Abstract
The title bis-piperidine, C26H28N2O3, was unexpectedly obtained via a dimerization mechanism promoted by acetic acid when performing the Dieckmann cyclization of a chiral amido ester. The S,S configuration was assigned by reference to the enantiomerically pure starting material. In the molecule, two core heterocycles are linked by a σ bond. One ring includes a keto–enol group, while the other presents an enone functionality. Both rings present a conformation intermediate between envelope and screw-boat, and the dihedral angle between the mean planes passing through the rings [48.9 (1)°] is large enough to avoid hindrance between ring substituents. The enol tautomeric form in one ring favors the formation of strong intermolecular O—H⋯O=C hydrogen bonds. The resulting one-dimensional supramolecular structure features single-stranded helices running along the 21 screw axis parallel to [100].
Related literature
For natural products having a bis-piperidine substructure, see: Gil et al. (1995 ▶); Torres et al. (2000 ▶); Matsunaga et al. (2004 ▶); Smith & Sulikowski (2010 ▶). For related structures of monocyclic piperidines, see: Didierjean et al. (2004 ▶); Romero et al. (2005 ▶). For the application of Dieckmann condensation in organic synthesis, see: Scheiber & Nemes (2008 ▶). For an example of self-condensation of a dione similar to that used for the synthesis of the title compound, see: Sugasawa & Oka (1954 ▶).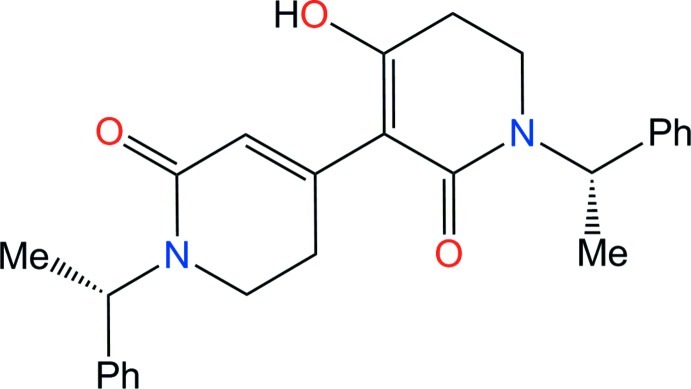
Experimental
Crystal data
C26H28N2O3
M r = 416.50
Orthorhombic,
a = 9.6647 (13) Å
b = 9.7281 (10) Å
c = 23.684 (3) Å
V = 2226.7 (5) Å3
Z = 4
Mo Kα radiation
μ = 0.08 mm−1
T = 296 K
0.60 × 0.60 × 0.08 mm
Data collection
Bruker P4 diffractometer
3173 measured reflections
2250 independent reflections
1843 reflections with I > 2σ(I)
R int = 0.019
3 standard reflections every 97 reflections intensity decay: 1.5%
Refinement
R[F 2 > 2σ(F 2)] = 0.039
wR(F 2) = 0.095
S = 1.04
2250 reflections
286 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.19 e Å−3
Δρmin = −0.15 e Å−3
Data collection: XSCANS (Siemens, 1996 ▶); cell refinement: XSCANS; data reduction: XSCANS; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: Mercury (Macrae et al., 2008 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536813004017/nr2039sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813004017/nr2039Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813004017/nr2039Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O4—H4⋯O2′i | 0.97 (4) | 1.67 (4) | 2.637 (3) | 177 (4) |
Symmetry code: (i) 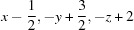 .
.
Acknowledgments
The authors wish to acknowledge CONACyT–Gobierno del Estado Tabasco and the Universidad Juárez Autónoma de Tabasco for financial support via projects TAB-2009-C18–122141 and UJAT-2009-C05–02, respectively.
