Abstract
The central pyrazole ring in the title compound, C17H16FN3S, adopts an envelope conformation with the methine C atom bearing the 4-fluorophenyl substituent as the flap atom. Whereas the tolyl ring is slightly twisted out of the least-squares plane through the pyrazole ring [dihedral angle = 13.51 (11)°], the fluorobenzene ring is almost perpendicular [dihedral angle = 80.21 (11)°]. The thioamide group is almost coplanar with the N—N bond of the ring [N—N—C—N torsion angle = 1.2 (3)°] and the amine group forms an intramolecular hydrogen bond with a ring N atom. In the crystal, supramolecular double layers in the bc plane are formed via N—H⋯S, N—H⋯F and C—H⋯F interactions.
Related literature
For the biological activity of pyrazolin-1-ylthiazoles, see: Abdel-Wahab et al. (2009 ▶, 2012 ▶); Chimenti et al. (2010 ▶). For related structures, see: Nonthason et al. (2011 ▶); Chantrapromma et al. (2012 ▶).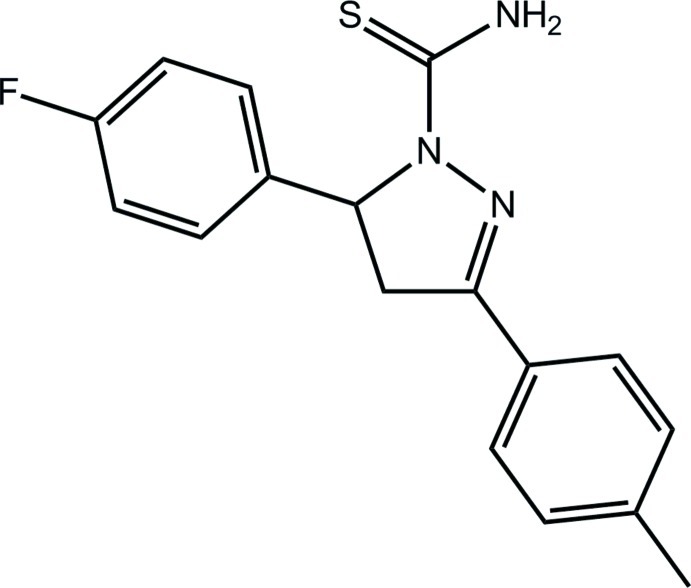
Experimental
Crystal data
C17H16FN3S
M r = 313.39
Monoclinic,

a = 14.4154 (10) Å
b = 11.3197 (9) Å
c = 9.5575 (8) Å
β = 103.991 (8)°
V = 1513.3 (2) Å3
Z = 4
Mo Kα radiation
μ = 0.22 mm−1
T = 295 K
0.30 × 0.20 × 0.10 mm
Data collection
Agilent SuperNova Dual diffractometer with an Atlas detector
Absorption correction: multi-scan (CrysAlis PRO; Agilent, 2011 ▶) T min = 0.772, T max = 1.000
9303 measured reflections
3500 independent reflections
2370 reflections with I > 2σ(I)
R int = 0.038
Refinement
R[F 2 > 2σ(F 2)] = 0.047
wR(F 2) = 0.122
S = 1.04
3500 reflections
200 parameters
H-atom parameters constrained
Δρmax = 0.18 e Å−3
Δρmin = −0.26 e Å−3
Data collection: CrysAlis PRO (Agilent, 2011 ▶); cell refinement: CrysAlis PRO; data reduction: CrysAlis PRO; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 2012 ▶) and DIAMOND (Brandenburg, 2006 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536813004194/hg5291sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813004194/hg5291Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813004194/hg5291Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N3—H31⋯N2 | 0.88 | 2.23 | 2.611 (3) | 106 |
| N3—H31⋯F1i | 0.88 | 2.41 | 3.255 (2) | 162 |
| N3—H32⋯S1ii | 0.88 | 2.83 | 3.538 (2) | 138 |
| C16—H16B⋯F1iii | 0.96 | 2.55 | 3.478 (3) | 163 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  .
.
