Abstract
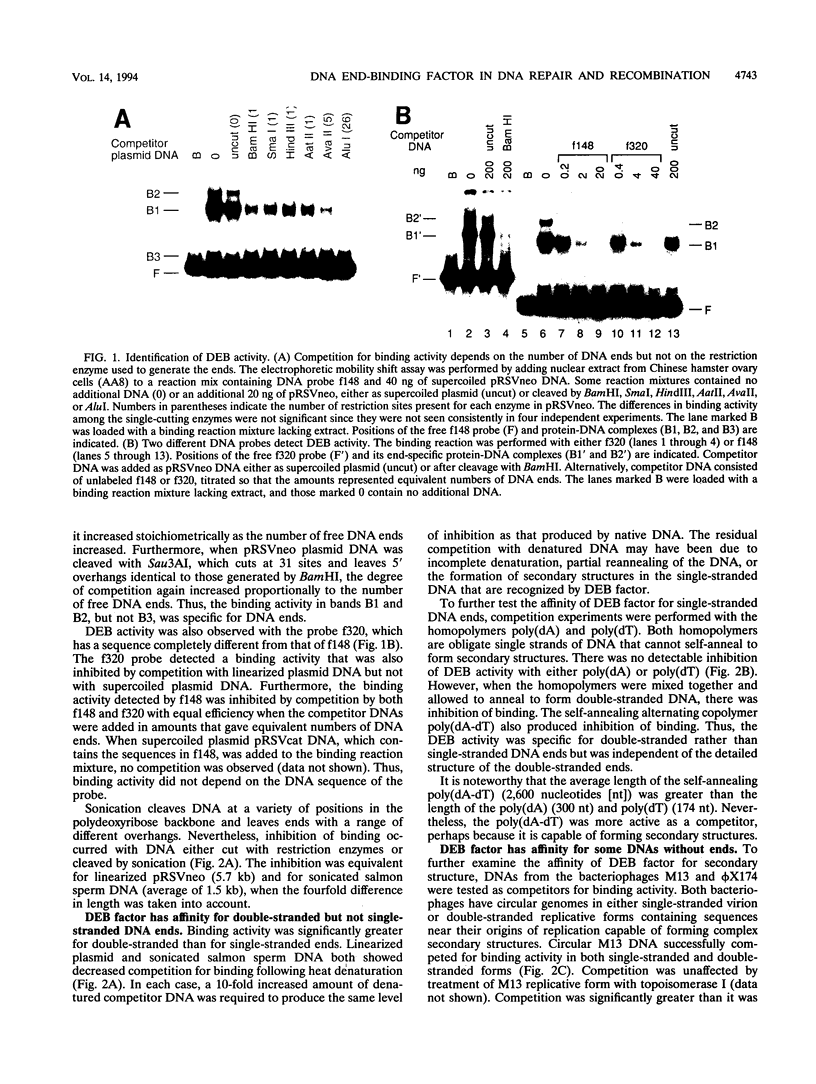
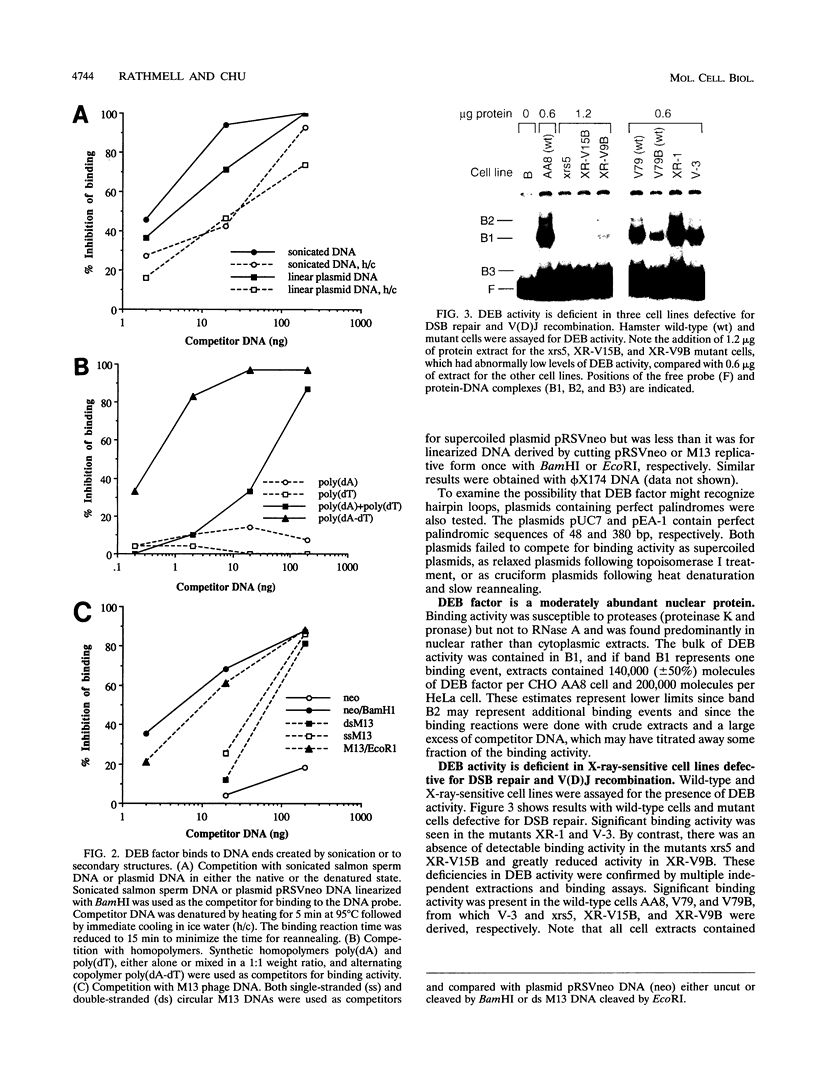
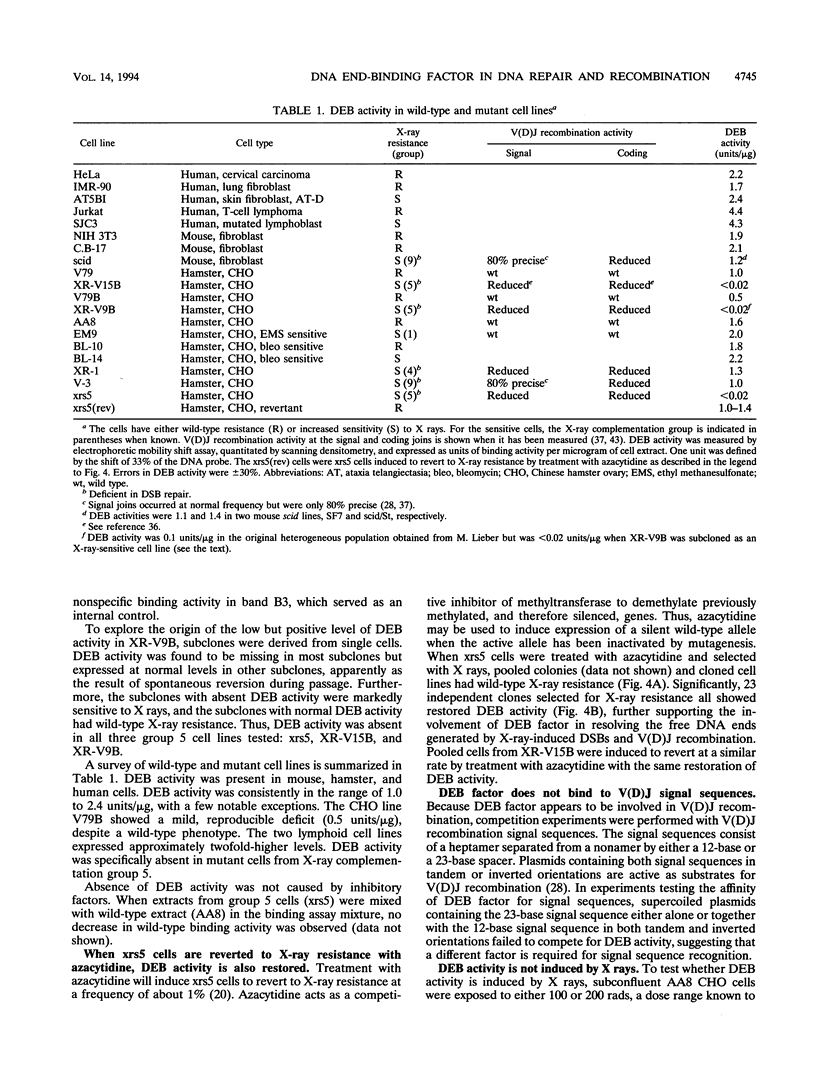
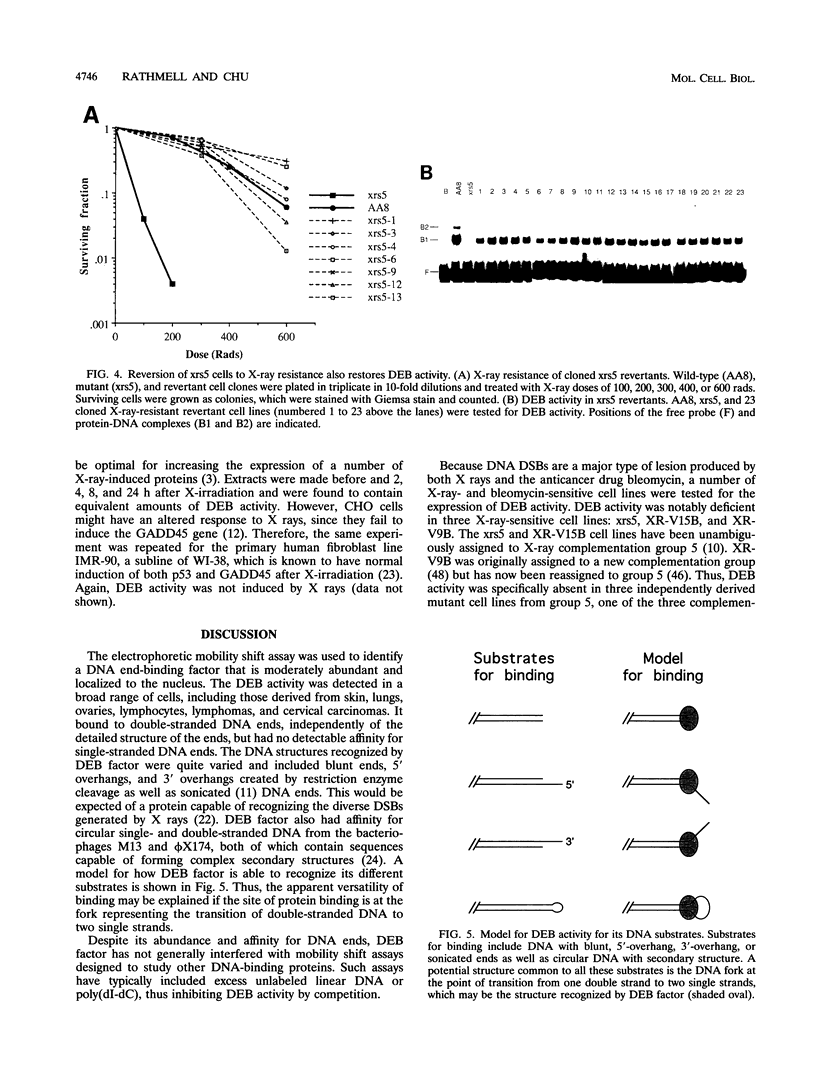
We have identified a nuclear factor that binds to double-stranded DNA ends, independently of the structure of the ends. It had equivalent affinities for DNA ends created by sonication or by restriction enzymes leaving 5', 3', or blunt ends but had no detectable affinity for single-stranded DNA ends. Since X rays induce DNA double-strand breaks, extracts from several complementation groups of X-ray-sensitive mammalian cells were tested for this DNA end-binding (DEB) activity. DEB activity was deficient in three independently derived cell lines from complementation group 5. Furthermore, when the cell lines reverted to X-ray resistance, expression of the DEB factor was restored to normal levels. Previous studies had shown that group 5 cells are defective for both double-strand break repair and V(D)J recombination. The residual V(D)J recombination activity in these cells produces abnormally large deletions at the sites of DNA joining (F. Pergola, M. Z. Zdzienicka, and M. R. Lieber, Mol. Cell. Biol. 13:3464-3471, 1993, and G. Taccioli, G. Rathbun, E. Oltz, T. Stamato, P. Jeggo, and F. Alt, Science 260:207-210, 1993), consistent with deficiency of a factor that protects DNA ends from degradation. Therefore, DEB factor may be involved in a biochemical pathway common to both double-strand break repair and V(D)J recombination.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benjamin R. C., Gill D. M. ADP-ribosylation in mammalian cell ghosts. Dependence of poly(ADP-ribose) synthesis on strand breakage in DNA. J Biol Chem. 1980 Nov 10;255(21):10493–10501. [PubMed] [Google Scholar]
- Biedermann K. A., Sun J. R., Giaccia A. J., Tosto L. M., Brown J. M. scid mutation in mice confers hypersensitivity to ionizing radiation and a deficiency in DNA double-strand break repair. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1394–1397. doi: 10.1073/pnas.88.4.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothman D. A., Bouvard I., Hughes E. N. Identification and characterization of X-ray-induced proteins in human cells. Cancer Res. 1989 Jun 1;49(11):2871–2878. [PubMed] [Google Scholar]
