Abstract
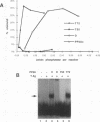
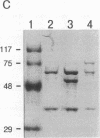
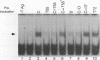
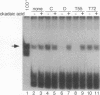
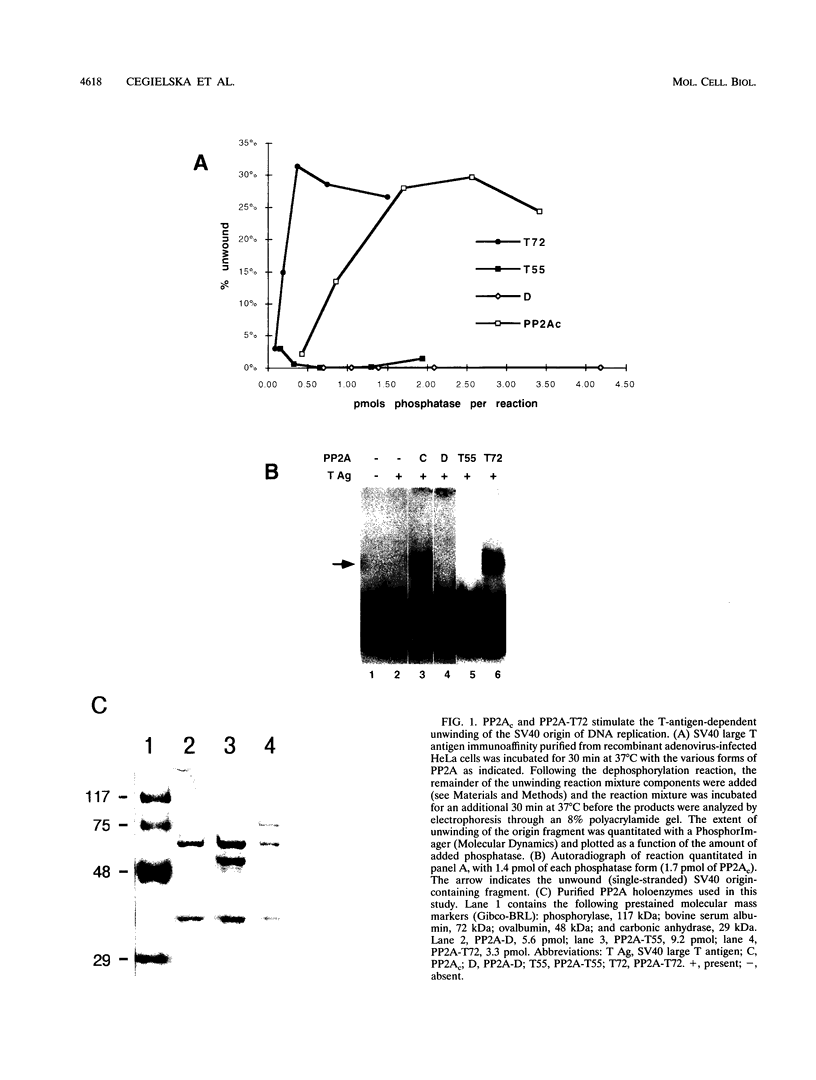
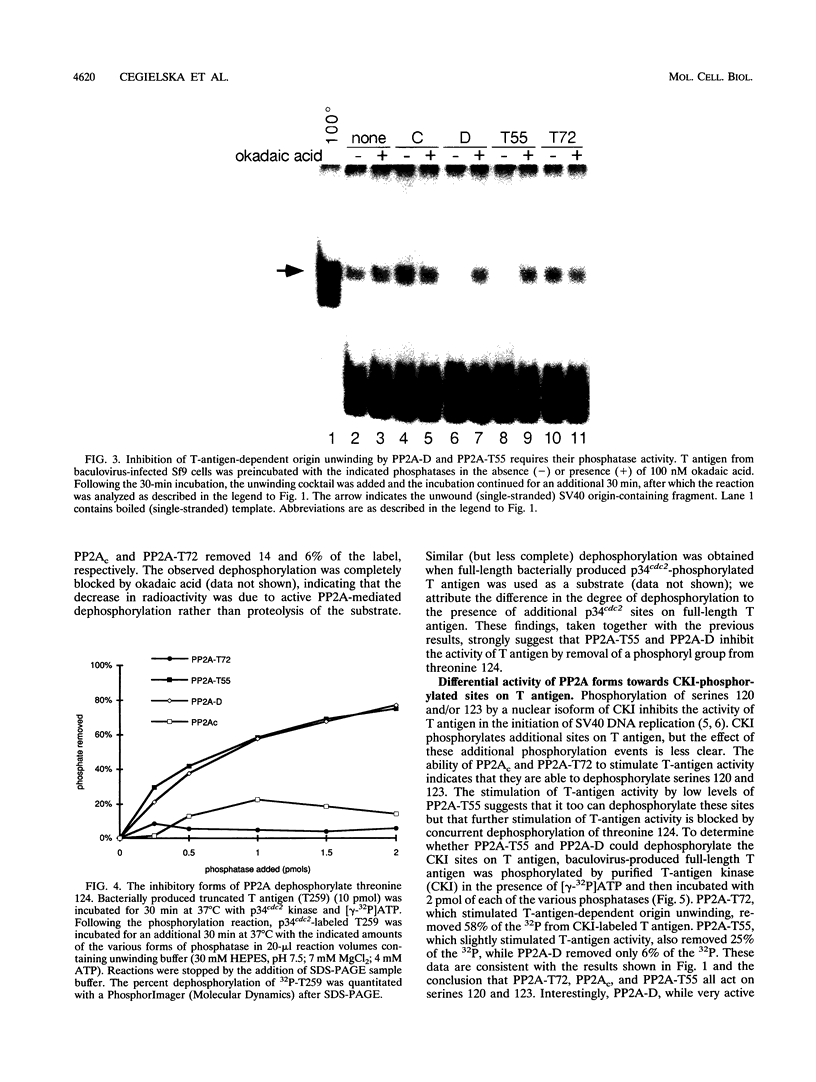
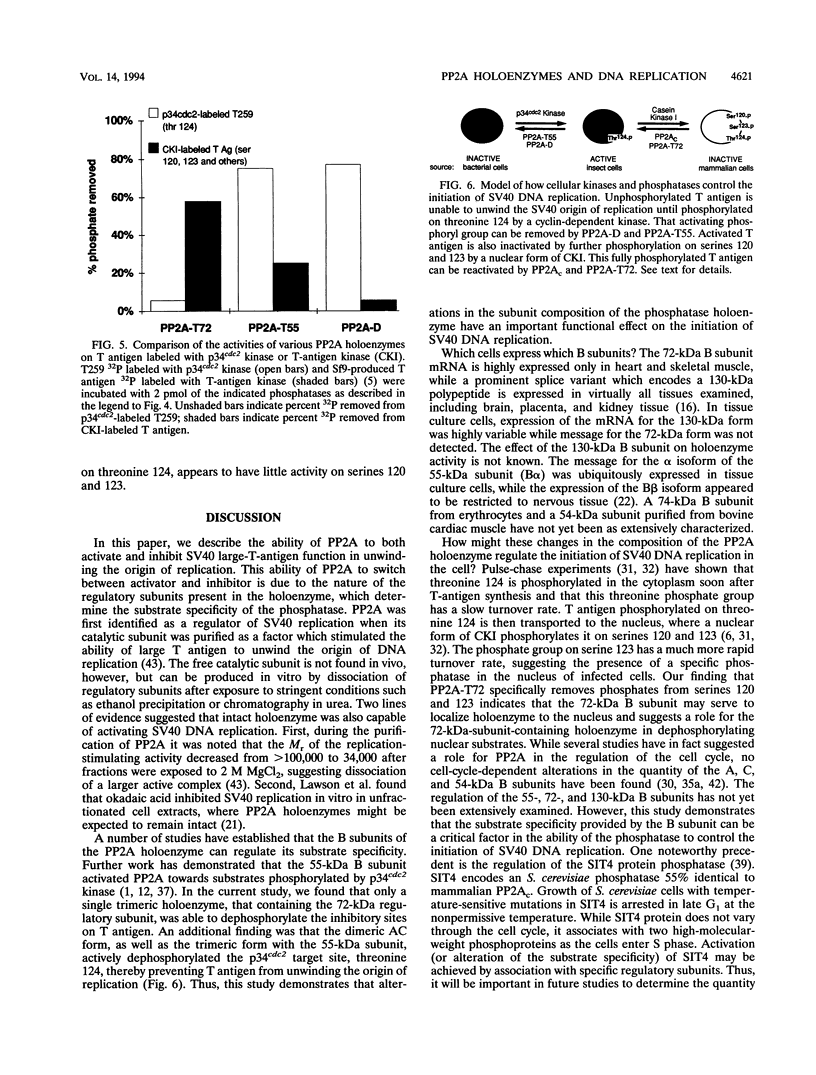
The ability of simian virus 40 (SV40) large T antigen to catalyze the initiation of viral DNA replication is regulated by its phosphorylation state. Previous studies have identified the free catalytic subunit of protein phosphatase 2A (PP2Ac) as the cellular phosphatase which can remove inhibitory phosphoryl groups from serines 120 and 123. The catalytic C subunit exists in the cell complexed with a 65-kDa A subunit and one of several B subunits. To determine if any of the holoenzymes could activate T antigen, we tested the ability of the heterodimeric AC and two heterotrimeric ABC forms to stimulate T-antigen function in unwinding the origin of SV40 DNA replication. Only free catalytic subunit C and the heterotrimeric form with a 72-kDa B subunit (PP2A-T72) could stimulate T-antigen-dependent origin unwinding. Both the dimeric form (PP2A-D) and the heterotrimer with a 55-kDa B subunit (PP2A-T55) actively inhibited T-antigen function. We found that PP2A-T72 activated T antigen by dephosphorylating serines 120 and 123, while PP2A-D and PP2A-T55 inactivated T antigen by dephosphorylating the p34cdc2 target site, threonine 124. Thus, alterations in the subunit composition of PP2A holoenzymes have significant functional consequences for the initiation of in vitro SV40 DNA replication. The regulatory B subunits of PP2A may play a role in regulating SV40 DNA replication in infected cells as well.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agostinis P., Derua R., Sarno S., Goris J., Merlevede W. Specificity of the polycation-stimulated (type-2A) and ATP,Mg-dependent (type-1) protein phosphatases toward substrates phosphorylated by P34cdc2 kinase. Eur J Biochem. 1992 Apr 1;205(1):241–248. doi: 10.1111/j.1432-1033.1992.tb16774.x. [DOI] [PubMed] [Google Scholar]