Acknowledgments
We thank the Ministry of Higher Education (Malaysia) for funding structural studies through the High-Impact Research scheme (UM.C/HIR-MOHE/SC/12).
supplementary crystallographic information
Comment
The title compound (I) was investigated crystallographically in connection with the established biological activities exhibited by pyrazolin-1-ylthiazoles (Abdel-Wahab et al., 2012; Abdel-Wahab et al., 2009; Chimenti et al., 2010).
The central pyrazolyl ring in (I), Fig. 1, adopts an envelope conformation with the methine-C7 atom being the flap atom. The tolyl ring is slightly twisted out of the least-squares plane through the pyrazolyl ring, forming a dihedral angle of 13.51 (11)°. By contrast, the fluorobenzene ring is almost perpendicular to the five-membered ring [dihedral angle = 80.21 (11)°]. Finally, the thioamide residue is co-planar with the N2—N1—C17—N3 torsion angle being 1.2 (3)°; the amine group is orientated towards the ring-N2 atom, forming a hydrogen bond, Table 1. Similar conformations have been observed in related structures bearing two six-membered rings (Nonthason et al., 2011; Chantrapromma et al., 2012).
The F atom proves to be pivotal in providing stability to the crystal structure of (I). Thus, in addition to forming an intramolecular N—H···N2 hydrogen bond, the amine-H31 atom forms a hydrogen bond to the F1 atom, Table 1. The second amine-H32 atom hydrogen bonds to a thione so that a supramolecular chain along the c axis ensues, Fig. 2. Chains stack along the b axis to form a row and centrosymmetrically related rows inter-digitate along the a axis allowing for the formation methylene-C16—H···F1 interactions and, therefore, double layers, Fig. 2.
Experimental
A mixture of 5-(4-fluorophenyl)-3-p-tolyl-4,5-dihydro-1H-pyrazole-1-carbothioamide (0.31 g, 0.001 M) and 2-bromo-1-(4-chlorophenyl)ethanone (0.23 g, 0.001 M) in anhydrous ethanol (30 ml) was heated under reflux for about 4 h. The resultant solid was filtered and dried. Re-crystallization was by slow evaporation of DMF solution of (I) which yielded colourless crystals in 61% yield. M.pt: 513–514 K.
Refinement
Nitrogen- and carbon-bound H-atoms were placed in calculated positions (N—H = 0.88 Å, and C—H 0.93 to 0.98 Å) and were included in the refinement in the riding model approximation, with Uiso(H) = 1.2–1.5Uequiv(N,C).
Figures
Fig. 1.
The molecular structure of (I) showing the atom-labelling scheme and displacement ellipsoids at the 50% probability level.
Fig. 2.

A view of the supramolecular chain along the c axis in (I) mediated by N—H···S and N—H···F hydrogen bonds, shown as orange and blue dashed lines, respectively.
Fig. 3.