supplementary crystallographic information
Comment
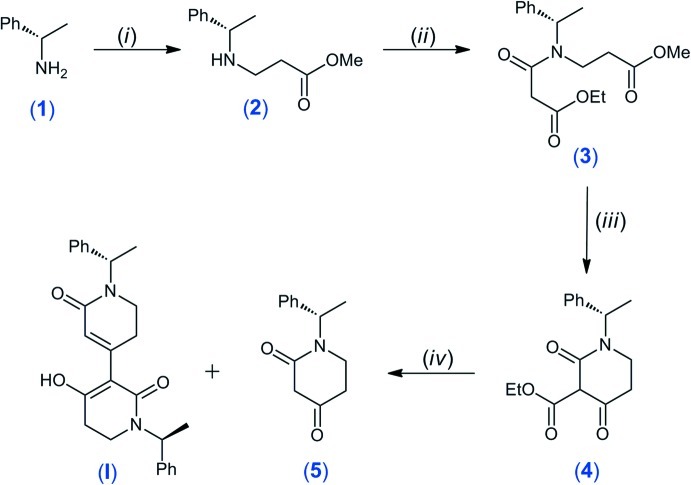
The title compound is a byproduct of the Dieckmann cyclization carried on the chiral amido ester 3 (Fig. 1). When concentrated acetic acid is used in the fourth synthetic step, a dimerization occurs during the decarboxylation process, affording the title molecule I as the major product, while the expected piperidine-2,4-dione 5 is obtained in low yield. This synthetic route for the preparation of this kind of piperidone derivatives is known to be successful in many cases (e.g. Scheiber & Nemes, 2008). However, it seems that the possible interference of secondary reactions like dimerization is poorly commented in the literature, probably because these reactions are seen as a trouble for the intended synthetic target. To the best of our knowledge, a single article clearly commented on this problem (Sugasawa & Oka, 1954). In this report, the authors added a note in the galley proofs, which is worth to quote in full: "in the course of the present work, we prepared N-benzyl-2,4-dioxopiperidine [···]. Our attempt to condense this ketone with ethyl cyanoacetate under Cope condition was not effected because this compound was found to undergo bimolecular self-condensation fairly rapidly, at a room temperature [···]. This tendency of the easy intermolecular self-condensation [of N-benzyl-2,4-dioxopiperidine] is so remarkable when compared with the stability of the corresponding 5-ethyl derivatives, which suffer no change when kept in a stoppered bottle at room temperature for a long time".
The synthesis of the title compound in good yield now confirms the observations done by Sugasawa & Oka 59 years ago.
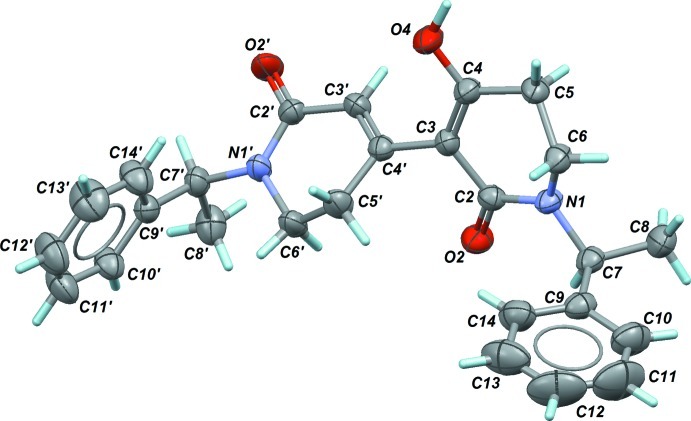
The molecular structure of I is built up from one ring including a keto-enol group (ring N1/C2···C6) bonded to a ring with the enone functionality (ring N1'/C2'···C6', see Fig. 2). Both rings present a conformation intermediate between envelope and screw-boat, with Cremer parameters being θ = 118.1° and φ = 101.0° for the keto-enol ring, and θ = 60.5° and φ = 278.5° for the enone ring. The dihedral angle between mean planes passing through these heterocycles, 48.9 (1)°, is large enough to avoid hindrance between atoms O2 and O4 in the first ring and H atoms at C3' and C5' in the other ring. Heterocycles in I have indeed conformations close to those observed in monocyclic related compounds which were X-ray characterized (e.g. Didierjean et al., 2004; Romero et al., 2005). In the solid state, the enolic tautomer of I seems to be favored over the di-ketone because the presence of a donor OH group allows the formation of stabilizing intermolecular O—H···O═C hydrogen bonds in the crystal. These strong interactions generate a supramolecular structure based on single stranded helices running along the 21 crystallographic screw axis in the [100] direction (Fig. 3).
The reported structure may be of interest in the field of natural products. It has been reported that the biosynthesis of some bis-piperidine alkaloids isolated from marine sponges, like halicyclamine A (Gil et al., 1995) or haliclonacyclamine C (Smith & Sulikowski, 2010) could involve the dimerization of dihydropyridines. Other natural products of interest also share the title compound bis-piperidine scaffold, with additional points of cyclization between the piperidine rings (Torres et al., 2000; Matsunaga et al., 2004).
Experimental
The synthesis is described in Fig. 1. A solution of 1 (41.2 mmol, 1 eq.) and methyl acrylate (49.6 mmol, 1.2 eq.) was stirred overnight at 298 K. The reaction mixture was concentrated under reduced pressure, and the crude purified by column chromatography (SiO2, CH2Cl2:MeOH, 97:3), to afford 2 as a colourless oil (98%). An amount of 2 (40.6 mmol, 1 eq.) was dissolved in diethyl malonate (40 ml) and the mixture refluxed until the reaction was complete (6 h). After concentration, the crude was chromatographed (Al2O3, n-hexane:AcOEt, 1:1), to afford 3, as a colourless oil (75%). A suspension of NaH (34.2 mmol, 2.5 eq.) in cyclohexane (100 ml) was refluxed for 20 min, and then, a solution of 3 (13.7 mmol, 1.1 eq. in 30 ml of anhydrous toluene) was added dropwise. After refluxing the mixture for 5 h, a solid was obtained, 4, which was filtered and dried in air. This solid was treated with acetic acid:water (30%, v/v) for the decarboxylation process. The mixture was refluxed until gas evolution stopped. After cooling down to 298 K, pH was adjusted to 7 with NaHCO3, and the mixture was washed with CH2Cl2 (3 × 50 ml). The organic phase was dried over Na2SO4, and concentrated. Compounds 5 and I were separated by column chromatography, (SiO2, CH2Cl2:MeOH, 95:5). The title compound I was obtained in 80% yield, and was recrystallized from AcOEt:n-hexane (1:1). m.p. = 444 K, [α]20D = -172.5 (c=1, CH2Cl2). Compound 5, a colourless oil, was isolated in low yield (< 20%). Key NMR and IR data are given in the archived CIF.
