2008 ▶); cell refinement: CrystalClear; data reduction: CrystalClear; program(s) used to solve structure: SHELXD (Schneider & Sheldrick, 2002 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 2012 ▶); software used to prepare material for publication: CrystalStructure (Rigaku, 2010 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812050866/lh5571sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812050866/lh5571Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812050866/lh5571Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C10—H10⋯O1i | 0.95 | 2.23 | 3.1575 (14) | 166 |
Symmetry code: (i) 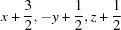 .
.
Acknowledgments
This work was supported by the Research for Promoting Technological Seeds from the Japan Science and Technology Agency (JST).
supplementary crystallographic information
Comment
The title compound, C10H8O2, is a benzaldehyde derivative whose structure is often observed in macrocyclic compounds (Berscheid et al. 1992; Mohr et al. 2003). Analogues of the title compound have already been reported as an ester (Nieger et al., 2004), as a ketone (Ranjith et al., 2010) and as an α,β-unsatuated ketone (Zhang et al., 2011). The molecule has a planar structure (atoms C1—C10/O1—O2 are essentailly co-planar with an r.m.s. deviation = 0.0192 Å), indicating an effective conjugation of the carbonyl group, the C1—C6 benzene ring and the lone pair of atom O2.
The molecular structure of the title compound is shown in Fig. 1. In the crystal, molecules form dimers across inversion centers (Fig. 2), owing to π–π stacking interactions. The intermolecular distance of C4···C6iii is 3.3026 (16) Å [Symmetry code:(iii) -x + 1, -y + 1, -z + 2.]. The molecules also form weak intermolecular C—H···O═C hydrogen bonds between the carbonyl oxygen and acetylene group to give a ladder-like structure where the distances of C10···O1i and O1···C10ii are 3.1575 (14) Å [Symmetry codes:(i) x + 3/2, -y + 1/2, z + 1/2 and (ii) x - 3/2, -y + 1/2, z - 1/2.].
Experimental
A mixture of 4-hydroxybenzaldehyde (1.22 g, 10 mmol) and 3-bromoprop-1-yne (3.57 g, 30 mmol) in 1-methylpyrrolidin-2-one (20 ml) was heated at 473K for 2 h in the presence of K2CO3 (4.15 g). The solution was poured into water and extracted by benzene (100 ml). The organic layer was washed with 5% NaOHaq, 5% Na2CO3aq and water, and was dried over Na2SO4. After removal of Na2SO4 and benzene, the residue was recrystallized by hexane to give 0.96 g (60%) of the title compound as a pale yellow powder. Single crystals with sufficient quality were prepared by sublimation at room temperature.
Refinement
The C-bound H atoms were placed at ideal positions and were refined as riding on their parent C atoms. Uiso(H) values of the H atoms were set at 1.2Ueq(parent atom).
Figures
Fig. 1.
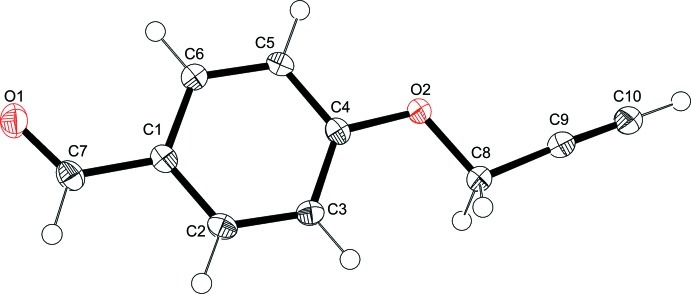
The molecular structure of the title compound with displacement ellipsoids drawn at the 50% probability level and H atoms are shown as small spheres.
Fig. 2.
