Abstract
We recently identified Vav as a Ras-activating guanine nucleotide exchange factor (GEF) stimulated by a T-cell antigen receptor-coupled protein tyrosine kinase (PTK). Here, we describe a novel, protein kinase-independent alternative pathway of Vav activation. Phorbol ester, 1,2-diacylglycerol, or ceramide treatment of intact T cells, Vav immunoprecipitates, or partially purified Vav generated by in vitro translation or COS-1 cell transfection stimulated the Ras exchange activity of Vav in the absence of detectable tyrosine phosphorylation. GEF activity of gel-purified Vav was similarly stimulated by phorbol myristate acetate (PMA). Stimulation was resistant to PTK and protein kinase C inhibitors but was blocked by calphostin, a PMA and diacylglycerol antagonist. In vitro-translated Vav lacking its cysteine-rich domain, or mutated at a single cysteine residue within this domain (C528A), was not stimulated by PMA but was fully activated by p56lck. This correlated with increased binding of radiolabeled phorbol ester to COS-1 cells expressing wild-type, but not C528A-mutated, Vav. Thus, Vav itself is a PMA-binding and -activated Ras GEF. Recombinant interleukin-1 alpha stimulated Vav via this pathway, suggesting that diglyceride-mediated Vav activation may couple PTK-independent receptors which stimulate production of lipid second messengers to Ras in hematopoietic cells.
Full text
PDF

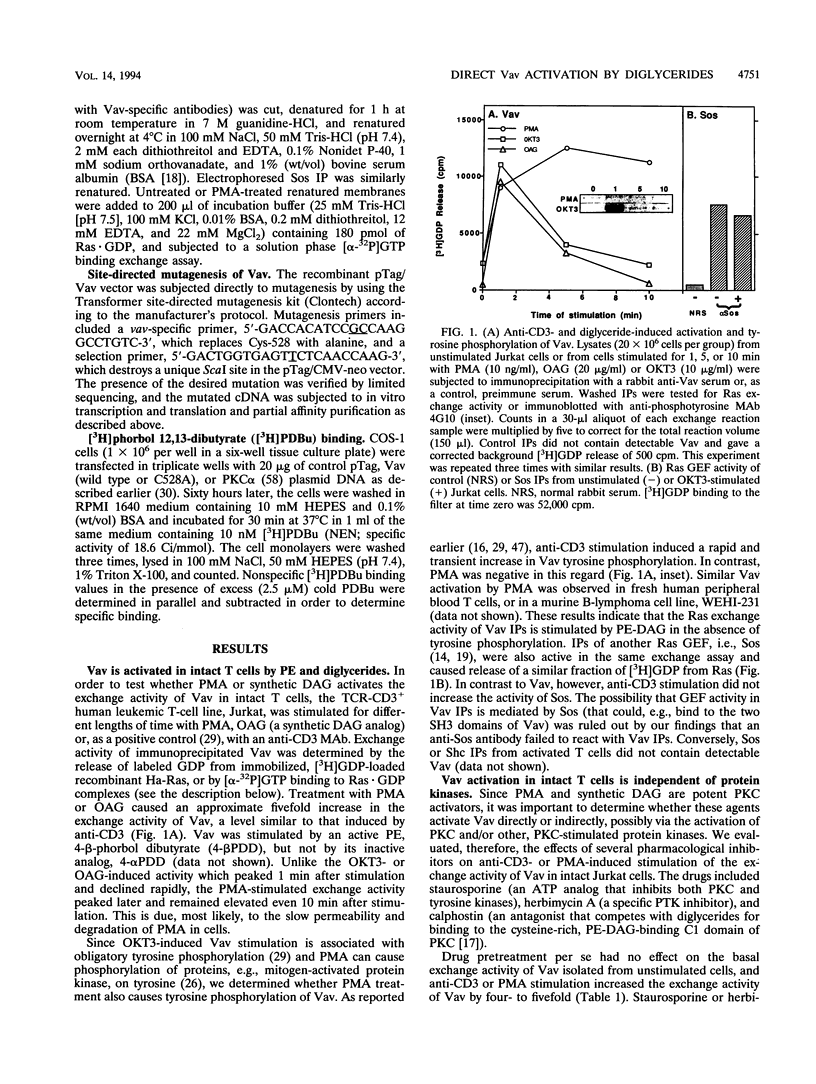

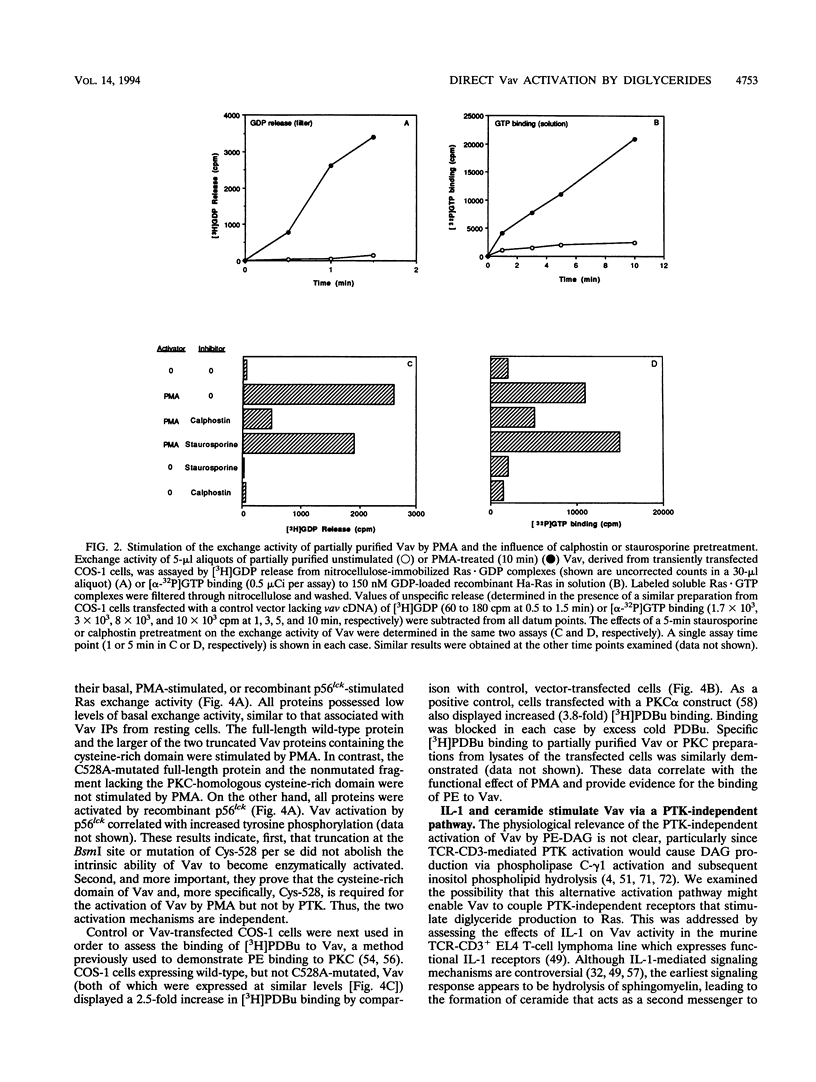
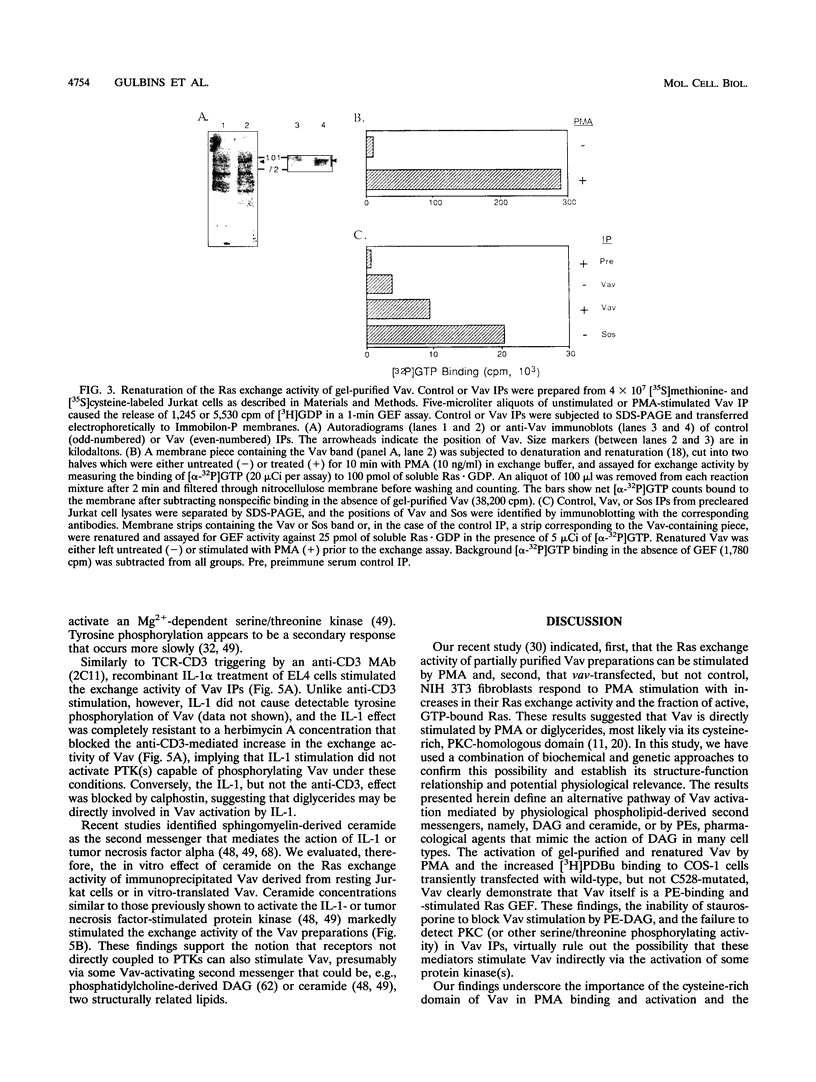
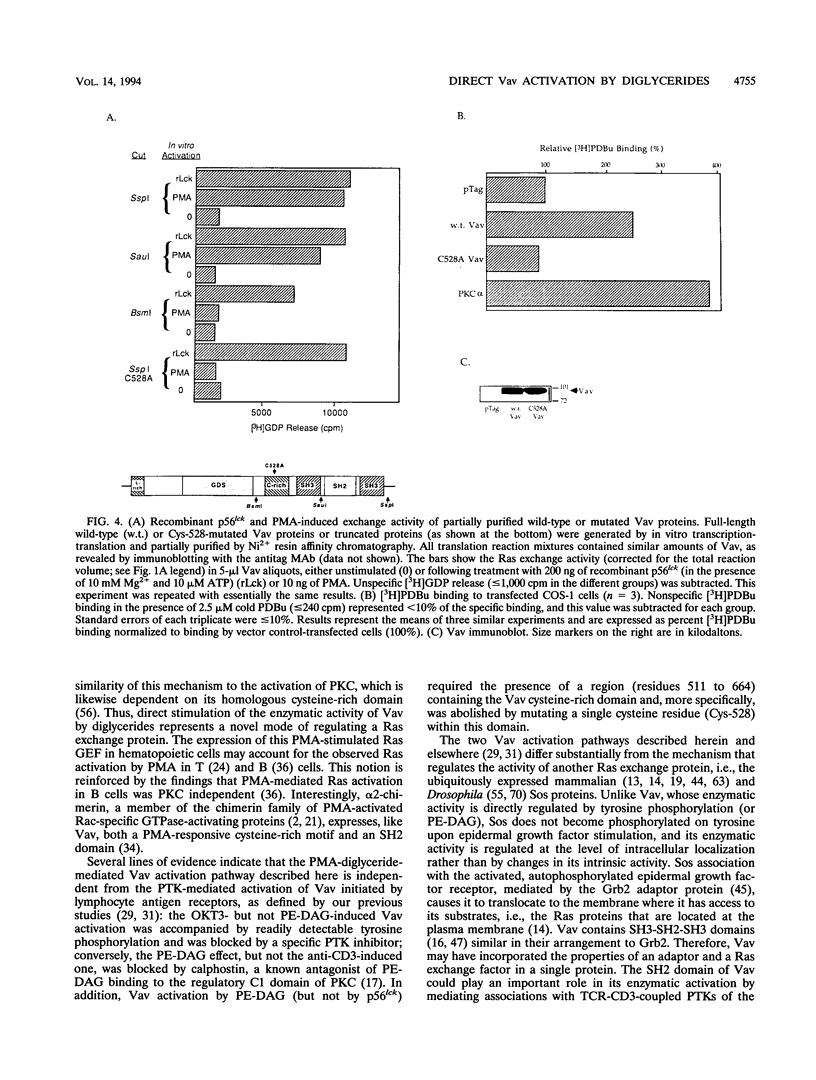


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Houston H., Allen J., Lints T., Harvey R. The hematopoietically expressed vav proto-oncogene shares homology with the dbl GDP-GTP exchange factor, the bcr gene and a yeast gene (CDC24) involved in cytoskeletal organization. Oncogene. 1992 Apr;7(4):611–618. [PubMed] [Google Scholar]
- Ahmed S., Lee J., Kozma R., Best A., Monfries C., Lim L. A novel functional target for tumor-promoting phorbol esters and lysophosphatidic acid. The p21rac-GTPase activating protein n-chimaerin. J Biol Chem. 1993 May 25;268(15):10709–10712. [PubMed] [Google Scholar]
- Alai M., Mui A. L., Cutler R. L., Bustelo X. R., Barbacid M., Krystal G. Steel factor stimulates the tyrosine phosphorylation of the proto-oncogene product, p95vav, in human hemopoietic cells. J Biol Chem. 1992 Sep 5;267(25):18021–18025. [PubMed] [Google Scholar]