Refinement
All C-bound H atoms were placed in idealized positions and refined as riding to their carrier atoms, with bond lengths fixed to 0.93 (aromatic CH), 0.96 (methyl CH3), 0.97 (methylene CH2) or 0.98 Å (methine CH). Isotropic displacement parameters were calculated as Uiso(H) = xUeq(carrier atom), with x = 1.5 (methyl groups) or x = 1.2 (other H atoms). H4 (hydroxyl group) was found in a difference map and refined with free coordinates and Uiso(H4) = 1.5Ueq(O4). The absolute configuration for C7 and C7' is based on the known configuration of the enantiomerically pure starting material, (S)-(–)-1-phenylethylamine, and 621 measured Friedel pairs were merged for refinement.
Figures
Fig. 1.
The synthesis of the title molecule, I. (i) Methyl acrylate, MeOH, 25 °C, 12 h. (ii) Diethyl malonate, reflux, 6 h. (iii) NaH, cyclohexane/toluene, reflux. (iv) AcOH/H2O (30%, v/v).
Fig. 2.
Molecular structure of the title compound, with 50% probability level displacement ellipsoids for non-H atoms.
Fig. 3.
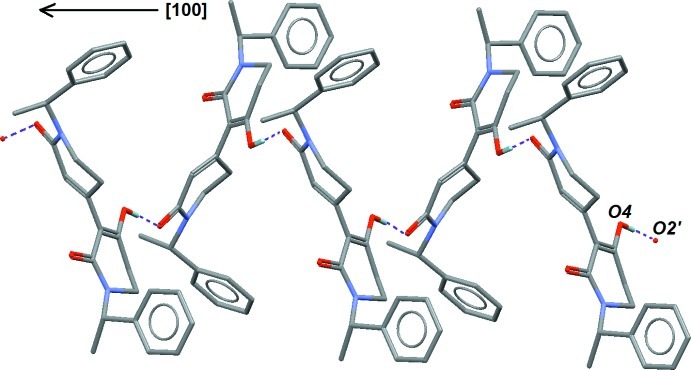
A chain of hydrogen-bonded molecules, along the screw axis parallel to [100].
Crystal data
| C26H28N2O3 | Dx = 1.242 Mg m−3 |
| Mr = 416.50 | Melting point: 444 K |
| Orthorhombic, P212121 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 62 reflections |
| a = 9.6647 (13) Å | θ = 4.7–12.0° |
| b = 9.7281 (10) Å | µ = 0.08 mm−1 |
| c = 23.684 (3) Å | T = 296 K |
| V = 2226.7 (5) Å3 | Plate, colourless |
| Z = 4 | 0.60 × 0.60 × 0.08 mm |
| F(000) = 888 |
Data collection
| Bruker P4 diffractometer | Rint = 0.019 |
| Radiation source: fine-focus sealed tube | θmax = 25.0°, θmin = 2.3° |
| Graphite monochromator | h = −11→2 |
| ω scans | k = −11→1 |
| 3173 measured reflections | l = −1→28 |
| 2250 independent reflections | 3 standard reflections every 97 reflections |
| 1843 reflections with I > 2σ(I) | intensity decay: 1.5% |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.039 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.095 | w = 1/[σ2(Fo2) + (0.0483P)2 + 0.2404P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.04 | (Δ/σ)max < 0.001 |
| 2250 reflections | Δρmax = 0.19 e Å−3 |
| 286 parameters | Δρmin = −0.15 e Å−3 |
| 0 restraints | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 0 constraints | Extinction coefficient: 0.0098 (15) |
| Primary atom site location: structure-invariant direct methods | Absolute structure: Assigned from synthesis. Friedel pairs (621) were merged |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.6918 (2) | 0.2061 (2) | 0.96330 (9) | 0.0342 (5) | |
| C2 | 0.7571 (3) | 0.3060 (3) | 0.93294 (10) | 0.0324 (6) | |
| O2 | 0.8401 (2) | 0.27815 (18) | 0.89463 (8) | 0.0451 (5) | |
