Abstract
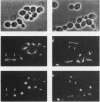
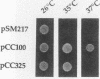
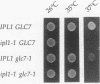
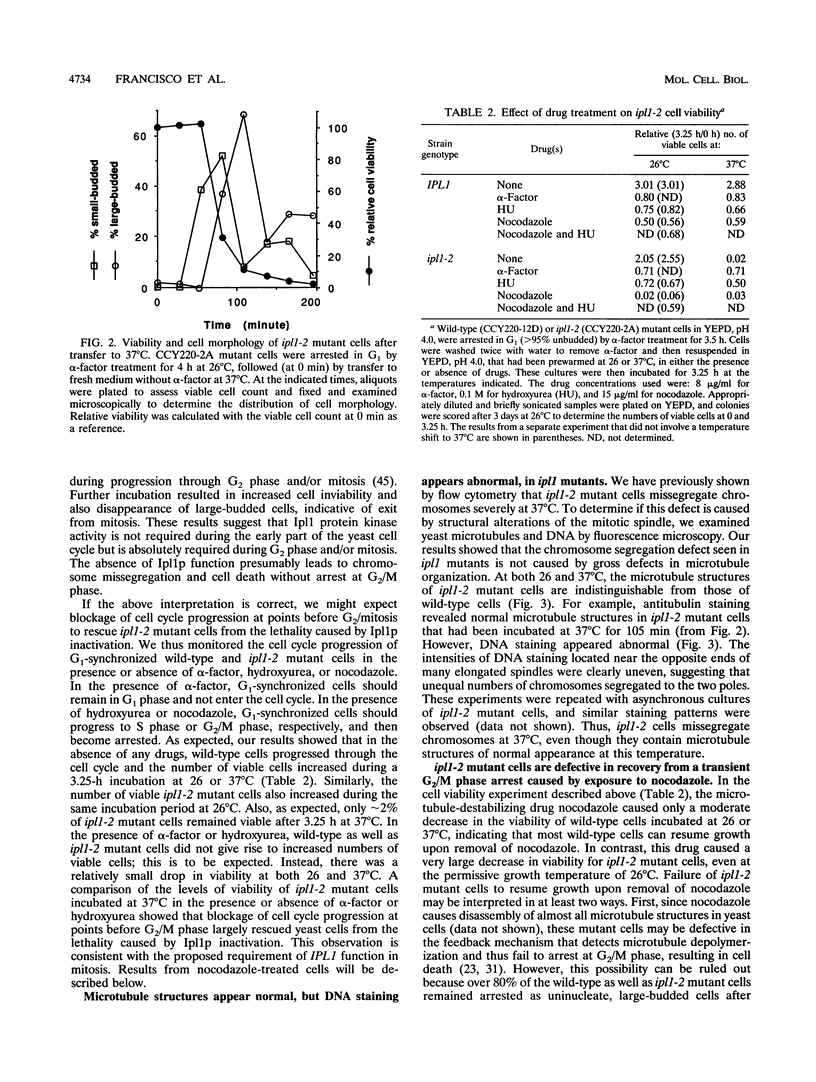
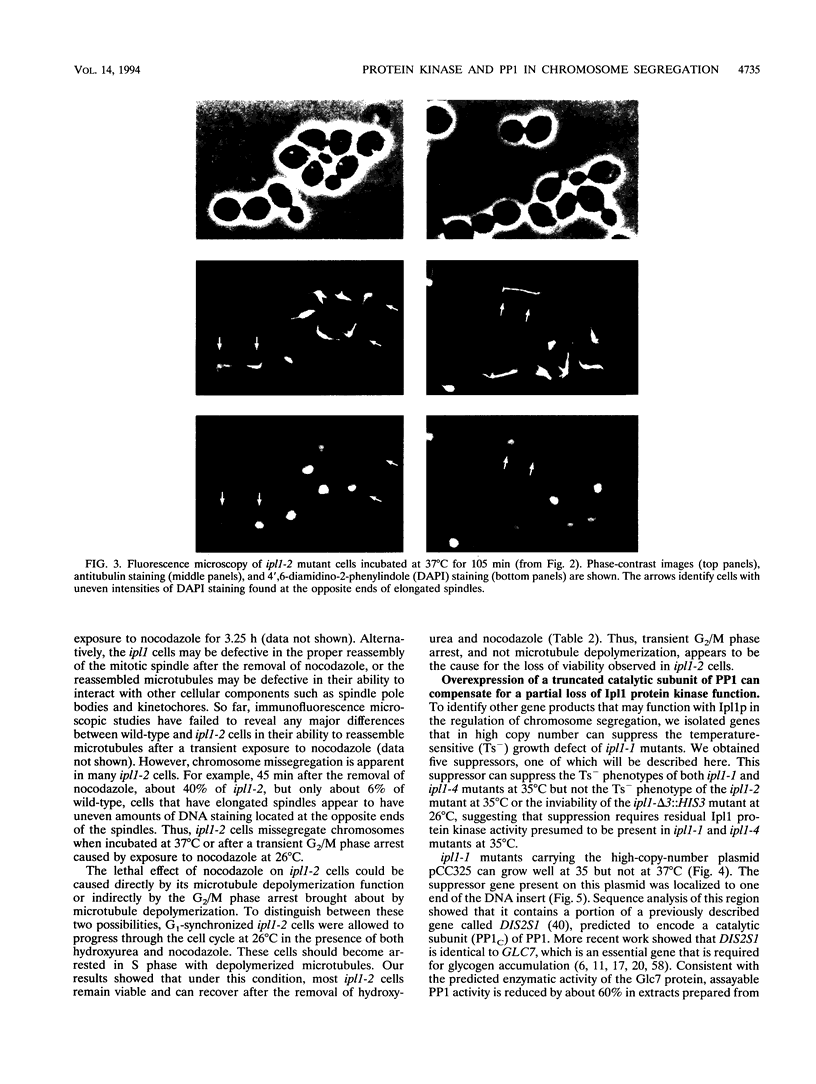
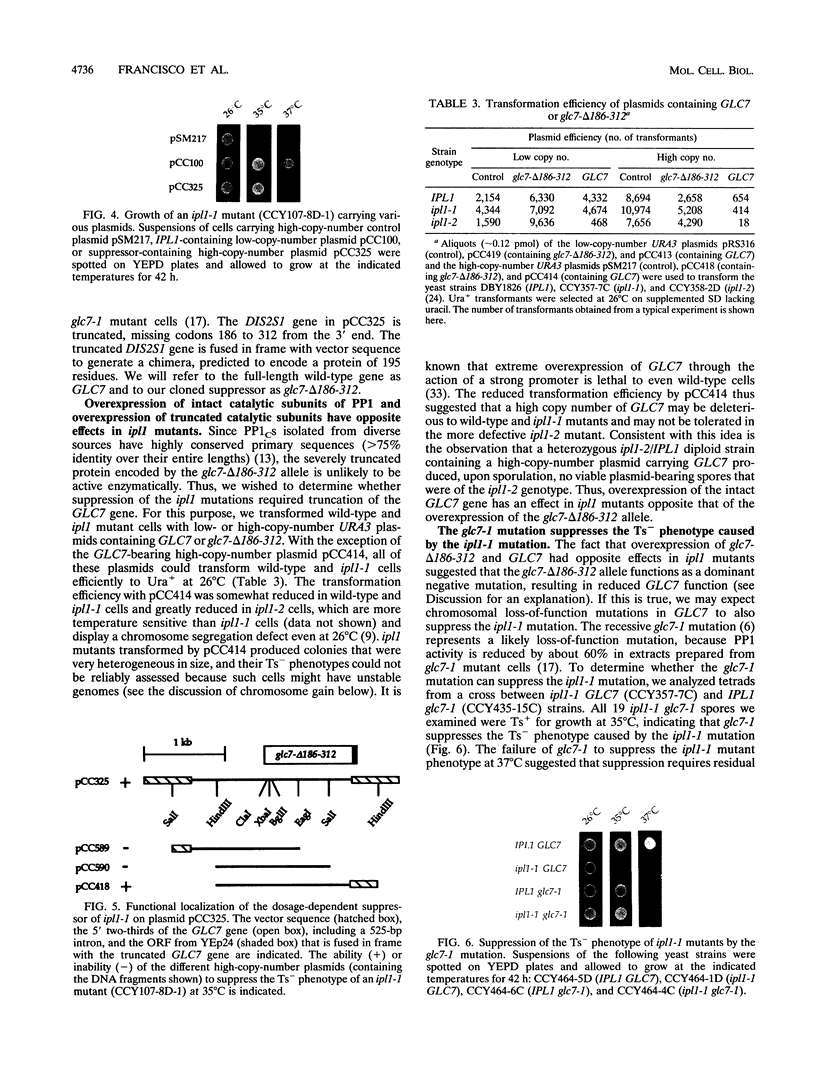
The IPL1 gene is required for high-fidelity chromosome segregation in the budding yeast Saccharomyces cerevisiae. Conditional ipl1ts mutants missegregate chromosomes severely at 37 degrees C. Here, we report that IPL1 encodes an essential putative protein kinase whose function is required during the later part of each cell cycle. At 26 degrees C, the permissive growth temperature, ipl1 mutant cells are defective in the recovery from a transient G2/M-phase arrest caused by the antimicrotubule drug nocodazole. In an effort to identify additional gene products that participate with the Ipl1 protein kinase in regulating chromosome segregation in yeast, a truncated version of the previously identified DIS2S1/GLC7 gene was isolated as a dosage-dependent suppressor of ipl1ts mutations. DIS2S1/GLC7 is predicted to encode a catalytic subunit (PP1C) of type 1 protein phosphatase. Overexpression of the full-length DIS2S1/GLC7 gene results in chromosome missegregation in wild-type cells and exacerbates the mutant phenotype in ipl1 cells. In addition, the glc7-1 mutation can partially suppress the ipl1-1 mutation. These results suggest that type 1 protein phosphatase acts in opposition to the Ipl1 protein kinase in vivo to ensure the high fidelity of chromosome segregation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axton J. M., Dombrádi V., Cohen P. T., Glover D. M. One of the protein phosphatase 1 isoenzymes in Drosophila is essential for mitosis. Cell. 1990 Oct 5;63(1):33–46. doi: 10.1016/0092-8674(90)90286-n. [DOI] [PubMed] [Google Scholar]
- Baitinger C., Alderton J., Poenie M., Schulman H., Steinhardt R. A. Multifunctional Ca2+/calmodulin-dependent protein kinase is necessary for nuclear envelope breakdown. J Cell Biol. 1990 Nov;111(5 Pt 1):1763–1773. doi: 10.1083/jcb.111.5.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen M., Stalmans W. The structure, role, and regulation of type 1 protein phosphatases. Crit Rev Biochem Mol Biol. 1992;27(3):227–281. doi: 10.3109/10409239209082564. [DOI] [PubMed] [Google Scholar]