- Altman A., Coggeshall K. M., Mustelin T. Molecular events mediating T cell activation. Adv Immunol. 1990;48:227–360. doi: 10.1016/s0065-2776(08)60756-7. [DOI] [PubMed] [Google Scholar]
- Amrein K. E., Flint N., Panholzer B., Burn P. Ras GTPase-activating protein: a substrate and a potential binding protein of the protein-tyrosine kinase p56lck. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3343–3346. doi: 10.1073/pnas.89.8.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier G., Telford D., Giampa L., Coggeshall K. M., Baier-Bitterlich G., Isakov N., Altman A. Molecular cloning and characterization of PKC theta, a novel member of the protein kinase C (PKC) gene family expressed predominantly in hematopoietic cells. J Biol Chem. 1993 Mar 5;268(7):4997–5004. [PubMed] [Google Scholar]
- Baldari C. T., Heguy A., Telford J. L. ras protein activity is essential for T-cell antigen receptor signal transduction. J Biol Chem. 1993 Feb 5;268(4):2693–2698. [PubMed] [Google Scholar]
- Baldari C. T., Macchia G., Telford J. L. Interleukin-2 promoter activation in T-cells expressing activated Ha-ras. J Biol Chem. 1992 Mar 5;267(7):4289–4291. [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Boguski M. S., Bairoch A., Attwood T. K., Michaels G. S. Proto-vav and gene expression. Nature. 1992 Jul 9;358(6382):113–113. doi: 10.1038/358113a0. [DOI] [PubMed] [Google Scholar]
- Boguski M. S., McCormick F. Proteins regulating Ras and its relatives. Nature. 1993 Dec 16;366(6456):643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- Bowtell D., Fu P., Simon M., Senior P. Identification of murine homologues of the Drosophila son of sevenless gene: potential activators of ras. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6511–6515. doi: 10.1073/pnas.89.14.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buday L., Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 1993 May 7;73(3):611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- Bustelo X. R., Barbacid M. Tyrosine phosphorylation of the vav proto-oncogene product in activated B cells. Science. 1992 May 22;256(5060):1196–1199. doi: 10.1126/science.256.5060.1196. [DOI] [PubMed] [Google Scholar]
- Bustelo X. R., Ledbetter J. A., Barbacid M. Product of vav proto-oncogene defines a new class of tyrosine protein kinase substrates. Nature. 1992 Mar 5;356(6364):68–71. doi: 10.1038/356068a0. [DOI] [PubMed] [Google Scholar]