| C3 | 0.7286 (3) | 0.4508 (3) | 0.94792 (10) | 0.0330 (6) | |
| C4 | 0.6613 (3) | 0.4827 (3) | 0.99592 (11) | 0.0360 (6) | |
| O4 | 0.6401 (2) | 0.61396 (19) | 1.01054 (8) | 0.0515 (6) | |
| H4 | 0.591 (4) | 0.629 (4) | 1.0457 (14) | 0.077* | |
| C5 | 0.6126 (3) | 0.3712 (3) | 1.03453 (11) | 0.0397 (7) | |
| H5A | 0.5315 | 0.4021 | 1.0551 | 0.048* | |
| H5B | 0.6845 | 0.3498 | 1.0617 | 0.048* | |
| C6 | 0.5774 (3) | 0.2438 (3) | 1.00066 (12) | 0.0395 (6) | |
| H6A | 0.5579 | 0.1683 | 1.0262 | 0.047* | |
| H6B | 0.4950 | 0.2608 | 0.9783 | 0.047* | |
| C7 | 0.7035 (3) | 0.0612 (3) | 0.94466 (11) | 0.0364 (7) | |
| H7A | 0.7807 | 0.0575 | 0.9179 | 0.044* | |
| C8 | 0.7425 (4) | −0.0316 (3) | 0.99393 (13) | 0.0523 (8) | |
| H8A | 0.8233 | 0.0043 | 1.0123 | 0.078* | |
| H8B | 0.7616 | −0.1225 | 0.9802 | 0.078* | |
| H8C | 0.6673 | −0.0352 | 1.0204 | 0.078* | |
| C9 | 0.5745 (3) | 0.0181 (3) | 0.91220 (11) | 0.0412 (7) | |
| C10 | 0.5143 (4) | −0.1103 (4) | 0.91817 (15) | 0.0656 (10) | |
| H10A | 0.5525 | −0.1738 | 0.9430 | 0.079* | |
| C11 | 0.3974 (5) | −0.1450 (5) | 0.8874 (2) | 0.0934 (15) | |
| H11A | 0.3575 | −0.2312 | 0.8920 | 0.112* | |
| C12 | 0.3405 (5) | −0.0541 (6) | 0.8505 (2) | 0.0936 (15) | |
| H12A | 0.2614 | −0.0779 | 0.8304 | 0.112* | |
| C13 | 0.3993 (4) | 0.0718 (5) | 0.84287 (17) | 0.0778 (12) | |
| H13A | 0.3616 | 0.1332 | 0.8170 | 0.093* | |
| C14 | 0.5151 (3) | 0.1078 (4) | 0.87365 (12) | 0.0557 (9) | |
| H14A | 0.5541 | 0.1942 | 0.8684 | 0.067* | |
| N1' | 0.8952 (2) | 0.7324 (2) | 0.82498 (8) | 0.0355 (5) | |
| C2' | 0.9292 (3) | 0.7510 (3) | 0.87967 (10) | 0.0340 (6) | |
| O2' | 1.0011 (2) | 0.8499 (2) | 0.89480 (8) | 0.0513 (6) | |
| C3' | 0.8771 (3) | 0.6493 (3) | 0.92029 (10) | 0.0370 (7) | |
| H3'A | 0.9110 | 0.6518 | 0.9570 | 0.044* | |
| C4' | 0.7837 (3) | 0.5535 (3) | 0.90715 (10) | 0.0321 (6) | |
| C5' | 0.7280 (3) | 0.5523 (3) | 0.84798 (11) | 0.0430 (7) | |
| H5'A | 0.7012 | 0.4593 | 0.8380 | 0.052* | |
| H5'B | 0.6462 | 0.6098 | 0.8461 | 0.052* | |
| C6' | 0.8320 (3) | 0.6029 (3) | 0.80695 (10) | 0.0445 (7) | |
| H6'A | 0.9038 | 0.5341 | 0.8024 | 0.053* | |
| H6'B | 0.7879 | 0.6161 | 0.7706 | 0.053* | |
| C7' | 0.9595 (3) | 0.8177 (3) | 0.78050 (10) | 0.0380 (7) | |
| H7'A | 0.9965 | 0.9002 | 0.7989 | 0.046* | |
| C8' | 1.0818 (3) | 0.7410 (3) | 0.75459 (13) | 0.0517 (8) | |
| H8'A | 1.1431 | 0.7112 | 0.7841 | 0.078* | |
| H8'B | 1.1307 | 0.8011 | 0.7294 | 0.078* | |
| H8'C | 1.0486 | 0.6625 | 0.7341 | 0.078* | |
| C9' | 0.8503 (3) | 0.8648 (3) | 0.73878 (11) | 0.0361 (6) | |
| C10' | 0.8695 (3) | 0.8573 (3) | 0.68070 (11) | 0.0454 (7) | |
| H10B | 0.9502 | 0.8189 | 0.6663 | 0.055* | |