A view of the crystal packing in projection down the c axis. The N—H···S, N—H···F and C—H···F interactions are shown as orange, blue and pink dashed lines, respectively.
Crystal data
| C17H16FN3S | F(000) = 656 |
| Mr = 313.39 | Dx = 1.376 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 2000 reflections |
| a = 14.4154 (10) Å | θ = 2.9–27.5° |
| b = 11.3197 (9) Å | µ = 0.22 mm−1 |
| c = 9.5575 (8) Å | T = 295 K |
| β = 103.991 (8)° | Prism, colourless |
| V = 1513.3 (2) Å3 | 0.30 × 0.20 × 0.10 mm |
| Z = 4 |
Data collection
| Agilent SuperNova Dual diffractometer with an Atlas detector | 3500 independent reflections |
| Radiation source: SuperNova (Mo) X-ray Source | 2370 reflections with I > 2σ(I) |
| Mirror monochromator | Rint = 0.038 |
| Detector resolution: 10.4041 pixels mm-1 | θmax = 27.6°, θmin = 2.9° |
| ω scan | h = −18→17 |
| Absorption correction: multi-scan (CrysAlis PRO; Agilent, 2011) | k = −10→14 |
| Tmin = 0.772, Tmax = 1.000 | l = −12→12 |
| 9303 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.047 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.122 | H-atom parameters constrained |
| S = 1.04 | w = 1/[σ2(Fo2) + (0.0448P)2 + 0.2973P] where P = (Fo2 + 2Fc2)/3 |
| 3500 reflections | (Δ/σ)max < 0.001 |
| 200 parameters | Δρmax = 0.18 e Å−3 |
| 0 restraints | Δρmin = −0.26 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.48991 (4) | 0.33372 (5) | 0.33938 (6) | 0.04739 (19) | |
| F1 | 0.76993 (11) | 0.35963 (12) | −0.10409 (14) | 0.0659 (4) | |
| N1 | 0.63664 (12) | 0.46879 (14) | 0.46526 (17) | 0.0384 (4) | |
| N2 | 0.71143 (11) | 0.50005 (15) | 0.58117 (17) | 0.0391 (4) | |
| N3 | 0.60198 (14) | 0.32075 (15) | 0.60342 (19) | 0.0491 (5) | |
| H31 | 0.6497 | 0.3462 | 0.6727 | 0.059* | |
| H32 | 0.5684 | 0.2591 | 0.6181 | 0.059* | |
| C1 | 0.73573 (16) | 0.40770 (19) | 0.0050 (2) | 0.0445 (5) | |
| C2 | 0.77815 (16) | 0.3771 (2) | 0.1434 (2) | 0.0483 (6) | |
| H2 | 0.8306 | 0.3266 | 0.1637 | 0.058* | |
| C3 | 0.74165 (14) | 0.42262 (19) | 0.2528 (2) | 0.0435 (5) | |
| H3 | 0.7697 | 0.4024 | 0.3479 | 0.052* | |
| C4 | 0.66378 (13) | 0.49796 (17) | 0.2226 (2) | 0.0341 (4) | |
| C5 | 0.62418 (15) | 0.52828 (18) | 0.0812 (2) | 0.0416 (5) | |
| H5 | 0.5728 | 0.5804 | 0.0602 | 0.050* | |
| C6 | 0.65938 (16) | 0.48273 (19) | −0.0307 (2) | 0.0469 (5) | |
| H6 | 0.6320 | 0.5025 | −0.1262 | 0.056* | |
| C7 | 0.62560 (14) | 0.55094 (17) | 0.3424 (2) | 0.0376 (5) | |
| H7 | 0.5584 | 0.5736 | 0.3066 | 0.045* | |
| C8 | 0.68480 (15) | 0.65640 (18) | 0.4168 (2) | 0.0406 (5) | |
| H8A | 0.7267 | 0.6865 | 0.3594 | 0.049* | |
| H8B | 0.6439 | 0.7198 | 0.4347 | 0.049* | |
| C9 | 0.74100 (14) | 0.60325 (18) | 0.5553 (2) | 0.0378 (5) | |