- Casnellie J. E. Protein kinase inhibitors: probes for the functions of protein phosphorylation. Adv Pharmacol. 1991;22:167–205. doi: 10.1016/s1054-3589(08)60035-6. [DOI] [PubMed] [Google Scholar]
- Celenza J. L., Carlson M. Renaturation of protein kinase activity of protein blots. Methods Enzymol. 1991;200:423–430. doi: 10.1016/0076-6879(91)00158-s. [DOI] [PubMed] [Google Scholar]
- Chardin P., Camonis J. H., Gale N. W., van Aelst L., Schlessinger J., Wigler M. H., Bar-Sagi D. Human Sos1: a guanine nucleotide exchange factor for Ras that binds to GRB2. Science. 1993 May 28;260(5112):1338–1343. doi: 10.1126/science.8493579. [DOI] [PubMed] [Google Scholar]
- Coppola J., Bryant S., Koda T., Conway D., Barbacid M. Mechanism of activation of the vav protooncogene. Cell Growth Differ. 1991 Feb;2(2):95–105. [PubMed] [Google Scholar]
- Diekmann D., Brill S., Garrett M. D., Totty N., Hsuan J., Monfries C., Hall C., Lim L., Hall A. Bcr encodes a GTPase-activating protein for p21rac. Nature. 1991 May 30;351(6325):400–402. doi: 10.1038/351400a0. [DOI] [PubMed] [Google Scholar]
- Downward J., Graves J. D., Warne P. H., Rayter S., Cantrell D. A. Stimulation of p21ras upon T-cell activation. Nature. 1990 Aug 23;346(6286):719–723. doi: 10.1038/346719a0. [DOI] [PubMed] [Google Scholar]
- Downward J. Ras regulation: putting back the GTP. Curr Biol. 1992 Jun;2(6):329–331. doi: 10.1016/0960-9822(92)90897-j. [DOI] [PubMed] [Google Scholar]
- Downward J. Regulatory mechanisms for ras proteins. Bioessays. 1992 Mar;14(3):177–184. doi: 10.1002/bies.950140308. [DOI] [PubMed] [Google Scholar]
- Duronio V., Welham M. J., Abraham S., Dryden P., Schrader J. W. p21ras activation via hemopoietin receptors and c-kit requires tyrosine kinase activity but not tyrosine phosphorylation of p21ras GTPase-activating protein. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1587–1591. doi: 10.1073/pnas.89.5.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore T., Martin G. S. Phorbol ester and diacylglycerol induce protein phosphorylation at tyrosine. Nature. 1983 Dec 1;306(5942):487–490. doi: 10.1038/306487a0. [DOI] [PubMed] [Google Scholar]
- Graves J. D., Downward J., Izquierdo-Pastor M., Rayter S., Warne P. H., Cantrell D. A. The growth factor IL-2 activates p21ras proteins in normal human T lymphocytes. J Immunol. 1992 Apr 15;148(8):2417–2422. [PubMed] [Google Scholar]