- Botstein D., Falco S. C., Stewart S. E., Brennan M., Scherer S., Stinchcomb D. T., Struhl K., Davis R. W. Sterile host yeasts (SHY): a eukaryotic system of biological containment for recombinant DNA experiments. Gene. 1979 Dec;8(1):17–24. doi: 10.1016/0378-1119(79)90004-0. [DOI] [PubMed] [Google Scholar]
- Browne C. L., Bower W. A., Palazzo R. E., Rebhun L. I. Inhibition of mitosis in fertilized sea urchin eggs by inhibition of the cyclic AMP-dependent protein kinase. Exp Cell Res. 1990 May;188(1):122–128. doi: 10.1016/0014-4827(90)90286-j. [DOI] [PubMed] [Google Scholar]
- Cannon J. F., Pringle J. R., Fiechter A., Khalil M. Characterization of glycogen-deficient glc mutants of Saccharomyces cerevisiae. Genetics. 1994 Feb;136(2):485–503. doi: 10.1093/genetics/136.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. F., Tatchell K. Characterization of Saccharomyces cerevisiae genes encoding subunits of cyclic AMP-dependent protein kinase. Mol Cell Biol. 1987 Aug;7(8):2653–2663. doi: 10.1128/mcb.7.8.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M., Botstein D. Two differentially regulated mRNAs with different 5' ends encode secreted with intracellular forms of yeast invertase. Cell. 1982 Jan;28(1):145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- Chan C. S., Botstein D. Isolation and characterization of chromosome-gain and increase-in-ploidy mutants in yeast. Genetics. 1993 Nov;135(3):677–691. doi: 10.1093/genetics/135.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester V. E. Heritable glycogen-storage deficiency in yeast and its induction by ultra-violet light. J Gen Microbiol. 1968 Apr;51(1):49–56. doi: 10.1099/00221287-51-1-49. [DOI] [PubMed] [Google Scholar]
- Clotet J., Posas F., Casamayor A., Schaaff-Gerstenschläger I., Ariño J. The gene DIS2S1 is essential in Saccharomyces cerevisiae and is involved in glycogen phosphorylase activation. Curr Genet. 1991 May;19(5):339–342. doi: 10.1007/BF00309593. [DOI] [PubMed] [Google Scholar]
- Cohen P. T., Brewis N. D., Hughes V., Mann D. J. Protein serine/threonine phosphatases; an expanding family. FEBS Lett. 1990 Aug 1;268(2):355–359. doi: 10.1016/0014-5793(90)81285-v. [DOI] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Dailey D., Schieven G. L., Lim M. Y., Marquardt H., Gilmore T., Thorner J., Martin G. S. Novel yeast protein kinase (YPK1 gene product) is a 40-kilodalton phosphotyrosyl protein associated with protein-tyrosine kinase activity. Mol Cell Biol. 1990 Dec;10(12):6244–6256. doi: 10.1128/mcb.10.12.