- Agostinis P., Goris J., Pinna L. A., Marchiori F., Perich J. W., Meyer H. E., Merlevede W. Synthetic peptides as model substrates for the study of the specificity of the polycation-stimulated protein phosphatases. Eur J Biochem. 1990 Apr 30;189(2):235–241. doi: 10.1111/j.1432-1033.1990.tb15482.x. [DOI] [PubMed] [Google Scholar]
- Agostinis P., Goris J., Waelkens E., Pinna L. A., Marchiori F., Merlevede W. Dephosphorylation of phosphoproteins and synthetic phosphopeptides. Study of the specificity of the polycation-stimulated and MgATP-dependent phosphorylase phosphatases. J Biol Chem. 1987 Jan 25;262(3):1060–1064. [PubMed] [Google Scholar]
- Cayla X., Ballmer-Hofer K., Merlevede W., Goris J. Phosphatase 2A associated with polyomavirus small-T or middle-T antigen is an okadaic acid-sensitive tyrosyl phosphatase. Eur J Biochem. 1993 May 15;214(1):281–286. doi: 10.1111/j.1432-1033.1993.tb17922.x. [DOI] [PubMed] [Google Scholar]
- Cegielska A., Moarefi I., Fanning E., Virshup D. M. T-antigen kinase inhibits simian virus 40 DNA replication by phosphorylation of intact T antigen on serines 120 and 123. J Virol. 1994 Jan;68(1):269–275. doi: 10.1128/jvi.68.1.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cegielska A., Virshup D. M. Control of simian virus 40 DNA replication by the HeLa cell nuclear kinase casein kinase I. Mol Cell Biol. 1993 Feb;13(2):1202–1211. doi: 10.1128/mcb.13.2.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Martin B. L., Brautigan D. L. Regulation of protein serine-threonine phosphatase type-2A by tyrosine phosphorylation. Science. 1992 Aug 28;257(5074):1261–1264. doi: 10.1126/science.1325671. [DOI] [PubMed] [Google Scholar]
- Chen Y. R., Lees-Miller S. P., Tegtmeyer P., Anderson C. W. The human DNA-activated protein kinase phosphorylates simian virus 40 T antigen at amino- and carboxy-terminal sites. J Virol. 1991 Oct;65(10):5131–5140. doi: 10.1128/jvi.65.10.5131-5140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Dobrowsky R. T., Kamibayashi C., Mumby M. C., Hannun Y. A. Ceramide activates heterotrimeric protein phosphatase 2A. J Biol Chem. 1993 Jul 25;268(21):15523–15530. [PubMed] [Google Scholar]
- Fanning E., Knippers R. Structure and function of simian virus 40 large tumor antigen. Annu Rev Biochem. 1992;61:55–85. doi: 10.1146/annurev.bi.61.070192.000415. [DOI] [PubMed] [Google Scholar]