- Gulbins E., Coggeshall K. M., Baier G., Katzav S., Burn P., Altman A. Tyrosine kinase-stimulated guanine nucleotide exchange activity of Vav in T cell activation. Science. 1993 May 7;260(5109):822–825. doi: 10.1126/science.8484124. [DOI] [PubMed] [Google Scholar]
- Gulbins E., Coggeshall K. M., Langlet C., Baier G., Bonnefoy-Berard N., Burn P., Wittinghofer A., Katzav S., Altman A. Activation of Ras in vitro and in intact fibroblasts by the Vav guanine nucleotide exchange protein. Mol Cell Biol. 1994 Feb;14(2):906–913. doi: 10.1128/mcb.14.2.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbins E., Langlet C., Baier G., Bonnefoy-Berard N., Herbert E., Altman A., Coggeshall K. M. Tyrosine phosphorylation and activation of Vav GTP/GDP exchange activity in antigen receptor-triggered B cells. J Immunol. 1994 Mar 1;152(5):2123–2129. [PubMed] [Google Scholar]
- Guy G. R., Chua S. P., Wong N. S., Ng S. B., Tan Y. H. Interleukin 1 and tumor necrosis factor activate common multiple protein kinases in human fibroblasts. J Biol Chem. 1991 Aug 5;266(22):14343–14352. [PubMed] [Google Scholar]
- Hall A. The cellular functions of small GTP-binding proteins. Science. 1990 Aug 10;249(4969):635–640. doi: 10.1126/science.2116664. [DOI] [PubMed] [Google Scholar]
- Hall C., Sin W. C., Teo M., Michael G. J., Smith P., Dong J. M., Lim H. H., Manser E., Spurr N. K., Jones T. A. Alpha 2-chimerin, an SH2-containing GTPase-activating protein for the ras-related protein p21rac derived by alternate splicing of the human n-chimerin gene, is selectively expressed in brain regions and testes. Mol Cell Biol. 1993 Aug;13(8):4986–4998. doi: 10.1128/mcb.13.8.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart M. J., Eva A., Evans T., Aaronson S. A., Cerione R. A. Catalysis of guanine nucleotide exchange on the CDC42Hs protein by the dbl oncogene product. Nature. 1991 Nov 28;354(6351):311–314. doi: 10.1038/354311a0. [DOI] [PubMed] [Google Scholar]
- Harwood A. E., Cambier J. C. B cell antigen receptor cross-linking triggers rapid protein kinase C independent activation of p21ras1. J Immunol. 1993 Nov 1;151(9):4513–4522. [PubMed] [Google Scholar]
- Isakov N., McMahon P., Altman A. Selective post-transcriptional down-regulation of protein kinase C isoenzymes in leukemic T cells chronically treated with phorbol ester. J Biol Chem. 1990 Feb 5;265(4):2091–2097. [PubMed] [Google Scholar]