| C11' | 0.7697 (4) | 0.9065 (4) | 0.64424 (13) | 0.0623 (10) | |
| H11B | 0.7848 | 0.9015 | 0.6055 | 0.075* | |
| C12' | 0.6498 (4) | 0.9619 (4) | 0.66362 (14) | 0.0677 (11) | |
| H12B | 0.5838 | 0.9950 | 0.6385 | 0.081* | |
| C13' | 0.6274 (4) | 0.9684 (4) | 0.72079 (15) | 0.0694 (10) | |
| H13B | 0.5451 | 1.0050 | 0.7345 | 0.083* | |
| C14' | 0.7266 (3) | 0.9210 (3) | 0.75817 (12) | 0.0540 (9) | |
| H14B | 0.7104 | 0.9267 | 0.7968 | 0.065* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0404 (13) | 0.0275 (11) | 0.0346 (11) | −0.0001 (11) | 0.0091 (10) | −0.0041 (9) |
| C2 | 0.0325 (14) | 0.0351 (14) | 0.0297 (12) | −0.0011 (13) | 0.0019 (12) | −0.0017 (12) |
| O2 | 0.0508 (12) | 0.0422 (11) | 0.0423 (10) | 0.0002 (10) | 0.0178 (10) | −0.0038 (9) |
| C3 | 0.0391 (15) | 0.0313 (14) | 0.0285 (13) | −0.0026 (13) | 0.0019 (13) | 0.0015 (11) |
| C4 | 0.0411 (15) | 0.0299 (13) | 0.0369 (14) | 0.0002 (13) | 0.0029 (14) | −0.0022 (12) |
| O4 | 0.0783 (15) | 0.0321 (10) | 0.0440 (11) | 0.0044 (12) | 0.0186 (12) | −0.0040 (9) |
| C5 | 0.0492 (17) | 0.0355 (14) | 0.0345 (13) | 0.0030 (15) | 0.0127 (13) | −0.0003 (12) |
| C6 | 0.0438 (15) | 0.0335 (13) | 0.0413 (13) | −0.0009 (15) | 0.0131 (14) | 0.0015 (12) |
| C7 | 0.0408 (16) | 0.0304 (14) | 0.0379 (15) | 0.0023 (13) | 0.0076 (13) | −0.0029 (12) |
| C8 | 0.069 (2) | 0.0383 (16) | 0.0498 (17) | 0.0068 (17) | 0.0001 (18) | 0.0023 (14) |
| C9 | 0.0424 (16) | 0.0387 (16) | 0.0425 (15) | −0.0041 (15) | 0.0104 (14) | −0.0084 (13) |
| C10 | 0.075 (2) | 0.055 (2) | 0.067 (2) | −0.022 (2) | 0.007 (2) | −0.0112 (18) |
| C11 | 0.090 (3) | 0.079 (3) | 0.112 (3) | −0.046 (3) | 0.012 (3) | −0.025 (3) |
| C12 | 0.061 (3) | 0.115 (4) | 0.105 (3) | −0.021 (3) | −0.015 (3) | −0.047 (3) |
| C13 | 0.064 (2) | 0.100 (3) | 0.069 (2) | 0.016 (3) | −0.022 (2) | −0.023 (2) |
| C14 | 0.060 (2) | 0.0543 (19) | 0.0529 (18) | 0.0013 (19) | −0.0122 (17) | −0.0087 (16) |
| N1' | 0.0458 (13) | 0.0324 (12) | 0.0283 (10) | −0.0111 (11) | −0.0044 (10) | 0.0032 (9) |
| C2' | 0.0389 (14) | 0.0290 (13) | 0.0341 (13) | −0.0029 (14) | −0.0037 (12) | −0.0004 (12) |
| O2' | 0.0703 (14) | 0.0451 (12) | 0.0385 (10) | −0.0271 (12) | −0.0081 (11) | −0.0027 (9) |
| C3' | 0.0493 (17) | 0.0367 (15) | 0.0251 (12) | −0.0032 (15) | −0.0030 (13) | −0.0004 (11) |
| C4' | 0.0361 (15) | 0.0295 (13) | 0.0308 (13) | 0.0006 (13) | 0.0040 (12) | −0.0010 (11) |
| C5' | 0.0483 (17) | 0.0441 (16) | 0.0364 (13) | −0.0171 (16) | −0.0055 (14) | 0.0013 (13) |
| C6' | 0.0631 (19) | 0.0415 (15) | 0.0290 (13) | −0.0164 (17) | −0.0049 (14) | −0.0005 (13) |
| C7' | 0.0437 (16) | 0.0348 (14) | 0.0354 (14) | −0.0078 (14) | 0.0014 (13) | 0.0067 (12) |