| C10 | 0.82031 (14) | 0.65987 (18) | 0.6583 (2) | 0.0381 (5) | |
| C11 | 0.87678 (15) | 0.5981 (2) | 0.7726 (2) | 0.0486 (6) | |
| H11 | 0.8658 | 0.5179 | 0.7826 | 0.058* | |
| C12 | 0.94914 (17) | 0.6537 (2) | 0.8718 (3) | 0.0526 (6) | |
| H12 | 0.9867 | 0.6101 | 0.9470 | 0.063* | |
| C13 | 0.96669 (15) | 0.7734 (2) | 0.8614 (2) | 0.0470 (5) | |
| C14 | 0.91224 (16) | 0.8339 (2) | 0.7453 (3) | 0.0519 (6) | |
| H14 | 0.9243 | 0.9136 | 0.7342 | 0.062* | |
| C15 | 0.83985 (15) | 0.7787 (2) | 0.6447 (2) | 0.0470 (5) | |
| H15 | 0.8041 | 0.8216 | 0.5673 | 0.056* | |
| C16 | 1.04181 (18) | 0.8345 (2) | 0.9752 (3) | 0.0673 (8) | |
| H16A | 1.0751 | 0.8910 | 0.9303 | 0.101* | |
| H16B | 1.0864 | 0.7772 | 1.0264 | 0.101* | |
| H16C | 1.0119 | 0.8744 | 1.0414 | 0.101* | |
| C17 | 0.58067 (14) | 0.37547 (17) | 0.4763 (2) | 0.0365 (5) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0422 (3) | 0.0508 (3) | 0.0468 (3) | −0.0061 (3) | 0.0063 (2) | −0.0054 (3) |
| F1 | 0.0882 (10) | 0.0730 (9) | 0.0427 (8) | 0.0155 (8) | 0.0279 (7) | −0.0051 (7) |
| N1 | 0.0401 (9) | 0.0438 (10) | 0.0305 (8) | −0.0056 (8) | 0.0071 (7) | −0.0003 (7) |
| N2 | 0.0387 (9) | 0.0462 (10) | 0.0315 (9) | −0.0038 (8) | 0.0066 (7) | −0.0025 (8) |
| N3 | 0.0545 (11) | 0.0474 (10) | 0.0437 (10) | −0.0086 (9) | 0.0088 (9) | 0.0077 (8) |
| C1 | 0.0549 (13) | 0.0448 (12) | 0.0370 (11) | −0.0018 (11) | 0.0170 (10) | −0.0035 (10) |
| C2 | 0.0448 (12) | 0.0556 (13) | 0.0442 (12) | 0.0135 (11) | 0.0099 (10) | 0.0022 (11) |
| C3 | 0.0438 (11) | 0.0547 (13) | 0.0296 (10) | 0.0095 (11) | 0.0043 (9) | 0.0018 (10) |
| C4 | 0.0342 (10) | 0.0357 (10) | 0.0315 (10) | −0.0021 (9) | 0.0062 (8) | 0.0008 (8) |
| C5 | 0.0433 (11) | 0.0431 (11) | 0.0346 (11) | 0.0075 (10) | 0.0020 (9) | 0.0040 (9) |
| C6 | 0.0594 (14) | 0.0487 (12) | 0.0283 (10) | 0.0028 (11) | 0.0023 (10) | 0.0040 (9) |
| C7 | 0.0360 (10) | 0.0428 (11) | 0.0325 (10) | 0.0030 (9) | 0.0056 (8) | 0.0044 (9) |
| C8 | 0.0455 (11) | 0.0403 (11) | 0.0360 (11) | 0.0006 (10) | 0.0100 (9) | −0.0010 (9) |
| C9 | 0.0370 (10) | 0.0422 (11) | 0.0359 (10) | 0.0003 (10) | 0.0124 (9) | −0.0027 (9) |
| C10 | 0.0362 (10) | 0.0427 (11) | 0.0372 (11) | −0.0007 (9) | 0.0124 (9) | −0.0038 (9) |
| C11 | 0.0509 (13) | 0.0447 (12) | 0.0469 (13) | −0.0063 (11) | 0.0052 (11) | −0.0013 (10) |
| C12 | 0.0521 (13) | 0.0542 (14) | 0.0449 (13) | 0.0011 (12) | −0.0010 (11) | −0.0012 (11) |
| C13 | 0.0385 (11) | 0.0510 (13) | 0.0509 (13) | 0.0004 (10) | 0.0098 (10) | −0.0134 (11) |
| C14 | 0.0434 (12) | 0.0413 (12) | 0.0695 (16) | −0.0045 (11) | 0.0106 (12) | −0.0052 (11) |
| C15 | 0.0423 (12) | 0.0465 (12) | 0.0513 (13) | 0.0009 (10) | 0.0093 (10) | 0.0026 (11) |