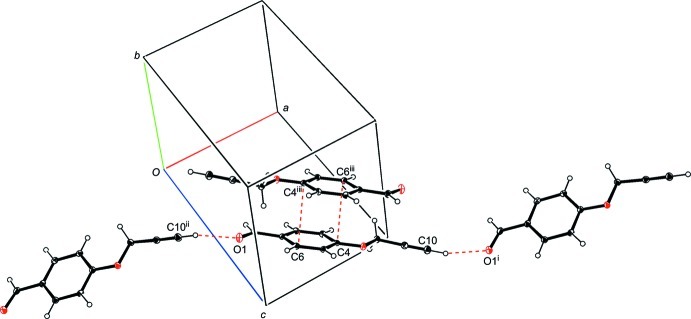
Part of the crystal structure showing the ladder-like array formed by π–π stacking interactions and weak C—H···O hydrogen bonds (dashed lines) [Symmetry codes: (i) x + 3/2, -y + 1/2, z + 1/2; (ii) x - 3/2, -y + 1/2, z - 1/2; (iii) -x + 1, -y + 1, -z + 2].
Crystal data
| C10H8O2 | F(000) = 336.00 |
| Mr = 160.17 | Dx = 1.327 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71075 Å |
| Hall symbol: -P 2yn | Cell parameters from 2598 reflections |
| a = 7.906 (3) Å | θ = 2.6–31.1° |
| b = 7.385 (2) Å | µ = 0.09 mm−1 |
| c = 14.036 (5) Å | T = 93 K |
| β = 102.025 (5)° | Prism, colorless |
| V = 801.5 (5) Å3 | 0.20 × 0.10 × 0.10 mm |
| Z = 4 |
Data collection
| Rigaku Saturn724+ diffractometer | 1669 reflections with F2 > 2σ(F2) |
| Detector resolution: 7.111 pixels mm-1 | Rint = 0.044 |
| ω scans | θmax = 27.5° |
| Absorption correction: numerical (NUMABS; Rigaku, 1999) | h = −10→10 |
| Tmin = 0.984, Tmax = 0.991 | k = −9→8 |
| 6309 measured reflections | l = −18→13 |
| 1832 independent reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.037 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.101 | H-atom parameters constrained |
| S = 1.06 | w = 1/[σ2(Fo2) + (0.0503P)2 + 0.2315P] where P = (Fo2 + 2Fc2)/3 |
| 1831 reflections | (Δ/σ)max = 0.001 |
| 141 parameters | Δρmax = 0.32 e Å−3 |
| 0 restraints | Δρmin = −0.17 e Å−3 |
| Primary atom site location: structure-invariant direct methods |
Special details
| Refinement. Refinement was performed using all reflections. except for one with very negative F2. The weighted R-factor (wR) and goodness of fit (S) are based on F2. R-factor (gt) are based on F. The threshold expression of F2 > 2.0 σ(F2) is used only for calculating R-factor (gt). |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | −0.02874 (9) | 0.43434 (11) | 0.86519 (6) | 0.0269 (3) | |
| O2 | 0.72238 (8) | 0.20161 (10) | 1.09425 (5) | 0.0184 (2) | |
| C1 | 0.26055 (12) | 0.32435 (14) | 0.90578 (7) | 0.0169 (3) | |
| C2 | 0.39397 (13) | 0.24978 (14) | 0.86774 (7) | 0.0183 (3) | |
| C3 | 0.55122 (13) | 0.20458 (14) | 0.92785 (7) | 0.0176 (3) | |
| C4 | 0.57337 (12) | 0.23630 (13) | 1.02780 (7) | 0.0160 (3) | |
| C5 | 0.43929 (12) | 0.30938 (13) | 1.06687 (7) | 0.0167 (3) | |
| C6 | 0.28397 (12) | 0.35345 (13) | 1.00640 (7) | 0.0171 (3) | |
| C7 | 0.09823 (13) | 0.37510 (14) | 0.83968 (7) | 0.0206 (3) | |
