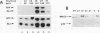Abstract
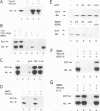
A basal repressor of class II gene transcription was identified, purified, and found to be identical to nonhistone chromosomal protein HMG2. HMG2 was shown to inhibit basal transcription under conditions in which transcription templates form soluble complexes with HMG2. Order-of-addition experiments clearly revealed that HMG2 acted after assembly of a TBP-TFIIA-promoter complex and before formation of the fourth phosphodiester bond by RNA polymerase II. Subsequently, an activity that efficiently counteracted repression of transcription by HMG2 in both TBP- and TFIID-containing transcription systems was isolated. Several lines of evidence suggested that antirepression was mediated by a TFIIH-associated factor. The antirepressor first coeluted with TFIIH, was depleted from this fraction by antibodies directed against the TFIIH subunit p62, was dependent on either ATP or dATP, and then was inhibited by the ATP analogs AMP-PNP and ATP gamma S. Relief of HMG2-mediated repression as well as basal promoter function of TFIIH may involve a helicase that coelutes with TFIIH and displays similar nucleotide specificities. Taken together, these data suggest novel consequences of chromatin-associated HMG proteins and they provide direct evidence for a role of TFIIH-associated enzymes in ATP-dependent antirepression of nonhistone chromosomal proteins.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auble D. T., Hahn S. An ATP-dependent inhibitor of TBP binding to DNA. Genes Dev. 1993 May;7(5):844–856. doi: 10.1101/gad.7.5.844. [DOI] [PubMed] [Google Scholar]
- Buratowski S., Hahn S., Guarente L., Sharp P. A. Five intermediate complexes in transcription initiation by RNA polymerase II. Cell. 1989 Feb 24;56(4):549–561. doi: 10.1016/0092-8674(89)90578-3. [DOI] [PubMed] [Google Scholar]
- Bustin M., Lehn D. A., Landsman D. Structural features of the HMG chromosomal proteins and their genes. Biochim Biophys Acta. 1990 Jul 30;1049(3):231–243. doi: 10.1016/0167-4781(90)90092-g. [DOI] [PubMed] [Google Scholar]
- Carballo M., Puigdomènech P., Palau J. DNA and histone H1 interact with different domains of HMG 1 and 2 proteins. EMBO J. 1983;2(10):1759–1764. doi: 10.1002/j.1460-2075.1983.tb01654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballo M., Puigdomènech P., Tancredi T., Palau J. Interaction between domains in chromosomal protein HMG-1. EMBO J. 1984 Jun;3(6):1255–1261. doi: 10.1002/j.1460-2075.1984.tb01960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers S. A., Rill R. L. Enrichment of transcribed and newly replicated DNA in soluble chromatin released from nuclei by mild micrococcal nuclease digestion. Biochim Biophys Acta. 1984 Jun 16;782(2):202–209. doi: 10.1016/0167-4781(84)90025-3. [DOI] [PubMed] [Google Scholar]
- Chiang C. M., Ge H., Wang Z., Hoffmann A., Roeder R. G. Unique TATA-binding protein-containing complexes and cofactors involved in transcription by RNA polymerases II and III. EMBO J. 1993 Jul;12(7):2749–2762. doi: 10.1002/j.1460-2075.1993.tb05936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conaway R. C., Conaway J. W. An RNA polymerase II transcription factor has an associated DNA-dependent ATPase (dATPase) activity strongly stimulated by the TATA region of promoters. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7356–7360. doi: 10.1073/pnas.86.19.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conaway R. C., Conaway J. W. General initiation factors for RNA polymerase II. Annu Rev Biochem. 1993;62:161–190. doi: 10.1146/annurev.bi.62.070193.001113. [DOI] [PubMed] [Google Scholar]
- Cortes P., Flores O., Reinberg D. Factors involved in specific transcription by mammalian RNA polymerase II: purification and analysis of transcription factor IIA and identification of transcription factor IIJ. Mol Cell Biol. 1992 Jan;12(1):413–421. doi: 10.1128/mcb.12.1.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynlacht B. D., Hoey T., Tjian R. Isolation of coactivators associated with the TATA-binding protein that mediate transcriptional activation. Cell. 1991 Aug 9;66(3):563–576. doi: 10.1016/0092-8674(81)90019-2. [DOI] [PubMed] [Google Scholar]