- Bosma M. J., Carroll A. M. The SCID mouse mutant: definition, characterization, and potential uses. Annu Rev Immunol. 1991;9:323–350. doi: 10.1146/annurev.iy.09.040191.001543. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cai Q. Q., Plet A., Imbert J., Lafage-Pochitaloff M., Cerdan C., Blanchard J. M. Chromosomal location and expression of the genes coding for Ku p70 and p80 in human cell lines and normal tissues. Cytogenet Cell Genet. 1994;65(4):221–227. doi: 10.1159/000133635. [DOI] [PubMed] [Google Scholar]
- Chu G., Chang E. Cisplatin-resistant cells express increased levels of a factor that recognizes damaged DNA. Proc Natl Acad Sci U S A. 1990 May;87(9):3324–3327. doi: 10.1073/pnas.87.9.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Chang E. Xeroderma pigmentosum group E cells lack a nuclear factor that binds to damaged DNA. Science. 1988 Oct 28;242(4878):564–567. doi: 10.1126/science.3175673. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E., Morgan W. F. Poly(ADP-ribose)polymerase: a perplexing participant in cellular responses to DNA breakage. Mutat Res. 1991 Jan;257(1):1–18. doi: 10.1016/0165-1110(91)90016-o. [DOI] [PubMed] [Google Scholar]
- Elsner H. I., Lindblad E. B. Ultrasonic degradation of DNA. DNA. 1989 Dec;8(10):697–701. doi: 10.1089/dna.1989.8.697. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Nebert D. W., Hollander M. C., Luethy J. D., Papathanasiou M., Fargnoli J., Holbrook N. J. Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol Cell Biol. 1989 Oct;9(10):4196–4203. doi: 10.1128/mcb.9.10.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaccia A. J., Lewis A. D., Denko N. C., Cholon A., Evans J. W., Waldren C. A., Stamato T. D., Brown J. M. The hypersensitivity of the Chinese hamster ovary variant BL-10 to bleomycin killing is due to a lack of glutathione S-transferase-alpha activity. Cancer Res. 1991 Aug 15;51(16):4463–4469. [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb T. M., Jackson S. P. The DNA-dependent protein kinase: requirement for DNA ends and association with Ku antigen. Cell. 1993 Jan 15;72(1):131–142. doi: 10.1016/0092-8674(93)90057-w. [DOI] [PubMed] [Google Scholar]