| C8 | 0.86477 (12) | 0.13260 (14) | 1.05624 (7) | 0.0185 (3) | |
| C9 | 1.00867 (12) | 0.09585 (15) | 1.13789 (7) | 0.0198 (3) | |
| C10 | 1.12987 (14) | 0.06479 (16) | 1.20170 (8) | 0.0242 (3) | |
| H2 | 0.3760 | 0.2314 | 0.7979 | 0.0271* | |
| H3 | 0.6428 | 0.1534 | 0.9001 | 0.0187* | |
| H5 | 0.4618 | 0.3309 | 1.1384 | 0.0247* | |
| H6 | 0.1910 | 0.4060 | 1.0336 | 0.0199* | |
| H7 | 0.1033 | 0.3559 | 0.7691 | 0.0267* | |
| H8A | 0.8996 | 0.2240 | 1.0127 | 0.0191* | |
| H8B | 0.8295 | 0.0199 | 1.0208 | 0.0222* | |
| H10 | 1.2291 | 0.0429 | 1.2516 | 0.0372* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0204 (4) | 0.0323 (5) | 0.0256 (5) | 0.0041 (3) | −0.0007 (3) | −0.0039 (3) |
| O2 | 0.0168 (4) | 0.0228 (4) | 0.0150 (4) | 0.0031 (3) | 0.0020 (3) | −0.0004 (3) |
| C1 | 0.0187 (5) | 0.0144 (5) | 0.0168 (5) | −0.0028 (4) | 0.0016 (4) | 0.0003 (4) |
| C2 | 0.0219 (5) | 0.0178 (5) | 0.0149 (5) | −0.0030 (4) | 0.0030 (4) | −0.0011 (4) |
| C3 | 0.0194 (5) | 0.0172 (5) | 0.0169 (5) | −0.0003 (4) | 0.0051 (4) | −0.0014 (4) |
| C4 | 0.0174 (5) | 0.0134 (5) | 0.0162 (5) | −0.0015 (4) | 0.0015 (4) | 0.0013 (4) |
| C5 | 0.0197 (5) | 0.0167 (5) | 0.0138 (5) | −0.0015 (4) | 0.0034 (4) | 0.0004 (4) |
| C6 | 0.0177 (5) | 0.0158 (5) | 0.0180 (5) | −0.0007 (4) | 0.0040 (4) | −0.0003 (4) |
| C7 | 0.0214 (5) | 0.0203 (5) | 0.0182 (5) | −0.0019 (4) | −0.0004 (4) | −0.0022 (4) |
| C8 | 0.0179 (5) | 0.0202 (5) | 0.0177 (5) | 0.0025 (4) | 0.0041 (4) | −0.0002 (4) |
| C9 | 0.0196 (5) | 0.0207 (5) | 0.0199 (5) | 0.0007 (4) | 0.0060 (4) | −0.0011 (4) |
| C10 | 0.0213 (5) | 0.0303 (6) | 0.0210 (5) | 0.0026 (5) | 0.0045 (4) | −0.0001 (5) |
Geometric parameters (Å, º)
| O1—C7 | 1.2154 (14) | C8—C9 | 1.4622 (13) |
| O2—C4 | 1.3659 (11) | C9—C10 | 1.1897 (14) |
| O2—C8 | 1.4360 (13) | C2—H2 | 0.970 |
| C1—C2 | 1.3913 (16) | C3—H3 | 0.968 |
| C1—C6 | 1.4021 (15) | C5—H5 | 0.995 |
| C1—C7 | 1.4670 (14) | C6—H6 | 0.977 |
| C2—C3 | 1.3899 (14) | C7—H7 | 1.010 |
| C3—C4 | 1.3968 (15) | C8—H8A | 0.988 |
| C4—C5 | 1.3993 (15) | C8—H8B | 0.980 |
| C5—C6 | 1.3784 (13) | C10—H10 | 0.950 |
| O1···C6 | 2.8906 (13) | C7···H8Biv | 3.4936 |
| O2···C10 | 3.4132 (15) | C7···H10iii | 2.9850 |
| C1···C4 | 2.7763 (14) | C8···H2viii | 3.5206 |
| C2···C5 | 2.7778 (17) | C8···H6xiii | 3.3423 |
| C3···C6 | 2.8035 (17) | C8···H8Avi | 3.4770 |
| C3···C8 | 2.7953 (15) | C8···H8Bvi | 3.0613 |
| O1···O2i | 3.5839 (13) | C9···H2viii | 2.9585 |
| O1···C6ii | 3.3603 (15) | C9···H3vi | 3.4464 |