| C8' | 0.0450 (16) | 0.0530 (18) | 0.0570 (17) | 0.0050 (17) | 0.0057 (15) | 0.0153 (16) |
| C9' | 0.0420 (15) | 0.0326 (14) | 0.0336 (13) | −0.0035 (14) | 0.0037 (13) | 0.0026 (12) |
| C10' | 0.0488 (17) | 0.0521 (18) | 0.0354 (13) | 0.0087 (16) | 0.0085 (15) | 0.0035 (13) |
| C11' | 0.072 (2) | 0.080 (2) | 0.0351 (15) | 0.010 (2) | −0.0004 (17) | 0.0130 (17) |
| C12' | 0.066 (2) | 0.083 (3) | 0.054 (2) | 0.022 (2) | −0.007 (2) | 0.0172 (19) |
| C13' | 0.052 (2) | 0.086 (3) | 0.070 (2) | 0.023 (2) | 0.0059 (19) | 0.006 (2) |
| C14' | 0.0559 (19) | 0.066 (2) | 0.0401 (15) | 0.0104 (19) | 0.0106 (16) | 0.0035 (15) |
Geometric parameters (Å, º)
| N1—C2 | 1.363 (3) | C14—H14A | 0.9300 |
| N1—C6 | 1.463 (3) | N1'—C2' | 1.348 (3) |
| N1—C7 | 1.482 (3) | N1'—C6' | 1.464 (3) |
| C2—O2 | 1.241 (3) | N1'—C7' | 1.478 (3) |
| C2—C3 | 1.478 (4) | C2'—O2' | 1.240 (3) |
| C3—C4 | 1.346 (4) | C2'—C3' | 1.469 (4) |
| C3—C4' | 1.488 (3) | C3'—C4' | 1.334 (4) |
| C4—O4 | 1.339 (3) | C3'—H3'A | 0.9300 |
| C4—C5 | 1.495 (4) | C4'—C5' | 1.501 (4) |
| O4—H4 | 0.97 (4) | C5'—C6' | 1.482 (4) |
| C5—C6 | 1.515 (4) | C5'—H5'A | 0.9700 |
| C5—H5A | 0.9700 | C5'—H5'B | 0.9700 |
| C5—H5B | 0.9700 | C6'—H6'A | 0.9700 |
| C6—H6A | 0.9700 | C6'—H6'B | 0.9700 |
| C6—H6B | 0.9700 | C7'—C9' | 1.517 (4) |
| C7—C8 | 1.523 (4) | C7'—C8' | 1.527 (4) |
| C7—C9 | 1.524 (4) | C7'—H7'A | 0.9800 |
| C7—H7A | 0.9800 | C8'—H8'A | 0.9600 |
| C8—H8A | 0.9600 | C8'—H8'B | 0.9600 |
| C8—H8B | 0.9600 | C8'—H8'C | 0.9600 |
| C8—H8C | 0.9600 | C9'—C10' | 1.390 (4) |
| C9—C10 | 1.385 (5) | C9'—C14' | 1.392 (4) |
| C9—C14 | 1.388 (4) | C10'—C11' | 1.380 (4) |
| C10—C11 | 1.386 (6) | C10'—H10B | 0.9300 |
| C10—H10A | 0.9300 | C11'—C12' | 1.357 (5) |
| C11—C12 | 1.359 (6) | C11'—H11B | 0.9300 |
| C11—H11A | 0.9300 | C12'—C13' | 1.373 (5) |
| C12—C13 | 1.362 (6) | C12'—H12B | 0.9300 |
| C12—H12A | 0.9300 | C13'—C14' | 1.384 (5) |
| C13—C14 | 1.381 (5) | C13'—H13B | 0.9300 |
| C13—H13A | 0.9300 | C14'—H14B | 0.9300 |
| C2—N1—C6 | 119.3 (2) | C2'—N1'—C6' | 119.8 (2) |
| C2—N1—C7 | 119.1 (2) | C2'—N1'—C7' | 120.4 (2) |
| C6—N1—C7 | 118.4 (2) | C6'—N1'—C7' | 116.79 (19) |
| O2—C2—N1 | 121.9 (2) | O2'—C2'—N1' | 121.2 (2) |
| O2—C2—C3 | 120.3 (2) | O2'—C2'—C3' | 121.7 (2) |
| N1—C2—C3 | 117.8 (2) | N1'—C2'—C3' | 117.1 (2) |
| C4—C3—C2 | 120.8 (2) | C4'—C3'—C2' | 123.3 (2) |
| C4—C3—C4' | 124.5 (2) | C4'—C3'—H3'A | 118.3 |
| C2—C3—C4' | 114.6 (2) | C2'—C3'—H3'A | 118.3 |
| O4—C4—C3 | 120.8 (2) | C3'—C4'—C3 | 124.0 (2) |
| O4—C4—C5 | 119.1 (2) | C3'—C4'—C5' | 117.8 (2) |
| C3—C4—C5 | 120.1 (2) | C3—C4'—C5' | 118.2 (2) |
| C4—O4—H4 | 116 (2) | C6'—C5'—C4' | 111.5 (2) |
| C4—C5—C6 | 109.9 (2) | C6'—C5'—H5'A | 109.3 |
| C4—C5—H5A | 109.7 | C4'—C5'—H5'A | 109.3 |