- Hwang B. J., Chu G. Purification and characterization of a human protein that binds to damaged DNA. Biochemistry. 1993 Feb 16;32(6):1657–1666. doi: 10.1021/bi00057a033. [DOI] [PubMed] [Google Scholar]
- Jaspers N. G., Bootsma D. Genetic heterogeneity in ataxia-telangiectasia studied by cell fusion. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2641–2644. doi: 10.1073/pnas.79.8.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeggo P. A., Hafezparast M., Thompson A. F., Broughton B. C., Kaur G. P., Zdzienicka M. Z., Athwal R. S. Localization of a DNA repair gene (XRCC5) involved in double-strand-break rejoining to human chromosome 2. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6423–6427. doi: 10.1073/pnas.89.14.6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeggo P. A., Holliday R. Azacytidine-induced reactivation of a DNA repair gene in Chinese hamster ovary cells. Mol Cell Biol. 1986 Aug;6(8):2944–2949. doi: 10.1128/mcb.6.8.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeggo P. A., Kemp L. M. X-ray-sensitive mutants of Chinese hamster ovary cell line. Isolation and cross-sensitivity to other DNA-damaging agents. Mutat Res. 1983 Dec;112(6):313–327. doi: 10.1016/0167-8817(83)90026-3. [DOI] [PubMed] [Google Scholar]
- Kapp D. S., Smith K. C. Chemical nature of chain breaks produced in DNA by x-irradiation in vitro. Radiat Res. 1970 Apr;42(1):34–49. [PubMed] [Google Scholar]
- Kastan M. B., Zhan Q., el-Deiry W. S., Carrier F., Jacks T., Walsh W. V., Plunkett B. S., Vogelstein B., Fornace A. J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992 Nov 13;71(4):587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Kuerbitz S. J., Plunkett B. S., Walsh W. V., Kastan M. B. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees-Miller S. P., Chen Y. R., Anderson C. W. Human cells contain a DNA-activated protein kinase that phosphorylates simian virus 40 T antigen, mouse p53, and the human Ku autoantigen. Mol Cell Biol. 1990 Dec;10(12):6472–6481. doi: 10.1128/mcb.10.12.6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber M. R., Hesse J. E., Lewis S., Bosma G. C., Rosenberg N., Mizuuchi K., Bosma M. J., Gellert M. The defect in murine severe combined immune deficiency: joining of signal sequences but not coding segments in V(D)J recombination. Cell. 1988 Oct 7;55(1):7–16. doi: 10.1016/0092-8674(88)90004-9. [DOI] [PubMed] [Google Scholar]
- Lieber M. R. The mechanism of V(D)J recombination: a balance of diversity, specificity, and stability. Cell. 1992 Sep 18;70(6):873–876. doi: 10.1016/0092-8674(92)90237-7. [DOI] [PubMed] [Google Scholar]
- Mimori T., Hardin J. A. Mechanism of interaction between Ku protein and DNA. J Biol Chem. 1986 Aug 5;261(22):10375–10379. [PubMed] [Google Scholar]
- Mimori T., Hardin J. A., Steitz J. A. Characterization of the DNA-binding protein antigen Ku recognized by autoantibodies from patients with rheumatic disorders. J Biol Chem. 1986 Feb 15;261(5):2274–2278. [PubMed] [Google Scholar]
- Oettinger M. A., Schatz D. G., Gorka C., Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990 Jun 22;248(4962):1517–1523. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- Ohgushi H., Yoshihara K., Kamiya T. Bovine thymus poly(adenosine diphosphate ribose) polymerase. Physical properties and binding to DNA. J Biol Chem. 1980 Jul 10;255(13):6205–6211. [PubMed] [Google Scholar]
- Osborn L., Kunkel S., Nabel G. J. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillard S., Strauss F. Analysis of the mechanism of interaction of simian Ku protein with DNA. Nucleic Acids Res. 1991 Oct 25;19(20):5619–5624. doi: 10.1093/nar/19.20.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson M., Chu G. Evidence that xeroderma pigmentosum cells from complementation group E are deficient in a homolog of yeast photolyase. Mol Cell Biol. 1989 Nov;9(11):5105–5112. doi: 10.1128/mcb.9.11.