| O1···C8i | 3.5397 (16) | C9···H6xiii | 3.2166 |
| O1···C9i | 3.4739 (17) | C9···H8Avi | 3.3479 |
| O1···C10iii | 3.1575 (14) | C9···H8Bvi | 2.9185 |
| O2···O1i | 3.5839 (13) | C10···H2viii | 3.0407 |
| O2···C1i | 3.5034 (17) | C10···H3vi | 2.9896 |
| O2···C2iv | 3.5282 (16) | C10···H3viii | 3.4607 |
| O2···C6i | 3.5729 (15) | C10···H5xiii | 3.5367 |
| O2···C7i | 3.4779 (15) | C10···H5xiv | 3.0372 |
| C1···O2i | 3.5034 (17) | C10···H6xiii | 3.5534 |
| C1···C4i | 3.5512 (17) | C10···H8Bvi | 3.2660 |
| C1···C5i | 3.5660 (16) | H2···O1xi | 3.5583 |
| C1···C8iv | 3.5880 (18) | H2···O2v | 2.9057 |
| C2···O2iv | 3.5282 (16) | H2···C7xi | 3.2950 |
| C2···C5i | 3.5602 (17) | H2···C8v | 3.5206 |
| C2···C10v | 3.5491 (18) | H2···C9v | 2.9585 |
| C3···C4iv | 3.4949 (17) | H2···C10v | 3.0407 |
| C3···C5i | 3.5907 (18) | H2···H5i | 3.5239 |
| C3···C6i | 3.5640 (17) | H2···H5v | 3.5908 |
| C4···C1i | 3.5512 (17) | H2···H7xi | 2.9435 |
| C4···C3iv | 3.4949 (17) | H2···H10v | 3.4275 |
| C4···C6i | 3.3026 (16) | H3···O1xiii | 3.4379 |
| C5···C1i | 3.5660 (16) | H3···C4iv | 3.5984 |
| C5···C2i | 3.5602 (17) | H3···C5iv | 3.5273 |
| C5···C3i | 3.5907 (18) | H3···C9vi | 3.4464 |
| C6···O1ii | 3.3603 (15) | H3···C10vi | 2.9896 |
| C6···O2i | 3.5729 (15) | H3···C10v | 3.4607 |
| C6···C3i | 3.5640 (17) | H3···H6i | 3.5598 |
| C6···C4i | 3.3026 (16) | H3···H7xi | 3.5107 |
| C7···O2i | 3.4779 (15) | H3···H10vi | 2.9257 |
| C8···O1i | 3.5397 (16) | H3···H10v | 3.2297 |
| C8···C1iv | 3.5880 (18) | H5···C1i | 3.5007 |
| C8···C8vi | 3.5082 (17) | H5···C2i | 3.3077 |
| C8···C9vi | 3.5225 (17) | H5···C3i | 3.5504 |
| C9···O1i | 3.4739 (17) | H5···C7viii | 3.1921 |
| C9···C8vi | 3.5225 (17) | H5···C10x | 3.5367 |
| C10···O1vii | 3.1575 (14) | H5···C10xii | 3.0372 |
| C10···C2viii | 3.5491 (18) | H5···H2i | 3.5239 |
| O1···H6 | 2.6337 | H5···H2viii | 3.5908 |
| O2···H3 | 2.6899 | H5···H7viii | 2.3799 |
| O2···H5 | 2.4642 | H5···H10x | 3.4131 |
| C1···H3 | 3.2914 | H5···H10xii | 2.8432 |
| C1···H5 | 3.3209 | H6···O1ii | 2.4110 |
| C2···H6 | 3.2988 | H6···O2i | 3.5493 |
| C2···H7 | 2.5468 | H6···C3i | 3.5012 |
| C3···H5 | 3.3126 | H6···C4i | 3.4439 |
| C3···H8A | 2.7677 | H6···C7ii | 3.5637 |
| C3···H8B | 2.6843 | H6···C8x | 3.3423 |
| C4···H2 | 3.2789 | H6···C9x | 3.2166 |
| C4···H6 | 3.2895 | H6···C10x | 3.5534 |
| C4···H8A | 2.6337 | H6···H3i | 3.5598 |
| C4···H8B | 2.5972 | H6···H6ii | 3.2783 |