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doonan J. H., Morris N. R. The bimG gene of Aspergillus nidulans, required for completion of anaphase, encodes a homolog of mammalian phosphoprotein phosphatase 1. Cell. 1989 Jun 16;57(6):987–996. doi: 10.1016/0092-8674(89)90337-1. [DOI] [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Feng Z. H., Wilson S. E., Peng Z. Y., Schlender K. K., Reimann E. M., Trumbly R. J. The yeast GLC7 gene required for glycogen accumulation encodes a type 1 protein phosphatase. J Biol Chem. 1991 Dec 15;266(35):23796–23801. [PubMed] [Google Scholar]
- Fernandez A., Brautigan D. L., Lamb N. J. Protein phosphatase type 1 in mammalian cell mitosis: chromosomal localization and involvement in mitotic exit. J Cell Biol. 1992 Mar;116(6):1421–1430. doi: 10.1083/jcb.116.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleig U. N., Gould K. L. Regulation of cdc2 activity in Schizosaccharomyces pombe: the role of phosphorylation. Semin Cell Biol. 1991 Aug;2(4):195–204. [PubMed] [Google Scholar]
- François J. M., Thompson-Jaeger S., Skroch J., Zellenka U., Spevak W., Tatchell K. GAC1 may encode a regulatory subunit for protein phosphatase type 1 in Saccharomyces cerevisiae. EMBO J. 1992 Jan;11(1):87–96. doi: 10.1002/j.1460-2075.1992.tb05031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Moreno S., Owen D. J., Sazer S., Nurse P. Phosphorylation at Thr167 is required for Schizosaccharomyces pombe p34cdc2 function. EMBO J. 1991 Nov;10(11):3297–3309. doi: 10.1002/j.1460-2075.1991.tb04894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hisamoto N., Sugimoto K., Matsumoto K. The Glc7 type 1 protein phosphatase of Saccharomyces cerevisiae is required for cell cycle progression in G2/M. Mol Cell Biol. 1994 May;14(5):3158–3165. doi: 10.1128/mcb.14.5.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt M. A., Totis L., Roberts B. T. S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell. 1991 Aug 9;66(3):507–517. doi: 10.1016/0092-8674(81)90014-3. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L. H., Eberly S. L., Chapman J. W., Araki H., Sugino A. The product of the Saccharomyces cerevisiae cell cycle gene DBF2 has homology with protein kinases and is periodically expressed in the cell cycle. Mol Cell Biol. 1990 Apr;10(4):1358–1366. doi: 10.1128/mcb.10.4.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita N., Ohkura H., Yanagida M. Distinct, essential roles of type 1 and 2A protein phosphatases in the control of the fission yeast cell division cycle. Cell. 1990 Oct 19;63(2):405–415. doi: 10.1016/0092-8674(90)90173-c. [DOI] [PubMed] [Google Scholar]