| C6—C5—H5A | 109.7 | C6'—C5'—H5'B | 109.3 |
| C4—C5—H5B | 109.7 | C4'—C5'—H5'B | 109.3 |
| C6—C5—H5B | 109.7 | H5'A—C5'—H5'B | 108.0 |
| H5A—C5—H5B | 108.2 | N1'—C6'—C5' | 112.2 (2) |
| N1—C6—C5 | 110.8 (2) | N1'—C6'—H6'A | 109.2 |
| N1—C6—H6A | 109.5 | C5'—C6'—H6'A | 109.2 |
| C5—C6—H6A | 109.5 | N1'—C6'—H6'B | 109.2 |
| N1—C6—H6B | 109.5 | C5'—C6'—H6'B | 109.2 |
| C5—C6—H6B | 109.5 | H6'A—C6'—H6'B | 107.9 |
| H6A—C6—H6B | 108.1 | N1'—C7'—C9' | 110.0 (2) |
| N1—C7—C8 | 110.8 (2) | N1'—C7'—C8' | 109.7 (2) |
| N1—C7—C9 | 110.5 (2) | C9'—C7'—C8' | 115.1 (2) |
| C8—C7—C9 | 115.2 (2) | N1'—C7'—H7'A | 107.2 |
| N1—C7—H7A | 106.6 | C9'—C7'—H7'A | 107.2 |
| C8—C7—H7A | 106.6 | C8'—C7'—H7'A | 107.2 |
| C9—C7—H7A | 106.6 | C7'—C8'—H8'A | 109.5 |
| C7—C8—H8A | 109.5 | C7'—C8'—H8'B | 109.5 |
| C7—C8—H8B | 109.5 | H8'A—C8'—H8'B | 109.5 |
| H8A—C8—H8B | 109.5 | C7'—C8'—H8'C | 109.5 |
| C7—C8—H8C | 109.5 | H8'A—C8'—H8'C | 109.5 |
| H8A—C8—H8C | 109.5 | H8'B—C8'—H8'C | 109.5 |
| H8B—C8—H8C | 109.5 | C10'—C9'—C14' | 117.5 (3) |
| C10—C9—C14 | 117.4 (3) | C10'—C9'—C7' | 122.4 (3) |
| C10—C9—C7 | 122.7 (3) | C14'—C9'—C7' | 120.1 (2) |
| C14—C9—C7 | 119.8 (3) | C11'—C10'—C9' | 120.5 (3) |
| C9—C10—C11 | 120.5 (4) | C11'—C10'—H10B | 119.8 |
| C9—C10—H10A | 119.7 | C9'—C10'—H10B | 119.8 |
| C11—C10—H10A | 119.7 | C12'—C11'—C10' | 121.5 (3) |
| C12—C11—C10 | 120.6 (4) | C12'—C11'—H11B | 119.3 |
| C12—C11—H11A | 119.7 | C10'—C11'—H11B | 119.3 |
| C10—C11—H11A | 119.7 | C11'—C12'—C13' | 119.1 (3) |
| C11—C12—C13 | 120.1 (4) | C11'—C12'—H12B | 120.4 |
| C11—C12—H12A | 120.0 | C13'—C12'—H12B | 120.4 |
| C13—C12—H12A | 120.0 | C12'—C13'—C14' | 120.4 (3) |
| C12—C13—C14 | 119.8 (4) | C12'—C13'—H13B | 119.8 |
| C12—C13—H13A | 120.1 | C14'—C13'—H13B | 119.8 |
| C14—C13—H13A | 120.1 | C13'—C14'—C9' | 121.0 (3) |
| C13—C14—C9 | 121.5 (4) | C13'—C14'—H14B | 119.5 |
| C13—C14—H14A | 119.2 | C9'—C14'—H14B | 119.5 |
| C9—C14—H14A | 119.2 | ||
| C6—N1—C2—O2 | −169.0 (2) | C6'—N1'—C2'—O2' | 169.0 (3) |
| C7—N1—C2—O2 | −9.4 (4) | C7'—N1'—C2'—O2' | 9.0 (4) |
| C6—N1—C2—C3 | 12.4 (4) | C6'—N1'—C2'—C3' | −11.1 (4) |
| C7—N1—C2—C3 | 172.0 (2) | C7'—N1'—C2'—C3' | −171.1 (2) |
| O2—C2—C3—C4 | −166.8 (3) | O2'—C2'—C3'—C4' | 170.0 (3) |
| N1—C2—C3—C4 | 11.8 (4) | N1'—C2'—C3'—C4' | −10.0 (4) |
| O2—C2—C3—C4' | 11.4 (4) | C2'—C3'—C4'—C3 | −179.2 (3) |
| N1—C2—C3—C4' | −170.0 (2) | C2'—C3'—C4'—C5' | −1.1 (4) |
| C2—C3—C4—O4 | 177.4 (3) | C4—C3—C4'—C3' | 59.3 (4) |
| C4'—C3—C4—O4 | −0.5 (4) | C2—C3—C4'—C3' | −118.7 (3) |
| C2—C3—C4—C5 | −0.8 (4) | C4—C3—C4'—C5' | −118.8 (3) |
| C4'—C3—C4—C5 | −178.8 (3) | C2—C3—C4'—C5' | 63.2 (3) |
| O4—C4—C5—C6 | 150.8 (3) | C3'—C4'—C5'—C6' | 30.6 (4) |