| C5···H3 | 3.3114 | H6···H8Ax | 2.6299 |
| C6···H2 | 3.2855 | H6···H8Ai | 2.8648 |
| C6···H7 | 3.3396 | H6···H8Biv | 3.2330 |
| C7···H2 | 2.6135 | H6···H10xii | 3.1175 |
| C7···H6 | 2.6767 | H7···O2v | 2.8373 |
| C8···H3 | 2.5110 | H7···C2ix | 3.4881 |
| C10···H8A | 3.1180 | H7···C4v | 3.4138 |
| C10···H8B | 3.1119 | H7···C5v | 3.1133 |
| H2···H3 | 2.3616 | H7···H2ix | 2.9435 |
| H2···H7 | 2.3008 | H7···H3ix | 3.5107 |
| H3···H8A | 2.3572 | H7···H5v | 2.3799 |
| H3···H8B | 2.2315 | H7···H10iii | 3.0118 |
| H5···H6 | 2.3967 | H8A···O1xiii | 2.7399 |
| O1···H2ix | 3.5583 | H8A···O1i | 3.1014 |
| O1···H3x | 3.4379 | H8A···C1xiii | 3.5673 |
| O1···H6ii | 2.4110 | H8A···C6xiii | 3.2041 |
| O1···H8Ax | 2.7399 | H8A···C6i | 3.4287 |
| O1···H8Ai | 3.1014 | H8A···C7xiii | 3.3458 |
| O1···H10iii | 2.2281 | H8A···C8vi | 3.4770 |
| O2···H2viii | 2.9057 | H8A···C9vi | 3.3479 |
| O2···H6i | 3.5493 | H8A···H6xiii | 2.6299 |
| O2···H7viii | 2.8373 | H8A···H6i | 2.8648 |
| C1···H5i | 3.5007 | H8A···H8Bvi | 2.9105 |
| C1···H8Ax | 3.5673 | H8B···C1iv | 2.8875 |
| C1···H8Biv | 2.8875 | H8B···C2iv | 3.2690 |
| C2···H5i | 3.3077 | H8B···C5iv | 3.2946 |
| C2···H7xi | 3.4881 | H8B···C6iv | 2.9001 |
| C2···H8Biv | 3.2690 | H8B···C7iv | 3.4936 |
| C3···H5i | 3.5504 | H8B···C8vi | 3.0613 |
| C3···H6i | 3.5012 | H8B···C9vi | 2.9185 |
| C4···H3iv | 3.5984 | H8B···C10vi | 3.2660 |
| C4···H6i | 3.4439 | H8B···H6iv | 3.2330 |
| C4···H7viii | 3.4138 | H8B···H8Avi | 2.9105 |
| C5···H3iv | 3.5273 | H8B···H8Bvi | 2.8905 |
| C5···H7viii | 3.1133 | H10···O1vii | 2.2281 |
| C5···H8Biv | 3.2946 | H10···C5xiv | 3.5569 |
| C5···H10xii | 3.5569 | H10···C7vii | 2.9850 |
| C6···H8Ax | 3.2041 | H10···H2viii | 3.4275 |
| C6···H8Ai | 3.4287 | H10···H3vi | 2.9257 |
| C6···H8Biv | 2.9001 | H10···H3viii | 3.2297 |
| C7···H2ix | 3.2950 | H10···H5xiii | 3.4131 |
| C7···H5v | 3.1921 | H10···H5xiv | 2.8432 |
| C7···H6ii | 3.5637 | H10···H6xiv | 3.1175 |
| C7···H8Ax | 3.3458 | H10···H7vii | 3.0118 |
| C4—O2—C8 | 116.38 (8) | C3—C2—H2 | 120.247 |
| C2—C1—C6 | 119.71 (9) | C2—C3—H3 | 119.929 |
| C2—C1—C7 | 119.45 (9) | C4—C3—H3 | 121.483 |
| C6—C1—C7 | 120.82 (10) | C4—C5—H5 | 117.787 |
| C1—C2—C3 | 121.06 (10) | C6—C5—H5 | 122.175 |
| C2—C3—C4 | 118.59 (10) | C1—C6—H6 | 120.143 |
| O2—C4—C3 | 124.34 (10) | C5—C6—H6 | 120.002 |
| O2—C4—C5 | 114.89 (9) | O1—C7—H7 | 122.939 |
| C3—C4—C5 | 120.77 (9) | C1—C7—H7 | 112.094 |
| C4—C5—C6 | 120.02 (10) | O2—C8—H8A | 109.216 |
