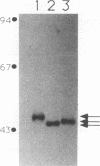
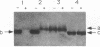
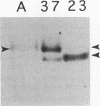
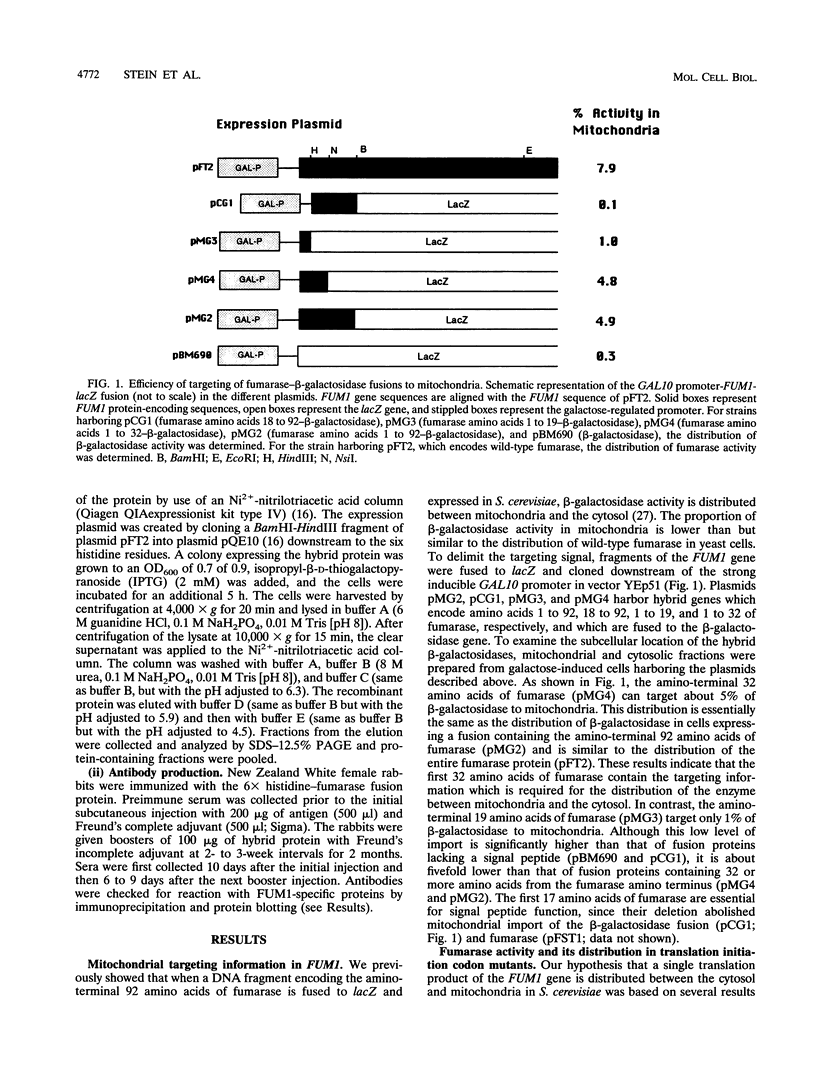
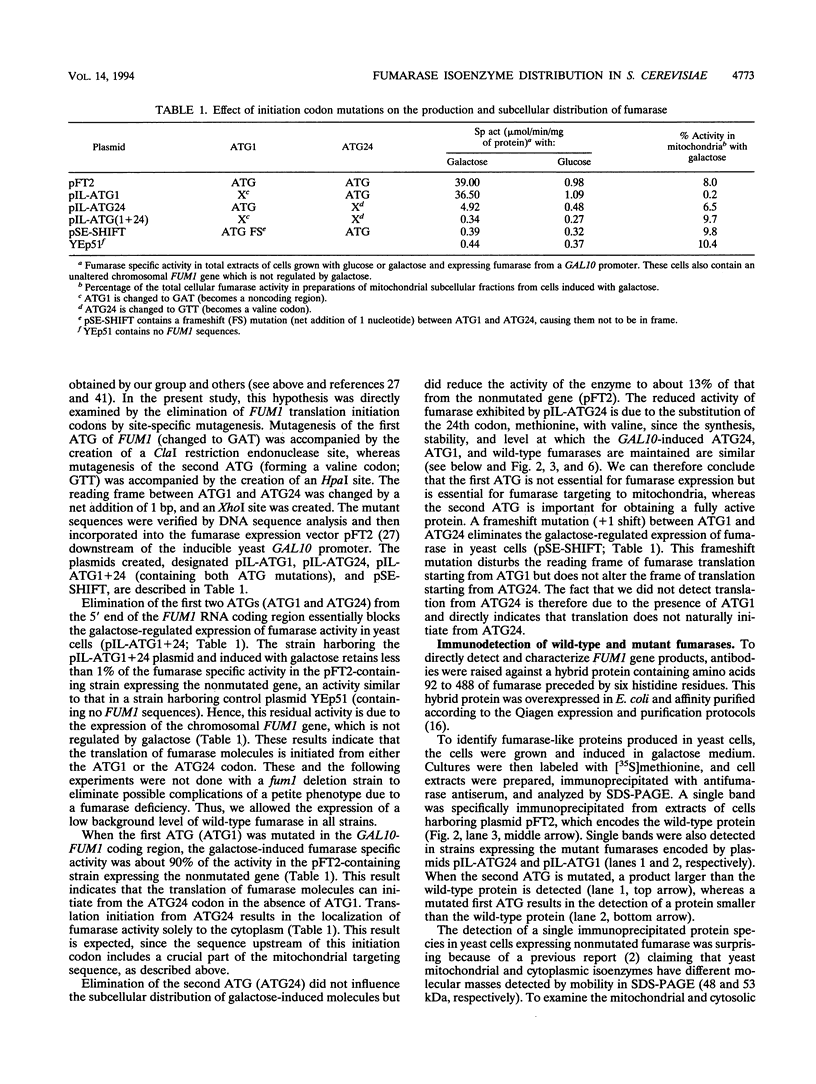
Abstract
The yeast mitochondrial and cytosolic isoenzymes of fumarase, which are encoded by a single nuclear gene (FUM1), follow a unique mechanism of protein subcellular localization and distribution. Translation of all FUM1 messages initiates only from the 5'-proximal AUG codon and results in a single translation product that contains the targeting sequence located within the first 32 amino acids of the precursor. All fumarase molecules synthesized in the cell are processed by the mitochondrial matrix signal peptidase; nevertheless, most of the enzyme (80 to 90%) ends up in the cytosol. The translocation and processing of fumarase are cotranslational. We suggest that in Saccharomyces cerevisiae, the single type of initial translation product of the FUM1 gene is first partially translocated, and then a subset of these molecules continues to be fully translocated into the organelle, whereas the rest are folded into an import-incompetent state and are released by the retrograde movement of fumarase into the cytosol.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beltzer J. P., Morris S. R., Kohlhaw G. B. Yeast LEU4 encodes mitochondrial and nonmitochondrial forms of alpha-isopropylmalate synthase. J Biol Chem. 1988 Jan 5;263(1):368–374. [PubMed] [Google Scholar]
- Boonyarat D., Doonan S. Purification and structural comparisons of the cytosolic and mitochondrial fumarases from baker's yeast. Int J Biochem. 1988;20(10):1125–1132. doi: 10.1016/0020-711x(88)90258-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carlson M., Botstein D. Two differentially regulated mRNAs with different 5' ends encode secreted with intracellular forms of yeast invertase. Cell. 1982 Jan;28(1):145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Martinez-Arias A., Shapira S. K., Chou J. Beta-galactosidase gene fusions for analyzing gene expression in escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- Daum G., Böhni P. C., Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13028–13033. [PubMed] [Google Scholar]
- Eisenberg D., Schwarz E., Komaromy M., Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984 Oct 15;179(1):125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- Fujiki M., Verner K. Coupling of cytosolic protein synthesis and mitochondrial protein import in yeast. Evidence for cotranslational import in vivo. J Biol Chem. 1993 Jan 25;268(3):1914–1920. [PubMed] [Google Scholar]
- Geli V., Yang M. J., Suda K., Lustig A., Schatz G. The MAS-encoded processing protease of yeast mitochondria. Overproduction and characterization of its two nonidentical subunits. J Biol Chem. 1990 Nov 5;265(31):19216–19222. [PubMed] [Google Scholar]