- Kinoshita N., Yamano H., Niwa H., Yoshida T., Yanagida M. Negative regulation of mitosis by the fission yeast protein phosphatase ppa2. Genes Dev. 1993 Jun;7(6):1059–1071. doi: 10.1101/gad.7.6.1059. [DOI] [PubMed] [Google Scholar]
- Kitada K., Johnson A. L., Johnston L. H., Sugino A. A multicopy suppressor gene of the Saccharomyces cerevisiae G1 cell cycle mutant gene dbf4 encodes a protein kinase and is identified as CDC5. Mol Cell Biol. 1993 Jul;13(7):4445–4457. doi: 10.1128/mcb.13.7.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lechner J., Carbon J. A 240 kd multisubunit protein complex, CBF3, is a major component of the budding yeast centromere. Cell. 1991 Feb 22;64(4):717–725. doi: 10.1016/0092-8674(91)90501-o. [DOI] [PubMed] [Google Scholar]
- Li R., Murray A. W. Feedback control of mitosis in budding yeast. Cell. 1991 Aug 9;66(3):519–531. doi: 10.1016/0092-8674(81)90015-5. [DOI] [PubMed] [Google Scholar]
- Lisziewicz J., Godany A., Förster H. H., Küntzel H. Isolation and nucleotide sequence of a Saccharomyces cerevisiae protein kinase gene suppressing the cell cycle start mutation cdc25. J Biol Chem. 1987 Feb 25;262(6):2549–2553. [PubMed] [Google Scholar]
- Liu H., Krizek J., Bretscher A. Construction of a GAL1-regulated yeast cDNA expression library and its application to the identification of genes whose overexpression causes lethality in yeast. Genetics. 1992 Nov;132(3):665–673. doi: 10.1093/genetics/132.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llamazares S., Moreira A., Tavares A., Girdham C., Spruce B. A., Gonzalez C., Karess R. E., Glover D. M., Sunkel C. E. polo encodes a protein kinase homolog required for mitosis in Drosophila. Genes Dev. 1991 Dec;5(12A):2153–2165. doi: 10.1101/gad.5.12a.2153. [DOI] [PubMed] [Google Scholar]
- Ma H., Kunes S., Schatz P. J., Botstein D. Plasmid construction by homologous recombination in yeast. Gene. 1987;58(2-3):201–216. doi: 10.1016/0378-1119(87)90376-3. [DOI] [PubMed] [Google Scholar]
- Mayer-Jaekel R. E., Ohkura H., Gomes R., Sunkel C. E., Baumgartner S., Hemmings B. A., Glover D. M. The 55 kd regulatory subunit of Drosophila protein phosphatase 2A is required for anaphase. Cell. 1993 Feb 26;72(4):621–633. doi: 10.1016/0092-8674(93)90080-a. [DOI] [PubMed] [Google Scholar]
