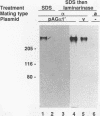
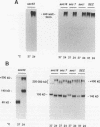
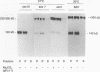
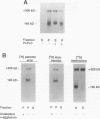
Abstract
Saccharomyces cerevisiae alpha-agglutinin is a cell wall-anchored adhesion glycoprotein. The previously identified 140-kDa form, which contains a glycosyl-phosphatidylinositol (GPI) anchor (D. Wojciechowicz, C.-F. Lu, J. Kurjan, and P. N. Lipke, Mol. Cell. Biol. 13:2554-2563, 1993), and additional forms of 80, 150, 250 to 300, and > 300 kDa had the properties of intermediates in a transport and cell wall anchorage pathway. N glycosylation and additional modifications resulted in successive increases in size during transport. The 150- and 250- to 300-kDa forms were membrane associated and are likely to be intermediates between the 140-kDa form and a cell surface GPI-anchored form of > 300 kDa. A soluble form of > 300 kDa that lacked the GPI anchor had properties of a periplasmic intermediate between the plasma membrane form and the > 300-kDa cell wall-anchored form. These results constitute experimental support for the hypothesis that GPI anchors act to localize alpha-agglutinin to the plasma membrane and that cell wall anchorage involves release from the GPI anchor to produce a periplasmic intermediate followed by linkage to the cell wall.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boone C., Sommer S. S., Hensel A., Bussey H. Yeast KRE genes provide evidence for a pathway of cell wall beta-glucan assembly. J Cell Biol. 1990 May;110(5):1833–1843. doi: 10.1083/jcb.110.5.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappellaro C., Hauser K., Mrśa V., Watzele M., Watzele G., Gruber C., Tanner W. Saccharomyces cerevisiae a- and alpha-agglutinin: characterization of their molecular interaction. EMBO J. 1991 Dec;10(13):4081–4088. doi: 10.1002/j.1460-2075.1991.tb04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Conzelmann A., Fankhauser C., Desponds C. Myoinositol gets incorporated into numerous membrane glycoproteins of Saccharomyces cerevisiae; incorporation is dependent on phosphomannomutase (sec53). EMBO J. 1990 Mar;9(3):653–661. doi: 10.1002/j.1460-2075.1990.tb08157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzelmann A., Riezman H., Desponds C., Bron C. A major 125-kd membrane glycoprotein of Saccharomyces cerevisiae is attached to the lipid bilayer through an inositol-containing phospholipid. EMBO J. 1988 Jul;7(7):2233–2240. doi: 10.1002/j.1460-2075.1988.tb03063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F., Hartwell L. H., Jackson C., Konopka J. B. Conjugation in Saccharomyces cerevisiae. Annu Rev Cell Biol. 1988;4:429–457. doi: 10.1146/annurev.cb.04.110188.002241. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Glycolipid anchoring of plasma membrane proteins. Annu Rev Cell Biol. 1990;6:1–39. doi: 10.1146/annurev.cb.06.110190.000245. [DOI] [PubMed] [Google Scholar]
- Eakle K. A., Bernstein M., Emr S. D. Characterization of a component of the yeast secretion machinery: identification of the SEC18 gene product. Mol Cell Biol. 1988 Oct;8(10):4098–4109. doi: 10.1128/mcb.8.10.4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early A. E., Williams J. G., Meyer H. E., Por S. B., Smith E., Williams K. L., Gooley A. A. Structural characterization of Dictyostelium discoideum prespore-specific gene D19 and of its product, cell surface glycoprotein PsA. Mol Cell Biol. 1988 Aug;8(8):3458–3466. doi: 10.1128/mcb.8.8.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund P. T. The structure and biosynthesis of glycosyl phosphatidylinositol protein anchors. Annu Rev Biochem. 1993;62:121–138. doi: 10.1146/annurev.bi.62.070193.001005. [DOI] [PubMed] [Google Scholar]