| C16 | 0.0527 (14) | 0.0653 (16) | 0.0745 (18) | −0.0040 (13) | −0.0025 (13) | −0.0231 (14) |
| C17 | 0.0384 (10) | 0.0378 (11) | 0.0365 (11) | 0.0033 (9) | 0.0151 (9) | −0.0034 (9) |
Geometric parameters (Å, º)
| S1—C17 | 1.679 (2) | C7—C8 | 1.538 (3) |
| F1—C1 | 1.369 (2) | C7—H7 | 0.9800 |
| N1—C17 | 1.349 (2) | C8—C9 | 1.501 (3) |
| N1—N2 | 1.392 (2) | C8—H8A | 0.9700 |
| N1—C7 | 1.476 (2) | C8—H8B | 0.9700 |
| N2—C9 | 1.288 (3) | C9—C10 | 1.464 (3) |
| N3—C17 | 1.332 (2) | C10—C11 | 1.383 (3) |
| N3—H31 | 0.8800 | C10—C15 | 1.386 (3) |
| N3—H32 | 0.8800 | C11—C12 | 1.381 (3) |
| C1—C2 | 1.361 (3) | C11—H11 | 0.9300 |
| C1—C6 | 1.367 (3) | C12—C13 | 1.386 (3) |
| C2—C3 | 1.379 (3) | C12—H12 | 0.9300 |
| C2—H2 | 0.9300 | C13—C14 | 1.377 (3) |
| C3—C4 | 1.383 (3) | C13—C16 | 1.505 (3) |
| C3—H3 | 0.9300 | C14—C15 | 1.385 (3) |
| C4—C5 | 1.377 (3) | C14—H14 | 0.9300 |
| C4—C7 | 1.509 (3) | C15—H15 | 0.9300 |
| C5—C6 | 1.389 (3) | C16—H16A | 0.9600 |
| C5—H5 | 0.9300 | C16—H16B | 0.9600 |
| C6—H6 | 0.9300 | C16—H16C | 0.9600 |
| C17—N1—N2 | 119.99 (16) | C9—C8—H8B | 111.3 |
| C17—N1—C7 | 127.16 (16) | C7—C8—H8B | 111.3 |
| N2—N1—C7 | 112.76 (15) | H8A—C8—H8B | 109.2 |
| C9—N2—N1 | 107.83 (16) | N2—C9—C10 | 120.57 (18) |
| C17—N3—H31 | 120.0 | N2—C9—C8 | 113.58 (17) |
| C17—N3—H32 | 120.0 | C10—C9—C8 | 125.81 (18) |
| H31—N3—H32 | 120.0 | C11—C10—C15 | 118.05 (19) |
| C2—C1—F1 | 118.6 (2) | C11—C10—C9 | 121.63 (19) |
| C2—C1—C6 | 123.1 (2) | C15—C10—C9 | 120.31 (19) |
| F1—C1—C6 | 118.25 (19) | C12—C11—C10 | 121.0 (2) |
| C1—C2—C3 | 118.5 (2) | C12—C11—H11 | 119.5 |
| C1—C2—H2 | 120.7 | C10—C11—H11 | 119.5 |
| C3—C2—H2 | 120.7 | C11—C12—C13 | 121.1 (2) |
| C2—C3—C4 | 120.74 (19) | C11—C12—H12 | 119.4 |
| C2—C3—H3 | 119.6 | C13—C12—H12 | 119.4 |
| C4—C3—H3 | 119.6 | C14—C13—C12 | 117.8 (2) |
| C5—C4—C3 | 118.76 (18) | C14—C13—C16 | 121.6 (2) |
| C5—C4—C7 | 120.31 (17) | C12—C13—C16 | 120.6 (2) |
| C3—C4—C7 | 120.86 (17) | C13—C14—C15 | 121.5 (2) |
| C4—C5—C6 | 121.39 (19) | C13—C14—H14 | 119.3 |
| C4—C5—H5 | 119.3 | C15—C14—H14 | 119.3 |
| C6—C5—H5 | 119.3 | C14—C15—C10 | 120.6 (2) |
| C1—C6—C5 | 117.44 (19) | C14—C15—H15 | 119.7 |
| C1—C6—H6 | 121.3 | C10—C15—H15 | 119.7 |
| C5—C6—H6 | 121.3 | C13—C16—H16A | 109.5 |
| N1—C7—C4 | 111.36 (16) | C13—C16—H16B | 109.5 |
| N1—C7—C8 | 100.38 (15) | H16A—C16—H16B | 109.5 |
| C4—C7—C8 | 113.31 (16) | C13—C16—H16C | 109.5 |
| N1—C7—H7 | 110.5 | H16A—C16—H16C | 109.5 |
| C4—C7—H7 | 110.5 | H16B—C16—H16C | 109.5 |
| C8—C7—H7 | 110.5 | N3—C17—N1 | 115.16 (18) |
| C9—C8—C7 | 102.56 (16) | N3—C17—S1 | 122.98 (16) |
| C9—C8—H8A | 111.3 | N1—C17—S1 | 121.85 (15) |
| C7—C8—H8A | 111.3 | ||
| C17—N1—N2—C9 | −167.33 (17) | N1—N2—C9—C10 | −179.64 (16) |