- Millar J. B., Russell P. The cdc25 M-phase inducer: an unconventional protein phosphatase. Cell. 1992 Feb 7;68(3):407–410. doi: 10.1016/0092-8674(92)90177-e. [DOI] [PubMed] [Google Scholar]
- Neigeborn L., Mitchell A. P. The yeast MCK1 gene encodes a protein kinase homolog that activates early meiotic gene expression. Genes Dev. 1991 Apr;5(4):533–548. doi: 10.1101/gad.5.4.533. [DOI] [PubMed] [Google Scholar]
- Nigg E. A. The substrates of the cdc2 kinase. Semin Cell Biol. 1991 Aug;2(4):261–270. [PubMed] [Google Scholar]
- Ohkura H., Kinoshita N., Miyatani S., Toda T., Yanagida M. The fission yeast dis2+ gene required for chromosome disjoining encodes one of two putative type 1 protein phosphatases. Cell. 1989 Jun 16;57(6):997–1007. doi: 10.1016/0092-8674(89)90338-3. [DOI] [PubMed] [Google Scholar]
- Ohkura H., Yanagida M. S. pombe gene sds22+ essential for a midmitotic transition encodes a leucine-rich repeat protein that positively modulates protein phosphatase-1. Cell. 1991 Jan 11;64(1):149–157. doi: 10.1016/0092-8674(91)90216-l. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Genetic applications of yeast transformation with linear and gapped plasmids. Methods Enzymol. 1983;101:228–245. doi: 10.1016/0076-6879(83)01017-4. [DOI] [PubMed] [Google Scholar]
- Osmani S. A., Pu R. T., Morris N. R. Mitotic induction and maintenance by overexpression of a G2-specific gene that encodes a potential protein kinase. Cell. 1988 Apr 22;53(2):237–244. doi: 10.1016/0092-8674(88)90385-6. [DOI] [PubMed] [Google Scholar]
- Pearson R. B., Kemp B. E. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- Pringle J. R., Preston R. A., Adams A. E., Stearns T., Drubin D. G., Haarer B. K., Jones E. W. Fluorescence microscopy methods for yeast. Methods Cell Biol. 1989;31:357–435. doi: 10.1016/s0091-679x(08)61620-9. [DOI] [PubMed] [Google Scholar]
- Ronne H., Carlberg M., Hu G. Z., Nehlin J. O. Protein phosphatase 2A in Saccharomyces cerevisiae: effects on cell growth and bud morphogenesis. Mol Cell Biol. 1991 Oct;11(10):4876–4884. doi: 10.1128/mcb.11.10.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. D., Novick P., Thomas J. H., Botstein D., Fink G. R. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60(2-3):237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S., Davis R. W. Replacement of chromosome segments with altered DNA sequences constructed in vitro. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4951–4955. doi: 10.1073/pnas.76.10.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer B., Philippsen P. CDC15, an essential cell cycle gene in Saccharomyces cerevisiae, encodes a protein kinase domain. Yeast. 1991 Apr;7(3):265–273. doi: 10.1002/yea.320070308. [DOI] [PubMed] [Google Scholar]