- Esmon B., Novick P., Schekman R. Compartmentalized assembly of oligosaccharides on exported glycoproteins in yeast. Cell. 1981 Aug;25(2):451–460. doi: 10.1016/0092-8674(81)90063-5. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Franzusoff A., Schekman R. Functional compartments of the yeast Golgi apparatus are defined by the sec7 mutation. EMBO J. 1989 Sep;8(9):2695–2702. doi: 10.1002/j.1460-2075.1989.tb08410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham T. R., Emr S. D. Compartmental organization of Golgi-specific protein modification and vacuolar protein sorting events defined in a yeast sec18 (NSF) mutant. J Cell Biol. 1991 Jul;114(2):207–218. doi: 10.1083/jcb.114.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick K. G., Boothroyd J. C., Rudner A. D., Pelham H. R. Genes that allow yeast cells to grow in the absence of the HDEL receptor. EMBO J. 1992 Nov;11(11):4187–4195. doi: 10.1002/j.1460-2075.1992.tb05512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser K., Tanner W. Purification of the inducible alpha-agglutinin of S. cerevisiae and molecular cloning of the gene. FEBS Lett. 1989 Sep 25;255(2):290–294. doi: 10.1016/0014-5793(89)81108-1. [DOI] [PubMed] [Google Scholar]
- Jones E. W. Tackling the protease problem in Saccharomyces cerevisiae. Methods Enzymol. 1991;194:428–453. doi: 10.1016/0076-6879(91)94034-a. [DOI] [PubMed] [Google Scholar]
- Kukuruzinska M. A., Bergh M. L., Jackson B. J. Protein glycosylation in yeast. Annu Rev Biochem. 1987;56:915–944. doi: 10.1146/annurev.bi.56.070187.004411. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lasky R. D., Ballou C. E. Cell-cell recognition in yeast: isolation of intact alpha-agglutinin from Saccharomyces kluyveri. Proc Natl Acad Sci U S A. 1988 Jan;85(2):349–353. doi: 10.1073/pnas.85.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipke P. N., Kurjan J. Sexual agglutination in budding yeasts: structure, function, and regulation of adhesion glycoproteins. Microbiol Rev. 1992 Mar;56(1):180–194. doi: 10.1128/mr.56.1.180-194.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipke P. N., Wojciechowicz D., Kurjan J. AG alpha 1 is the structural gene for the Saccharomyces cerevisiae alpha-agglutinin, a cell surface glycoprotein involved in cell-cell interactions during mating. Mol Cell Biol. 1989 Aug;9(8):3155–3165. doi: 10.1128/mcb.9.8.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller G., Bandlow W. Two lipid-anchored cAMP-binding proteins in the yeast Saccharomyces cerevisiae are unrelated to the R subunit of cytoplasmic protein kinase A. Eur J Biochem. 1991 Dec 5;202(2):299–308. doi: 10.1111/j.1432-1033.1991.tb16376.x. [DOI] [PubMed] [Google Scholar]
- Novick P., Ferro S., Schekman R. Order of events in the yeast secretory pathway. Cell. 1981 Aug;25(2):461–469. doi: 10.1016/0092-8674(81)90064-7. [DOI] [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980 Aug;21(1):205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Novick P., Schekman R. Export of major cell surface proteins is blocked in yeast secretory mutants. J Cell Biol. 1983 Feb;96(2):541–547. doi: 10.1083/jcb.96.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuoffer C., Jenö P., Conzelmann A., Riezman H. Determinants for glycophospholipid anchoring of the Saccharomyces cerevisiae GAS1 protein to the plasma membrane. Mol Cell Biol. 1991 Jan;11(1):27–37. doi: 10.1128/mcb.11.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Rothblatt J., Schekman R. A hitchhiker's guide to analysis of the secretory pathway in yeast. Methods Cell Biol. 1989;32:3–36. doi: 10.1016/s0091-679x(08)61165-6. [DOI] [PubMed] [Google Scholar]