- Izquierdo M., Cantrell D. A. Protein tyrosine kinases couple the interleukin-2 receptor to p21ras. Eur J Immunol. 1993 Jan;23(1):131–135. doi: 10.1002/eji.1830230121. [DOI] [PubMed] [Google Scholar]
- Izquierdo M., Downward J., Graves J. D., Cantrell D. A. Role of protein kinase C in T-cell antigen receptor regulation of p21ras: evidence that two p21ras regulatory pathways coexist in T cells. Mol Cell Biol. 1992 Jul;12(7):3305–3312. doi: 10.1128/mcb.12.7.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo M., Leevers S. J., Marshall C. J., Cantrell D. p21ras couples the T cell antigen receptor to extracellular signal-regulated kinase 2 in T lymphocytes. J Exp Med. 1993 Oct 1;178(4):1199–1208. doi: 10.1084/jem.178.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June C. H., Fletcher M. C., Ledbetter J. A., Schieven G. L., Siegel J. N., Phillips A. F., Samelson L. E. Inhibition of tyrosine phosphorylation prevents T-cell receptor-mediated signal transduction. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7722–7726. doi: 10.1073/pnas.87.19.7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzav S., Cleveland J. L., Heslop H. E., Pulido D. Loss of the amino-terminal helix-loop-helix domain of the vav proto-oncogene activates its transforming potential. Mol Cell Biol. 1991 Apr;11(4):1912–1920. doi: 10.1128/mcb.11.4.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzav S., Martin-Zanca D., Barbacid M. vav, a novel human oncogene derived from a locus ubiquitously expressed in hematopoietic cells. EMBO J. 1989 Aug;8(8):2283–2290. doi: 10.1002/j.1460-2075.1989.tb08354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Batzer A., Daly R., Yajnik V., Skolnik E., Chardin P., Bar-Sagi D., Margolis B., Schlessinger J. Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signalling. Nature. 1993 May 6;363(6424):85–88. doi: 10.1038/363085a0. [DOI] [PubMed] [Google Scholar]
- Lowenstein E. J., Daly R. J., Batzer A. G., Li W., Margolis B., Lammers R., Ullrich A., Skolnik E. Y., Bar-Sagi D., Schlessinger J. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 1992 Aug 7;70(3):431–442. doi: 10.1016/0092-8674(92)90167-b. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Zhang K., DeClue J. E., Willumsen B. M. Regulation of p21ras activity. Trends Genet. 1991 Nov-Dec;7(11-12):346–351. doi: 10.1016/0168-9525(91)90253-m. [DOI] [PubMed] [Google Scholar]