- Gillman E. C., Slusher L. B., Martin N. C., Hopper A. K. MOD5 translation initiation sites determine N6-isopentenyladenosine modification of mitochondrial and cytoplasmic tRNA. Mol Cell Biol. 1991 May;11(5):2382–2390. doi: 10.1128/mcb.11.5.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick B. S., Brandt A., Cunningham K., Müller S., Hallberg R. L., Schatz G. Cytochromes c1 and b2 are sorted to the intermembrane space of yeast mitochondria by a stop-transfer mechanism. Cell. 1992 May 29;69(5):809–822. doi: 10.1016/0092-8674(92)90292-k. [DOI] [PubMed] [Google Scholar]
- Johnston M., Davis R. W. Sequences that regulate the divergent GAL1-GAL10 promoter in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Aug;4(8):1440–1448. doi: 10.1128/mcb.4.8.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keruchenko J. S., Keruchenko I. D., Gladilin K. L., Zaitsev V. N., Chirgadze N. Y. Purification, characterization and preliminary X-ray study of fumarase from Saccharomyces cerevisiae. Biochim Biophys Acta. 1992 Jul 13;1122(1):85–92. doi: 10.1016/0167-4838(92)90131-v. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Koll H., Guiard B., Rassow J., Ostermann J., Horwich A. L., Neupert W., Hartl F. U. Antifolding activity of hsp60 couples protein import into the mitochondrial matrix with export to the intermembrane space. Cell. 1992 Mar 20;68(6):1163–1175. doi: 10.1016/0092-8674(92)90086-r. [DOI] [PubMed] [Google Scholar]
- Natsoulis G., Hilger F., Fink G. R. The HTS1 gene encodes both the cytoplasmic and mitochondrial histidine tRNA synthetases of S. cerevisiae. Cell. 1986 Jul 18;46(2):235–243. doi: 10.1016/0092-8674(86)90740-3. [DOI] [PubMed] [Google Scholar]
- Nelson N., Schatz G. Energy-dependent processing of cytoplasmically made precursors to mitochondrial proteins. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4365–4369. doi: 10.1073/pnas.76.9.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi C. E., Weiss J. Bidirectional movement of a nascent polypeptide across microsomal membranes reveals requirements for vectorial translocation of proteins. Cell. 1992 Oct 2;71(1):87–96. doi: 10.1016/0092-8674(92)90268-h. [DOI] [PubMed] [Google Scholar]
- Peleg Y., Rokem J. S., Goldberg I., Pines O. Inducible overexpression of the FUM1 gene in Saccharomyces cerevisiae: localization of fumarase and efficient fumaric acid bioconversion to L-malic acid. Appl Environ Microbiol. 1990 Sep;56(9):2777–2783. doi: 10.1128/aem.56.9.2777-2783.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. Distinct repressible mRNAs for cytoplasmic and secreted yeast invertase are encoded by a single gene. Cell. 1981 Aug;25(2):525–536. doi: 10.1016/0092-8674(81)90071-4. [DOI] [PubMed] [Google Scholar]
- Reid G. A., Schatz G. Import of proteins into mitochondria. Yeast cells grown in the presence of carbonyl cyanide m-chlorophenylhydrazone accumulate massive amounts of some mitochondrial precursor polypeptides. J Biol Chem. 1982 Nov 10;257(21):13056–13061. [PubMed] [Google Scholar]