- Roy A., Lu C. F., Marykwas D. L., Lipke P. N., Kurjan J. The AGA1 product is involved in cell surface attachment of the Saccharomyces cerevisiae cell adhesion glycoprotein a-agglutinin. Mol Cell Biol. 1991 Aug;11(8):4196–4206. doi: 10.1128/mcb.11.8.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schekman R. Protein localization and membrane traffic in yeast. Annu Rev Cell Biol. 1985;1:115–143. doi: 10.1146/annurev.cb.01.110185.000555. [DOI] [PubMed] [Google Scholar]
- Schreuder M. P., Brekelmans S., van den Ende H., Klis F. M. Targeting of a heterologous protein to the cell wall of Saccharomyces cerevisiae. Yeast. 1993 Apr;9(4):399–409. doi: 10.1002/yea.320090410. [DOI] [PubMed] [Google Scholar]
- Sijmons P. C., Nederbragt A. J., Klis F. M., Van den Ende H. Isolation and composition of the constitutive agglutinins from haploid Saccharomyces cerevisiae cells. Arch Microbiol. 1987 Sep;148(3):208–212. doi: 10.1007/BF00414813. [DOI] [PubMed] [Google Scholar]
- Tanner W., Lehle L. Protein glycosylation in yeast. Biochim Biophys Acta. 1987 Apr 27;906(1):81–99. doi: 10.1016/0304-4157(87)90006-2. [DOI] [PubMed] [Google Scholar]
- Terrance K., Heller P., Wu Y. S., Lipke P. N. Identification of glycoprotein components of alpha-agglutinin, a cell adhesion protein from Saccharomyces cerevisiae. J Bacteriol. 1987 Feb;169(2):475–482. doi: 10.1128/jb.169.2.475-482.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrance K., Lipke P. N. Sexual agglutination in Saccharomyces cerevisiae. J Bacteriol. 1981 Dec;148(3):889–896. doi: 10.1128/jb.148.3.889-896.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vai M., Popolo L., Grandori R., Lacanà E., Alberghina L. The cell cycle modulated glycoprotein GP115 is one of the major yeast proteins containing glycosylphosphatidylinositol. Biochim Biophys Acta. 1990 May 8;1038(3):277–285. doi: 10.1016/0167-4838(90)90237-a. [DOI] [PubMed] [Google Scholar]
- Van Rinsum J., Klis F. M., van den Ende H. Cell wall glucomannoproteins of Saccharomyces cerevisiae mnn9. Yeast. 1991 Oct;7(7):717–726. doi: 10.1002/yea.320070707. [DOI] [PubMed] [Google Scholar]
- Weinstock K., Ballou C. E. Cell-cell recognition in yeast. Molecular nature of the sexual agglutinin from Saccharomyces kluyveri 17-cells. J Biol Chem. 1986 Dec 5;261(34):16174–16179. [PubMed] [Google Scholar]
- Wilcox C. A., Fuller R. S. Posttranslational processing of the prohormone-cleaving Kex2 protease in the Saccharomyces cerevisiae secretory pathway. J Cell Biol. 1991 Oct;115(2):297–307. doi: 10.1083/jcb.115.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- Wilson D. W., Wilcox C. A., Flynn G. C., Chen E., Kuang W. J., Henzel W. J., Block M. R., Ullrich A., Rothman J. E. A fusion protein required for vesicle-mediated transport in both mammalian cells and yeast. Nature. 1989 Jun 1;339(6223):355–359. doi: 10.1038/339355a0. [DOI] [PubMed] [Google Scholar]
- Wojciechowicz D., Lu C. F., Kurjan J., Lipke P. N. Cell surface anchorage and ligand-binding domains of the Saccharomyces cerevisiae cell adhesion protein alpha-agglutinin, a member of the immunoglobulin superfamily. Mol Cell Biol. 1993 Apr;13(4):2554–2563. doi: 10.1128/mcb.13.4.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik H., Fernandez M. P., Bowers B., Cabib E. Saccharomyces cerevisiae mannoproteins form an external cell wall layer that determines wall porosity. J Bacteriol. 1984 Sep;159(3):1018–1026. doi: 10.1128/jb.159.3.1018-1026.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nobel H., Lipke P. N. Is there a role for GPIs in yeast cell-wall assembly? Trends Cell Biol. 1994 Feb;4(2):42–45. doi: 10.1016/0962-8924(94)90003-5. [DOI] [PubMed] [Google Scholar]