- Ferrigno P., Langan T. A., Cohen P. Protein phosphatase 2A1 is the major enzyme in vertebrate cell extracts that dephosphorylates several physiological substrates for cyclin-dependent protein kinases. Mol Biol Cell. 1993 Jul;4(7):669–677. doi: 10.1091/mbc.4.7.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grässer F. A., Mann K., Walter G. Removal of serine phosphates from simian virus 40 large T antigen increases its ability to stimulate DNA replication in vitro but has no effect on ATPase and DNA binding. J Virol. 1987 Nov;61(11):3373–3380. doi: 10.1128/jvi.61.11.3373-3380.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H., Damuni Z. Autophosphorylation-activated protein kinase phosphorylates and inactivates protein phosphatase 2A. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2500–2504. doi: 10.1073/pnas.90.6.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy A. M., Zolnierowicz S., Stapleton A. E., Goebl M., DePaoli-Roach A. A., Pringle J. R. CDC55, a Saccharomyces cerevisiae gene involved in cellular morphogenesis: identification, characterization, and homology to the B subunit of mammalian type 2A protein phosphatase. Mol Cell Biol. 1991 Nov;11(11):5767–5780. doi: 10.1128/mcb.11.11.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix P., Mayer-Jackel R. E., Cron P., Goris J., Hofsteenge J., Merlevede W., Hemmings B. A. Structure and expression of a 72-kDa regulatory subunit of protein phosphatase 2A. Evidence for different size forms produced by alternative splicing. J Biol Chem. 1993 Jul 15;268(20):15267–15276. [PubMed] [Google Scholar]
- Hendrix P., Turowski P., Mayer-Jaekel R. E., Goris J., Hofsteenge J., Merlevede W., Hemmings B. A. Analysis of subunit isoforms in protein phosphatase 2A holoenzymes from rabbit and Xenopus. J Biol Chem. 1993 Apr 5;268(10):7330–7337. [PubMed] [Google Scholar]
- Höss A., Moarefi I., Scheidtmann K. H., Cisek L. J., Corden J. L., Dornreiter I., Arthur A. K., Fanning E. Altered phosphorylation pattern of simian virus 40 T antigen expressed in insect cells by using a baculovirus vector. J Virol. 1990 Oct;64(10):4799–4807. doi: 10.1128/jvi.64.10.4799-4807.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaoka T., Imazu M., Usui H., Kinohara N., Takeda M. Resolution and reassociation of three distinct components from pig heart phosphoprotein phosphatase. J Biol Chem. 1983 Feb 10;258(3):1526–1535. [PubMed] [Google Scholar]