- Margolis B., Hu P., Katzav S., Li W., Oliver J. M., Ullrich A., Weiss A., Schlessinger J. Tyrosine phosphorylation of vav proto-oncogene product containing SH2 domain and transcription factor motifs. Nature. 1992 Mar 5;356(6364):71–74. doi: 10.1038/356071a0. [DOI] [PubMed] [Google Scholar]
- Maruyama I. N., Brenner S. A phorbol ester/diacylglycerol-binding protein encoded by the unc-13 gene of Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5729–5733. doi: 10.1073/pnas.88.13.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias S., Dressler K. A., Kolesnick R. N. Characterization of a ceramide-activated protein kinase: stimulation by tumor necrosis factor alpha. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10009–10013. doi: 10.1073/pnas.88.22.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias S., Younes A., Kan C. C., Orlow I., Joseph C., Kolesnick R. N. Activation of the sphingomyelin signaling pathway in intact EL4 cells and in a cell-free system by IL-1 beta. Science. 1993 Jan 22;259(5094):519–522. doi: 10.1126/science.8424175. [DOI] [PubMed] [Google Scholar]
- Mills G. B., Stewart D. J., Mellors A., Gelfand E. W. Interleukin 2 does not induce phosphatidylinositol hydrolysis in activated T cells. J Immunol. 1986 Apr 15;136(8):3019–3024. [PubMed] [Google Scholar]
- Mustelin T., Burn P. Regulation of src family tyrosine kinases in lymphocytes. Trends Biochem Sci. 1993 Jun;18(6):215–220. doi: 10.1016/0968-0004(93)90192-p. [DOI] [PubMed] [Google Scholar]
- Mustelin T., Coggeshall K. M., Isakov N., Altman A. T cell antigen receptor-mediated activation of phospholipase C requires tyrosine phosphorylation. Science. 1990 Mar 30;247(4950):1584–1587. doi: 10.1126/science.2138816. [DOI] [PubMed] [Google Scholar]
- O'Neill L. A., Bird T. A., Saklatvala J. How does interleukin 1 activate cells? Interleukin 1 signal transduction. Immunol Today. 1990 Nov;11(11):392–394. doi: 10.1016/0167-5699(90)90155-3. [DOI] [PubMed] [Google Scholar]
- Ohno S., Akita Y., Konno Y., Imajoh S., Suzuki K. A novel phorbol ester receptor/protein kinase, nPKC, distantly related to the protein kinase C family. Cell. 1988 Jun 3;53(5):731–741. doi: 10.1016/0092-8674(88)90091-8. [DOI] [PubMed] [Google Scholar]