| C7—N1—N2—C9 | 9.5 (2) | N1—N2—C9—C8 | 2.3 (2) |
| F1—C1—C2—C3 | −177.94 (19) | C7—C8—C9—N2 | −12.1 (2) |
| C6—C1—C2—C3 | 0.9 (3) | C7—C8—C9—C10 | 169.92 (18) |
| C1—C2—C3—C4 | −0.2 (3) | N2—C9—C10—C11 | 11.6 (3) |
| C2—C3—C4—C5 | −1.0 (3) | C8—C9—C10—C11 | −170.55 (19) |
| C2—C3—C4—C7 | −178.1 (2) | N2—C9—C10—C15 | −167.37 (19) |
| C3—C4—C5—C6 | 1.5 (3) | C8—C9—C10—C15 | 10.5 (3) |
| C7—C4—C5—C6 | 178.58 (19) | C15—C10—C11—C12 | 1.4 (3) |
| C2—C1—C6—C5 | −0.4 (3) | C9—C10—C11—C12 | −177.6 (2) |
| F1—C1—C6—C5 | 178.42 (19) | C10—C11—C12—C13 | 0.9 (4) |
| C4—C5—C6—C1 | −0.8 (3) | C11—C12—C13—C14 | −2.7 (4) |
| C17—N1—C7—C4 | −79.3 (2) | C11—C12—C13—C16 | 176.3 (2) |
| N2—N1—C7—C4 | 104.12 (18) | C12—C13—C14—C15 | 2.3 (3) |
| C17—N1—C7—C8 | 160.42 (18) | C16—C13—C14—C15 | −176.7 (2) |
| N2—N1—C7—C8 | −16.1 (2) | C13—C14—C15—C10 | −0.1 (3) |
| C5—C4—C7—N1 | 149.56 (18) | C11—C10—C15—C14 | −1.8 (3) |
| C3—C4—C7—N1 | −33.4 (2) | C9—C10—C15—C14 | 177.28 (19) |
| C5—C4—C7—C8 | −98.2 (2) | N2—N1—C17—N3 | 1.2 (3) |
| C3—C4—C7—C8 | 78.8 (2) | C7—N1—C17—N3 | −175.12 (18) |
| N1—C7—C8—C9 | 15.56 (19) | N2—N1—C17—S1 | −179.70 (14) |
| C4—C7—C8—C9 | −103.26 (18) | C7—N1—C17—S1 | 4.0 (3) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N3—H31···N2 | 0.88 | 2.23 | 2.611 (3) | 106 |
| N3—H31···F1i | 0.88 | 2.41 | 3.255 (2) | 162 |
| N3—H32···S1ii | 0.88 | 2.83 | 3.538 (2) | 138 |
| C16—H16B···F1iii | 0.96 | 2.55 | 3.478 (3) | 163 |
Symmetry codes: (i) x, y, z+1; (ii) x, −y+1/2, z+1/2; (iii) −x+2, −y+1, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HG5291).
References
- Abdel-Wahab, B. F., Abdel-Aziz, H. A. & Ahmed, E. M. (2009). Eur. J. Med. Chem. 44, 2632–2635. [DOI] [PubMed]
- Abdel-Wahab, B. F., Abdel-Latif, E., Mohamed, H. A. & Awad, G. E. A. (2012). Eur. J. Med. Chem. 52, 263–268. [DOI] [PubMed]
- Agilent (2011). CrysAlis PRO Agilent Technologies, Yarnton, England.
- Brandenburg, K. (2006). DIAMOND Crystal Impact GbR, Bonn, Germany.
- Chantrapromma, S., Nonthason, P., Suwunwong, T. & Fun, H.-K. (2012). Acta Cryst. E68, o830–o831. [DOI] [PMC free article] [PubMed]
- Chimenti, F., Carradori, S., Secci, D., Bolasco, A., Bizzarri, B., Chimenti, P., Granese, A., Yáñez, M. & Orallo, F. (2010). Eur. J. Med. Chem. 45, 800–804. [DOI] [PubMed]
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Nonthason, P., Suwunwong, T., Chantrapromma, S. & Fun, H.-K. (2011). Acta Cryst. E67, o3501–o3502. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536813004194/hg5291sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813004194/hg5291Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813004194/hg5291Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