- Eckstein F. Nucleoside phosphorothioates. Annu Rev Biochem. 1985;54:367–402. doi: 10.1146/annurev.bi.54.070185.002055. [DOI] [PubMed] [Google Scholar]
- Fischer L., Gerard M., Chalut C., Lutz Y., Humbert S., Kanno M., Chambon P., Egly J. M. Cloning of the 62-kilodalton component of basic transcription factor BTF2. Science. 1992 Sep 4;257(5075):1392–1395. doi: 10.1126/science.1529339. [DOI] [PubMed] [Google Scholar]
- Flores O., Lu H., Reinberg D. Factors involved in specific transcription by mammalian RNA polymerase II. Identification and characterization of factor IIH. J Biol Chem. 1992 Feb 5;267(4):2786–2793. [PubMed] [Google Scholar]
- Ha I., Roberts S., Maldonado E., Sun X., Kim L. U., Green M., Reinberg D. Multiple functional domains of human transcription factor IIB: distinct interactions with two general transcription factors and RNA polymerase II. Genes Dev. 1993 Jun;7(6):1021–1032. doi: 10.1101/gad.7.6.1021. [DOI] [PubMed] [Google Scholar]
- Isackson P. J., Fishback J. L., Bidney D. L., Reeck G. R. Preferential affinity of high molecular weight high mobility group non-histone chromatin proteins for single-stranded DNA. J Biol Chem. 1979 Jul 10;254(13):5569–5572. [PubMed] [Google Scholar]
- Kim W. Y., Dahmus M. E. Purification of RNA polymerase IIO from calf thymus. J Biol Chem. 1988 Dec 15;263(35):18880–18885. [PubMed] [Google Scholar]
- Kretzschmar M., Meisterernst M., Scheidereit C., Li G., Roeder R. G. Transcriptional regulation of the HIV-1 promoter by NF-kappa B in vitro. Genes Dev. 1992 May;6(5):761–774. doi: 10.1101/gad.6.5.761. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M., Stelzer G., Roeder R. G., Meisterernst M. RNA polymerase II cofactor PC2 facilitates activation of transcription by GAL4-AH in vitro. Mol Cell Biol. 1994 Jun;14(6):3927–3937. doi: 10.1128/mcb.14.6.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl L., Salmond B., Tran L. Concentrations of high-mobility-group proteins in the nucleus and cytoplasm of several rat tissues. J Cell Biol. 1984 Aug;99(2):648–654. doi: 10.1083/jcb.99.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. K., DeJong J., Hashimoto S., Horikoshi M., Roeder R. G. TFIIA induces conformational changes in TFIID via interactions with the basic repeat. Mol Cell Biol. 1992 Nov;12(11):5189–5196. doi: 10.1128/mcb.12.11.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado E., Ha I., Cortes P., Weis L., Reinberg D. Factors involved in specific transcription by mammalian RNA polymerase II: role of transcription factors IIA, IID, and IIB during formation of a transcription-competent complex. Mol Cell Biol. 1990 Dec;10(12):6335–6347. doi: 10.1128/mcb.10.12.6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisterernst M., Roeder R. G. Family of proteins that interact with TFIID and regulate promoter activity. Cell. 1991 Nov 1;67(3):557–567. doi: 10.1016/0092-8674(91)90530-c. [DOI] [PubMed] [Google Scholar]
- Merino A., Madden K. R., Lane W. S., Champoux J. J., Reinberg D. DNA topoisomerase I is involved in both repression and activation of transcription. Nature. 1993 Sep 16;365(6443):227–232. doi: 10.1038/365227a0. [DOI] [PubMed] [Google Scholar]
- Nakajima N., Horikoshi M., Roeder R. G. Factors involved in specific transcription by mammalian RNA polymerase II: purification, genetic specificity, and TATA box-promoter interactions of TFIID. Mol Cell Biol. 1988 Oct;8(10):4028–4040. doi: 10.1128/mcb.8.10.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvin J. D., Sharp P. A. DNA topology and a minimal set of basal factors for transcription by RNA polymerase II. Cell. 1993 May 7;73(3):533–540. doi: 10.1016/0092-8674(93)90140-l. [DOI] [PubMed] [Google Scholar]
- Paull T. T., Haykinson M. J., Johnson R. C. The nonspecific DNA-binding and -bending proteins HMG1 and HMG2 promote the assembly of complex nucleoprotein structures. Genes Dev. 1993 Aug;7(8):1521–1534. doi: 10.1101/gad.7.8.1521. [DOI] [PubMed] [Google Scholar]