- Olivier J. P., Raabe T., Henkemeyer M., Dickson B., Mbamalu G., Margolis B., Schlessinger J., Hafen E., Pawson T. A Drosophila SH2-SH3 adaptor protein implicated in coupling the sevenless tyrosine kinase to an activator of Ras guanine nucleotide exchange, Sos. Cell. 1993 Apr 9;73(1):179–191. doi: 10.1016/0092-8674(93)90170-u. [DOI] [PubMed] [Google Scholar]
- Ono Y., Fujii T., Igarashi K., Kuno T., Tanaka C., Kikkawa U., Nishizuka Y. Phorbol ester binding to protein kinase C requires a cysteine-rich zinc-finger-like sequence. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4868–4871. doi: 10.1073/pnas.86.13.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker P. J., Coussens L., Totty N., Rhee L., Young S., Chen E., Stabel S., Waterfield M. D., Ullrich A. The complete primary structure of protein kinase C--the major phorbol ester receptor. Science. 1986 Aug 22;233(4766):853–859. doi: 10.1126/science.3755547. [DOI] [PubMed] [Google Scholar]
- Ravichandran K. S., Lee K. K., Songyang Z., Cantley L. C., Burn P., Burakoff S. J. Interaction of Shc with the zeta chain of the T cell receptor upon T cell activation. Science. 1993 Nov 5;262(5135):902–905. doi: 10.1126/science.8235613. [DOI] [PubMed] [Google Scholar]
- Rayter S. I., Woodrow M., Lucas S. C., Cantrell D. A., Downward J. p21ras mediates control of IL-2 gene promoter function in T cell activation. EMBO J. 1992 Dec;11(12):4549–4556. doi: 10.1002/j.1460-2075.1992.tb05556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T. M. Cell biology. A signal chain of events. Nature. 1992 Dec 10;360(6404):534–535. doi: 10.1038/360534a0. [DOI] [PubMed] [Google Scholar]
- Rosoff P. M., Savage N., Dinarello C. A. Interleukin-1 stimulates diacylglycerol production in T lymphocytes by a novel mechanism. Cell. 1988 Jul 1;54(1):73–81. doi: 10.1016/0092-8674(88)90181-x. [DOI] [PubMed] [Google Scholar]
- Rozakis-Adcock M., Fernley R., Wade J., Pawson T., Bowtell D. The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator mSos1. Nature. 1993 May 6;363(6424):83–85. doi: 10.1038/363083a0. [DOI] [PubMed] [Google Scholar]
- Saltzman E. M., Thom R. R., Casnellie J. E. Activation of a tyrosine protein kinase is an early event in the stimulation of T lymphocytes by interleukin-2. J Biol Chem. 1988 May 25;263(15):6956–6959. [PubMed] [Google Scholar]
- Satoh T., Nakafuku M., Kaziro Y. Function of Ras as a molecular switch in signal transduction. J Biol Chem. 1992 Dec 5;267(34):24149–24152. [PubMed] [Google Scholar]