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergola F., Zdzienicka M. Z., Lieber M. R. V(D)J recombination in mammalian cell mutants defective in DNA double-strand break repair. Mol Cell Biol. 1993 Jun;13(6):3464–3471. doi: 10.1128/mcb.13.6.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz D. G., Oettinger M. A., Baltimore D. The V(D)J recombination activating gene, RAG-1. Cell. 1989 Dec 22;59(6):1035–1048. doi: 10.1016/0092-8674(89)90760-5. [DOI] [PubMed] [Google Scholar]
- Singh H., Sen R., Baltimore D., Sharp P. A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986 Jan 9;319(6049):154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stamato T. D., Weinstein R., Giaccia A., Mackenzie L. Isolation of cell cycle-dependent gamma ray-sensitive Chinese hamster ovary cell. Somatic Cell Genet. 1983 Mar;9(2):165–173. doi: 10.1007/BF01543175. [DOI] [PubMed] [Google Scholar]
- Taccioli G. E., Rathbun G., Oltz E., Stamato T., Jeggo P. A., Alt F. W. Impairment of V(D)J recombination in double-strand break repair mutants. Science. 1993 Apr 9;260(5105):207–210. doi: 10.1126/science.8469973. [DOI] [PubMed] [Google Scholar]
- Taccioli G. E., Rathbun G., Shinkai Y., Oltz E. M., Cheng H., Whitmore G., Stamato T., Jeggo P., Alt F. W. Activities involved in V(D)J recombination. Curr Top Microbiol Immunol. 1992;182:107–114. doi: 10.1007/978-3-642-77633-5_13. [DOI] [PubMed] [Google Scholar]
- Whitmore G. F., Varghese A. J., Gulyas S. Cell cycle responses of two X-ray sensitive mutants defective in DNA repair. Int J Radiat Biol. 1989 Nov;56(5):657–665. doi: 10.1080/09553008914551881. [DOI] [PubMed] [Google Scholar]
- Zdzienicka M. Z., Tran Q., van der Schans G. P., Simons J. W. Characterization of an X-ray-hypersensitive mutant of V79 Chinese hamster cells. Mutat Res. 1988 Nov;194(3):239–249. doi: 10.1016/0167-8817(88)90025-9. [DOI] [PubMed] [Google Scholar]
- Zdzienicka M. Z., van Wessel N., van der Schans G. P. A fourth complementation group among ionizing radiation-sensitive Chinese hamster cell mutants defective in DNA double-strand break repair. Radiat Res. 1992 Sep;131(3):309–314. [PubMed] [Google Scholar]