- Kamibayashi C., Estes R., Slaughter C., Mumby M. C. Subunit interactions control protein phosphatase 2A. Effects of limited proteolysis, N-ethylmaleimide, and heparin on the interaction of the B subunit. J Biol Chem. 1991 Jul 15;266(20):13251–13260. [PubMed] [Google Scholar]
- Lawson R., Cohen P., Lane D. P. Simian virus 40 large T-antigen-dependent DNA replication is activated by protein phosphatase 2A in vitro. J Virol. 1990 May;64(5):2380–2383. doi: 10.1128/jvi.64.5.2380-2383.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Jaekel R. E., Ohkura H., Gomes R., Sunkel C. E., Baumgartner S., Hemmings B. A., Glover D. M. The 55 kd regulatory subunit of Drosophila protein phosphatase 2A is required for anaphase. Cell. 1993 Feb 26;72(4):621–633. doi: 10.1016/0092-8674(93)90080-a. [DOI] [PubMed] [Google Scholar]
- Mayer R. E., Hendrix P., Cron P., Matthies R., Stone S. R., Goris J., Merlevede W., Hofsteenge J., Hemmings B. A. Structure of the 55-kDa regulatory subunit of protein phosphatase 2A: evidence for a neuronal-specific isoform. Biochemistry. 1991 Apr 16;30(15):3589–3597. doi: 10.1021/bi00229a001. [DOI] [PubMed] [Google Scholar]
- McVey D., Brizuela L., Mohr I., Marshak D. R., Gluzman Y., Beach D. Phosphorylation of large tumour antigen by cdc2 stimulates SV40 DNA replication. Nature. 1989 Oct 12;341(6242):503–507. doi: 10.1038/341503a0. [DOI] [PubMed] [Google Scholar]
- McVey D., Ray S., Gluzman Y., Berger L., Wildeman A. G., Marshak D. R., Tegtmeyer P. cdc2 phosphorylation of threonine 124 activates the origin-unwinding functions of simian virus 40 T antigen. J Virol. 1993 Sep;67(9):5206–5215. doi: 10.1128/jvi.67.9.5206-5215.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey D., Strauss M., Gluzman Y. Properties of the DNA-binding domain of the simian virus 40 large T antigen. Mol Cell Biol. 1989 Dec;9(12):5525–5536. doi: 10.1128/mcb.9.12.