- Shero J. H., Hieter P. A suppressor of a centromere DNA mutation encodes a putative protein kinase (MCK1). Genes Dev. 1991 Apr;5(4):549–560. doi: 10.1101/gad.5.4.549. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon A. A., Cohen P. T., Stark M. J. Saccharomyces cerevisiae protein phosphatase 2A performs an essential cellular function and is encoded by two genes. EMBO J. 1990 Dec;9(13):4339–4346. doi: 10.1002/j.1460-2075.1990.tb07883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone E. M., Yamano H., Kinoshita N., Yanagida M. Mitotic regulation of protein phosphatases by the fission yeast sds22 protein. Curr Biol. 1993 Jan;3(1):13–26. doi: 10.1016/0960-9822(93)90140-j. [DOI] [PubMed] [Google Scholar]
- Stuart J. S., Frederick D. L., Varner C. M., Tatchell K. The mutant type 1 protein phosphatase encoded by glc7-1 from Saccharomyces cerevisiae fails to interact productively with the GAC1-encoded regulatory subunit. Mol Cell Biol. 1994 Feb;14(2):896–905. doi: 10.1128/mcb.14.2.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi M., Yanagida M. A mitotic role for a novel fission yeast protein kinase dsk1 with cell cycle stage dependent phosphorylation and localization. Mol Biol Cell. 1993 Mar;4(3):247–260. doi: 10.1091/mbc.4.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Cameron S., Sass P., Zoller M., Wigler M. Three different genes in S. cerevisiae encode the catalytic subunits of the cAMP-dependent protein kinase. Cell. 1987 Jul 17;50(2):277–287. doi: 10.1016/0092-8674(87)90223-6. [DOI] [PubMed] [Google Scholar]
- Toyn J. H., Araki H., Sugino A., Johnston L. H. The cell-cycle-regulated budding yeast gene DBF2, encoding a putative protein kinase, has a homologue that is not under cell-cycle control. Gene. 1991 Jul 31;104(1):63–70. doi: 10.1016/0378-1119(91)90465-n. [DOI] [PubMed] [Google Scholar]
- Wek R. C., Cannon J. F., Dever T. E., Hinnebusch A. G. Truncated protein phosphatase GLC7 restores translational activation of GCN4 expression in yeast mutants defective for the eIF-2 alpha kinase GCN2. Mol Cell Biol. 1992 Dec;12(12):5700–5710. doi: 10.1128/mcb.12.12.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]