| C3—C4—C5—C6 | −30.9 (4) | C3—C4'—C5'—C6' | −151.1 (2) |
| C2—N1—C6—C5 | −44.4 (3) | C2'—N1'—C6'—C5' | 40.8 (4) |
| C7—N1—C6—C5 | 155.8 (2) | C7'—N1'—C6'—C5' | −158.4 (2) |
| C4—C5—C6—N1 | 51.5 (3) | C4'—C5'—C6'—N1' | −48.9 (3) |
| C2—N1—C7—C8 | 131.3 (3) | C2'—N1'—C7'—C9' | −135.9 (3) |
| C6—N1—C7—C8 | −68.8 (3) | C6'—N1'—C7'—C9' | 63.5 (3) |
| C2—N1—C7—C9 | −99.8 (3) | C2'—N1'—C7'—C8' | 96.5 (3) |
| C6—N1—C7—C9 | 60.1 (3) | C6'—N1'—C7'—C8' | −64.1 (3) |
| N1—C7—C9—C10 | −140.3 (3) | N1'—C7'—C9'—C10' | −133.8 (3) |
| C8—C7—C9—C10 | −13.8 (4) | C8'—C7'—C9'—C10' | −9.2 (4) |
| N1—C7—C9—C14 | 41.5 (3) | N1'—C7'—C9'—C14' | 47.8 (3) |
| C8—C7—C9—C14 | 168.0 (3) | C8'—C7'—C9'—C14' | 172.3 (3) |
| C14—C9—C10—C11 | −1.3 (5) | C14'—C9'—C10'—C11' | 1.1 (5) |
| C7—C9—C10—C11 | −179.6 (3) | C7'—C9'—C10'—C11' | −177.3 (3) |
| C9—C10—C11—C12 | 0.5 (6) | C9'—C10'—C11'—C12' | −0.7 (5) |
| C10—C11—C12—C13 | 0.9 (7) | C10'—C11'—C12'—C13' | −0.3 (6) |
| C11—C12—C13—C14 | −1.4 (7) | C11'—C12'—C13'—C14' | 0.9 (6) |
| C12—C13—C14—C9 | 0.5 (5) | C12'—C13'—C14'—C9' | −0.5 (6) |
| C10—C9—C14—C13 | 0.8 (5) | C10'—C9'—C14'—C13' | −0.5 (5) |
| C7—C9—C14—C13 | 179.1 (3) | C7'—C9'—C14'—C13' | 178.0 (3) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O4—H4···O2′i | 0.97 (4) | 1.67 (4) | 2.637 (3) | 177 (4) |
Symmetry code: (i) x−1/2, −y+3/2, −z+2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: NR2039).
References
- Didierjean, C., Marin, J., Wenger, E., Briand, J.-P., Aubry, A. & Guichard, G. (2004). Acta Cryst. C60, o200–o203. [DOI] [PubMed]
- Gil, L., Baucherel, X., Martin, M.-T., Marazano, C. & Das, B. C. (1995). Tetrahedron Lett. 36, 6231–6234.
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
- Matsunaga, S., Miyata, Y., van Soest, R. W. M. & Fusetani, N. (2004). J. Nat. Prod. 67, 1758–1760. [DOI] [PubMed]
- Romero, N., Terán, J. L., Gnecco, D. & Bernès, S. (2005). Acta Cryst. E61, o2924–o2926.
- Scheiber, P. & Nemes, P. (2008). Arkivoc, iii, 194–199.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Siemens (1996). XSCANS Siemens Analytical X-ray Instruments Inc., Madison, Wisconsin, USA.
- Smith, B. J. & Sulikowski, G. A. (2010). Angew. Chem. Int. Ed. 49, 1599–1602. [DOI] [PubMed]
- Sugasawa, S. & Oka, K. (1954). Chem. Pharm. Bull. 2, 85–88. [DOI] [PubMed]
- Torres, Y. R., Berlinck, R. G. S., Magalhães, A., Schefer, A. B., Ferreira, A. G., Hajdu, E. & Muricy, G. (2000). J. Nat. Prod. 63, 1098–1105. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536813004017/nr2039sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813004017/nr2039Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813004017/nr2039Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report