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moarefi I. F., Small D., Gilbert I., Höpfner M., Randall S. K., Schneider C., Russo A. A., Ramsperger U., Arthur A. K., Stahl H. Mutation of the cyclin-dependent kinase phosphorylation site in simian virus 40 (SV40) large T antigen specifically blocks SV40 origin DNA unwinding. J Virol. 1993 Aug;67(8):4992–5002. doi: 10.1128/jvi.67.8.4992-5002.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr I. J., Stillman B., Gluzman Y. Regulation of SV40 DNA replication by phosphorylation of T antigen. EMBO J. 1987 Jan;6(1):153–160. doi: 10.1002/j.1460-2075.1987.tb04733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby M. C., Walter G. Protein serine/threonine phosphatases: structure, regulation, and functions in cell growth. Physiol Rev. 1993 Oct;73(4):673–699. doi: 10.1152/physrev.1993.73.4.673. [DOI] [PubMed] [Google Scholar]
- Scheidtmann K. H. Phosphorylation of simian virus 40 large T antigen: cytoplasmic and nuclear phophorylation sites differ in their metabolic stability. Virology. 1986 Apr 15;150(1):85–95. [PubMed] [Google Scholar]
- Scheidtmann K. H., Schickedanz J., Walter G., Lanford R. E., Butel J. S. Differential phosphorylation of cytoplasmic and nuclear variants of simian virus 40 large T antigen encoded by simian virus 40-adenovirus 7 hybrid viruses. J Virol. 1984 May;50(2):636–640. doi: 10.1128/jvi.50.2.636-640.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Virshup D. M., Kelly T. J. Protein phosphatase 2A dephosphorylates simian virus 40 large T antigen specifically at residues involved in regulation of DNA-binding activity. J Virol. 1991 Apr;65(4):2098–2101. doi: 10.1128/jvi.65.4.2098-2101.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J., Fanning E. Mutations in the phosphorylation sites of simian virus 40 (SV40) T antigen alter its origin DNA-binding specificity for sites I or II and affect SV40 DNA replication activity. J Virol. 1988 May;62(5):1598–1605. doi: 10.1128/jvi.62.5.1598-1605.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra D., Asins G., Calvet V. E., Hegardt F. G. Purification and properties of a protein inhibitor that inhibits phosphatase 2A activity when hydroxymethylglutaryl coenzyme A reductase is the substrate. J Biol Chem. 1989 Sep 5;264(25):14681–14685. [PubMed] [Google Scholar]