| C1—C6—C5 | 119.85 (10) | O2—C8—H8B | 109.121 |
| O1—C7—C1 | 124.97 (10) | C9—C8—H8A | 109.879 |
| O2—C8—C9 | 108.48 (9) | C9—C8—H8B | 109.452 |
| C8—C9—C10 | 177.37 (12) | H8A—C8—H8B | 110.659 |
| C1—C2—H2 | 118.687 | C9—C10—H10 | 177.936 |
| C4—O2—C8—C9 | −177.31 (7) | C7—C1—C6—C5 | −178.20 (9) |
| C8—O2—C4—C3 | 1.73 (13) | C1—C2—C3—C4 | −0.28 (15) |
| C8—O2—C4—C5 | −177.84 (8) | C2—C3—C4—O2 | −178.56 (9) |
| C2—C1—C6—C5 | 0.50 (15) | C2—C3—C4—C5 | 0.99 (15) |
| C6—C1—C2—C3 | −0.46 (15) | O2—C4—C5—C6 | 178.63 (8) |
| C2—C1—C7—O1 | 176.92 (10) | C3—C4—C5—C6 | −0.96 (14) |
| C7—C1—C2—C3 | 178.26 (9) | C4—C5—C6—C1 | 0.20 (14) |
| C6—C1—C7—O1 | −4.38 (16) |
Symmetry codes: (i) −x+1, −y+1, −z+2; (ii) −x, −y+1, −z+2; (iii) x−3/2, −y+1/2, z−1/2; (iv) −x+1, −y, −z+2; (v) x−1/2, −y+1/2, z−1/2; (vi) −x+2, −y, −z+2; (vii) x+3/2, −y+1/2, z+1/2; (viii) x+1/2, −y+1/2, z+1/2; (ix) −x+1/2, y+1/2, −z+3/2; (x) x−1, y, z; (xi) −x+1/2, y−1/2, −z+3/2; (xii) −x+3/2, y+1/2, −z+5/2; (xiii) x+1, y, z; (xiv) −x+3/2, y−1/2, −z+5/2.
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C10—H10···O1vii | 0.95 | 2.23 | 3.1575 (14) | 166 |
Symmetry code: (vii) x+3/2, −y+1/2, z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: LH5571).
References
- Berscheid, R., Nieger, M. & Vogtle, F. (1992). Chem. Ber. 125, 2539–2552.
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Mohr, F., Eisler, D. J., McArdle, C. P., Atieh, K., Jennings, M. C. & Puddephatt, R. J. (2003). J. Organomet. Chem. 670, 27–36.
- Nieger, M., Michel, I. & Vogtle, F. (2004). Private communication (deposition number 246025). CCDC, Cambridge, England.
- Ranjith, S., Thirunarayanan, A., Raja, S., Rajakumar, P. & SubbiahPandi, A. (2010). Acta Cryst. E66, o2261–o2262. [DOI] [PMC free article] [PubMed]
- Rigaku (1999). NUMABS Rigaku Corporation, Tokyo, Japan.
- Rigaku (2008). CrystalClear Rigaku Corporation, Tokyo, Japan.
- Rigaku (2010). CrystalStructure Rigaku Corporation, Tokyo, Japan.
- Schneider, T. R. & Sheldrick, G. M. (2002). Acta Cryst. D58, 1772–1779. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Zhang, C.-H., Zhao, J.-M. & Chen, B.-G. (2011). Acta Cryst. E67, o150.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812050866/lh5571sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812050866/lh5571Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812050866/lh5571Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report