- Satoh T., Nakafuku M., Miyajima A., Kaziro Y. Involvement of ras p21 protein in signal-transduction pathways from interleukin 2, interleukin 3, and granulocyte/macrophage colony-stimulating factor, but not from interleukin 4. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3314–3318. doi: 10.1073/pnas.88.8.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Uehara Y., Kaziro Y. Inhibition of interleukin 3 and granulocyte-macrophage colony-stimulating factor stimulated increase of active ras.GTP by herbimycin A, a specific inhibitor of tyrosine kinases. J Biol Chem. 1992 Feb 5;267(4):2537–2541. [PubMed] [Google Scholar]
- Schütze S., Potthoff K., Machleidt T., Berkovic D., Wiegmann K., Krönke M. TNF activates NF-kappa B by phosphatidylcholine-specific phospholipase C-induced "acidic" sphingomyelin breakdown. Cell. 1992 Nov 27;71(5):765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- Shou C., Farnsworth C. L., Neel B. G., Feig L. A. Molecular cloning of cDNAs encoding a guanine-nucleotide-releasing factor for Ras p21. Nature. 1992 Jul 23;358(6384):351–354. doi: 10.1038/358351a0. [DOI] [PubMed] [Google Scholar]
- Simon M. A., Dodson G. S., Rubin G. M. An SH3-SH2-SH3 protein is required for p21Ras1 activation and binds to sevenless and Sos proteins in vitro. Cell. 1993 Apr 9;73(1):169–177. doi: 10.1016/0092-8674(93)90169-q. [DOI] [PubMed] [Google Scholar]
- Weiss A., Littman D. R. Signal transduction by lymphocyte antigen receptors. Cell. 1994 Jan 28;76(2):263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Weiss A. T cell antigen receptor signal transduction: a tale of tails and cytoplasmic protein-tyrosine kinases. Cell. 1993 Apr 23;73(2):209–212. doi: 10.1016/0092-8674(93)90221-b. [DOI] [PubMed] [Google Scholar]
- Williams L. T. Missing links between receptors and Ras. Curr Biol. 1992 Nov;2(11):601–603. doi: 10.1016/0960-9822(92)90169-b. [DOI] [PubMed] [Google Scholar]
- Woodrow M. A., Rayter S., Downward J., Cantrell D. A. p21ras function is important for T cell antigen receptor and protein kinase C regulation of nuclear factor of activated T cells. J Immunol. 1993 May 1;150(9):3853–3861. [PubMed] [Google Scholar]
- Woodrow M., Clipstone N. A., Cantrell D. p21ras and calcineurin synergize to regulate the nuclear factor of activated T cells. J Exp Med. 1993 Nov 1;178(5):1517–1522. doi: 10.1084/jem.178.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]