- Roise D., Theiler F., Horvath S. J., Tomich J. M., Richards J. H., Allison D. S., Schatz G. Amphiphilicity is essential for mitochondrial presequence function. EMBO J. 1988 Mar;7(3):649–653. doi: 10.1002/j.1460-2075.1988.tb02859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberger O., Hirst T. R., Pines O. Targeting and assembly of an oligomeric bacterial enterotoxoid in the endoplasmic reticulum of Saccharomyces cerevisiae. Mol Microbiol. 1991 Nov;5(11):2663–2671. doi: 10.1111/j.1365-2958.1991.tb01975.x. [DOI] [PubMed] [Google Scholar]
- Shani O., Pines O. The relationship between disulphide bond formation, processing and secretion of lipo-beta-lactamase in yeast. Mol Microbiol. 1992 Jan;6(2):189–195. doi: 10.1111/j.1365-2958.1992.tb02000.x. [DOI] [PubMed] [Google Scholar]
- Slusher L. B., Gillman E. C., Martin N. C., Hopper A. K. mRNA leader length and initiation codon context determine alternative AUG selection for the yeast gene MOD5. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9789–9793. doi: 10.1073/pnas.88.21.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Yoshida T., Tuboi S. Evidence that rat liver mitochondrial and cytosolic fumarases are synthesized from one species of mRNA by alternative translational initiation at two in-phase AUG codons. Eur J Biochem. 1992 Jul 15;207(2):767–772. doi: 10.1111/j.1432-1033.1992.tb17107.x. [DOI] [PubMed] [Google Scholar]
- Tuboi S., Suzuki T., Sato M., Yoshida T. Rat liver mitochondrial and cytosolic fumarases with identical amino acid sequences are encoded from a single mRNA with two alternative in-phase AUG initiation sites. Adv Enzyme Regul. 1990;30:289–304. doi: 10.1016/0065-2571(90)90023-u. [DOI] [PubMed] [Google Scholar]
- Villarejo M. R., Zabin I. Beta-galactosidase from termination and deletion mutant strains. J Bacteriol. 1974 Oct;120(1):466–474. doi: 10.1128/jb.120.1.466-474.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Tzagoloff A. Mitochondrial and cytoplasmic fumarases in Saccharomyces cerevisiae are encoded by a single nuclear gene FUM1. J Biol Chem. 1987 Sep 5;262(25):12275–12282. [PubMed] [Google Scholar]
- Yaffe M. P., Schatz G. Two nuclear mutations that block mitochondrial protein import in yeast. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4819–4823. doi: 10.1073/pnas.81.15.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Jensen R. E., Yaffe M. P., Oppliger W., Schatz G. Import of proteins into yeast mitochondria: the purified matrix processing protease contains two subunits which are encoded by the nuclear MAS1 and MAS2 genes. EMBO J. 1988 Dec 1;7(12):3857–3862. doi: 10.1002/j.1460-2075.1988.tb03271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H., Donahue T. F. Control of translation initiation in Saccharomyces cerevisiae. Mol Microbiol. 1992 Jun;6(11):1413–1419. doi: 10.1111/j.1365-2958.1992.tb00861.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 1986 Jun;5(6):1335–1342. doi: 10.1002/j.1460-2075.1986.tb04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]