- Shenolikar S., Nairn A. C. Protein phosphatases: recent progress. Adv Second Messenger Phosphoprotein Res. 1991;23:1–121. [PubMed] [Google Scholar]
- Sola M. M., Langan T., Cohen P. p34cdc2 phosphorylation sites in histone H1 are dephosphorylated by protein phosphatase 2A1. Biochim Biophys Acta. 1991 Sep 3;1094(2):211–216. doi: 10.1016/0167-4889(91)90011-l. [DOI] [PubMed] [Google Scholar]
- Sontag E., Fedorov S., Kamibayashi C., Robbins D., Cobb M., Mumby M. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the map kinase pathway and induces cell proliferation. Cell. 1993 Dec 3;75(5):887–897. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- Sutton A., Immanuel D., Arndt K. T. The SIT4 protein phosphatase functions in late G1 for progression into S phase. Mol Cell Biol. 1991 Apr;11(4):2133–2148. doi: 10.1128/mcb.11.4.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T., Shiomi K., Togashi S., Takeichi M. Mutation of twins encoding a regulator of protein phosphatase 2A leads to pattern duplication in Drosophila imaginal discs. Genes Dev. 1993 Mar;7(3):429–440. doi: 10.1101/gad.7.3.429. [DOI] [PubMed] [Google Scholar]
- Virshup D. M., Kauffman M. G., Kelly T. J. Activation of SV40 DNA replication in vitro by cellular protein phosphatase 2A. EMBO J. 1989 Dec 1;8(12):3891–3898. doi: 10.1002/j.1460-2075.1989.tb08568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virshup D. M., Kelly T. J. Purification of replication protein C, a cellular protein involved in the initial stages of simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1989 May;86(10):3584–3588. doi: 10.1073/pnas.86.10.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virshup D. M., Russo A. A., Kelly T. J. Mechanism of activation of simian virus 40 DNA replication by protein phosphatase 2A. Mol Cell Biol. 1992 Nov;12(11):4883–4895. doi: 10.1128/mcb.12.11.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waelkens E., Goris J., Merlevede W. Purification and properties of polycation-stimulated phosphorylase phosphatases from rabbit skeletal muscle. J Biol Chem. 1987 Jan 25;262(3):1049–1059. [PubMed] [Google Scholar]