- Postnikov Y. V., Shick V. V., Belyavsky A. V., Khrapko K. R., Brodolin K. L., Nikolskaya T. A., Mirzabekov A. D. Distribution of high mobility group proteins 1/2, E and 14/17 and linker histones H1 and H5 on transcribed and non-transcribed regions of chicken erythrocyte chromatin. Nucleic Acids Res. 1991 Feb 25;19(4):717–725. doi: 10.1093/nar/19.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond T. J., Finch J. T., Rushton B., Rhodes D., Klug A. Structure of the nucleosome core particle at 7 A resolution. Nature. 1984 Oct 11;311(5986):532–537. doi: 10.1038/311532a0. [DOI] [PubMed] [Google Scholar]
- Roeder R. G. The complexities of eukaryotic transcription initiation: regulation of preinitiation complex assembly. Trends Biochem Sci. 1991 Nov;16(11):402–408. doi: 10.1016/0968-0004(91)90164-q. [DOI] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell. 1985 Nov;43(1):165–175. doi: 10.1016/0092-8674(85)90021-2. [DOI] [PubMed] [Google Scholar]
- Schaeffer L., Roy R., Humbert S., Moncollin V., Vermeulen W., Hoeijmakers J. H., Chambon P., Egly J. M. DNA repair helicase: a component of BTF2 (TFIIH) basic transcription factor. Science. 1993 Apr 2;260(5104):58–63. doi: 10.1126/science.8465201. [DOI] [PubMed] [Google Scholar]
- Serizawa H., Conaway J. W., Conaway R. C. Phosphorylation of C-terminal domain of RNA polymerase II is not required in basal transcription. Nature. 1993 May 27;363(6427):371–374. doi: 10.1038/363371a0. [DOI] [PubMed] [Google Scholar]
- Serizawa H., Conaway R. C., Conaway J. W. Multifunctional RNA polymerase II initiation factor delta from rat liver. Relationship between carboxyl-terminal domain kinase, ATPase, and DNA helicase activities. J Biol Chem. 1993 Aug 15;268(23):17300–17308. [PubMed] [Google Scholar]
- Singh J., Dixon G. H. High mobility group proteins 1 and 2 function as general class II transcription factors. Biochemistry. 1990 Jul 3;29(26):6295–6302. doi: 10.1021/bi00478a026. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Sumimoto H., Ohkuma Y., Yamamoto T., Horikoshi M., Roeder R. G. Factors involved in specific transcription by mammalian RNA polymerase II: identification of general transcription factor TFIIG. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9158–9162. doi: 10.1073/pnas.87.23.9158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremethick D. J., Molloy P. L. High mobility group proteins 1 and 2 stimulate transcription in vitro by RNA polymerases II and III. J Biol Chem. 1986 May 25;261(15):6986–6992. [PubMed] [Google Scholar]
- Tyree C. M., George C. P., Lira-DeVito L. M., Wampler S. L., Dahmus M. E., Zawel L., Kadonaga J. T. Identification of a minimal set of proteins that is sufficient for accurate initiation of transcription by RNA polymerase II. Genes Dev. 1993 Jul;7(7A):1254–1265. doi: 10.1101/gad.7.7a.1254. [DOI] [PubMed] [Google Scholar]
- Wang W., Gralla J. D., Carey M. The acidic activator GAL4-AH can stimulate polymerase II transcription by promoting assembly of a closed complex requiring TFIID and TFIIA. Genes Dev. 1992 Sep;6(9):1716–1727. doi: 10.1101/gad.6.9.1716. [DOI] [PubMed] [Google Scholar]
- Watt F., Molloy P. L. High mobility group proteins 1 and 2 stimulate binding of a specific transcription factor to the adenovirus major late promoter. Nucleic Acids Res. 1988 Feb 25;16(4):1471–1486. doi: 10.1093/nar/16.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeda G., van Ham R. C., Vermeulen W., Bootsma D., van der Eb A. J., Hoeijmakers J. H. A presumed DNA helicase encoded by ERCC-3 is involved in the human repair disorders xeroderma pigmentosum and Cockayne's syndrome. Cell. 1990 Aug 24;62(4):777–791. doi: 10.1016/0092-8674(90)90122-u. [DOI] [PubMed] [Google Scholar]
- Zawel L., Reinberg D. Initiation of transcription by RNA polymerase II: a multi-step process. Prog Nucleic Acid Res Mol Biol. 1993;44:67–108. doi: 10.1016/s0079-6603(08)60217-2. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Boyer T. G., Berk A. J. Factors (TAFs) required for activated transcription interact with TATA box-binding protein conserved core domain. Genes Dev. 1993 Feb;7(2):180–187. doi: 10.1101/gad.7.2.180. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Lieberman P. M., Boyer T. G., Berk A. J. Holo-TFIID supports transcriptional stimulation by diverse activators and from a TATA-less promoter. Genes Dev. 1992 Oct;6(10):1964–1974. doi: 10.1101/gad.6.10.1964. [DOI] [PubMed] [Google Scholar]