Abstract
The fate of pluripotent mesenchymal stem cells (MSC) is determined through integration of chemical, spatial, and physical signals. The suppression of MSC adipogenesis by mechanical stimuli, which requires Akt-induced inhibition of glycogen synthase kinase 3β (GSK3β) with β-catenin activation, can be enhanced by repetitive dosing within a single day. Here, we demonstrate that reapplication of cyclic strain within a 24-hour period leads to amplification of both Akt activation and its subsequent inhibition of GSK3β, such that total cycle number can be reduced while still inhibiting adipogenesis. Amplification of Akt signaling is facilitated by a dynamic restructuring of the cell in response to mechanical signals, as evidenced by a transient increase in focal adhesion (FA) number and increased RhoA activity. Preventing FA assembly or development of tension blocks activation of Akt by mechanical signals, but not by insulin. This indicates that the FA infrastructure is essential to the physical, but not necessarily the chemical, sensitivity, and responsiveness of the cell. Exploiting the transient nature of cytoskeletal remodeling may represent a process to enhance cell responsiveness to mechanical input and ultimately define the fate of MSCs with a minimal input.
Keywords: Akt, Adipocyte, Focal adhesion, Mechanical force
Introduction
Stem cell lineage commitment occurs within a microenvironment replete with growth factors [1], nutrients, morphologic cues [2], and a dynamic mechanical environment, some combination of which biases the fate selection of these progenitors. In the bone marrow, exercise is translated into a complex combination of mechanical strain, acceleration, pressure, and fluid shear [3] and can be perceived by resident mesenchymal stem cells (MSCs) as a regulatory signal to limit adipogenesis and promote the formation of higher order tissues such as bone and muscle [4, 5]. When considered in the converse, the absence of mechanical signals is permissive to the formation of fat both in vivo [6] and in vitro [7]; this indicates that physically based stimuli can restrict adipogenesis by directly defining MSC fate, a mechanism divergent from metabolic expenditures which offset the net energy of caloric intake.
Mechanical repression of adipogenesis is dependent on preservation of β-catenin activity through inhibition of glycogen synthase kinase 3β (GSK3β) via phosphorylation [8]. Physiologically, the regulatory effect of β-catenin signaling on MSC lineage is validated by finding that an activating mutation in the low-density lipoprotein receptor–related protein 5 receptor that binds Wnt leads to enhanced bone formation while decreasing adipogenesis [9, 10]. In contrast to Wnt signaling, which allows β-catenin activation through a sustained sequestration of GSK3β [11], only the distal portion of this signal pathway is conscripted during the MSC mechanoresponse: mechanical activation of β-catenin is achieved through a transient inactivation of GSK3β through serine phosphorylation [8, 12]. Thus, interrupting either GSK3β inactivation or β-catenin activation prevents the ability of mechanical signals to restrain adipogenesis.
An organism’s response to mechanical signals is evident at multiple levels, scaling from molecular events to cellular processes to tissue level adaptations. Not only are mechanical parameters such as intensity and duration critically important in defining the cellular response, the “scheduling” of events through the day also modulate phenotypic outcomes. Previous work has demonstrated that a daily application of mechanical stimuli delivered as 2% strain at 10 cycles per minute for 6 hours inhibited adipogenesis as shown by decreased expression of peroxisome proliferator-activated receptor γ (PPARγ) and adiponectin, in both marrow-derived MSCs and in the C3H10T1/2 cell lines [7, 8]. Further, a daily repetition of mechanical treatment was necessary to ensure that the transient GSK3β/β-catenin activation prevents the default to adipogenesis that occurs over several days [7].
Mechanical information is ubiquitous to all cells at all stages of development, homeostasis, senescence, and apoptosis. Cells respond to both internally or externally generated forces [13], each of which can regulate stem cell lineage commitment [2, 14]. Transient intracellular and pericellular structural adaptations to exogenous stimuli can quantitatively and qualitatively change signaling responses [15], an attribute that may be mediated by dynamic restructuring of cytoskeletal elements at the interface between cells and the surface upon which they adhere. Focal adhesions (FAs) can assemble within 60 minutes [16] and have been previously noted to form consequent to mechanical force [17, 18]. These multiprotein complexes are thought to provide a mechanical connection between the actin contractile cytoskeleton, where tension is generated, and the external environment [19]. However, any role in the adaptive response to exogenous stimuli remains unclear. As noted, increasing the number of mechanical bouts within a 6–9-hour period improves MSC sensitivity to mechanical signals, even with very low magnitude signals [20], causing a further repression of adipocyte differentiation. Herein, we postulate that specific physical adaptation engendered by development of FAs allow for an amplification of those signal pathways responsible for restricting adipogenesis.
Materials and Methods
Reagents
Fetal bovine serum was from Atlanta Biologicals (Atlanta, GA). Culture media, trypsin-EDTA reagent, and antibiotics were from Invitrogen. Insulin, dexamethasone, blebbistatin, and ML7 were from Sigma-Aldrich (www.sigmaaldrich.com). Rock inhibitor was from Calbiochem (www.merck-chemicals.com/life-science-research/calbiochem).
Cells and Culture Conditions
Marrow-derived mesenchymal stem cells (mdMSCs) [21] were maintained in growth medium (10% fetal bovine serum and 100 µg/ml penicillin/streptomycin). For experiments, the cells were plated at a density of 6,000–10,000 cells per square centimeter in collagen-I coated silicone membrane plates and cultured for 2 days before beginning experiments. Adipogenic medium included 0.1 µM dexamethasone and 5 µg/ml insulin.
Mechanical Strain
Uniform biaxial strain was applied to mdMSCs plated on six-well Bioflex Collagen-I coated plates using the Flexcell FX-4000 system (Flexcell International, Hillsborough, NC). A daily regimen of 2% or 1% strain was delivered at 10 cycles per minute for the indicated cycle number.
Western Blotting
Whole cell lysates were prepared with lysis buffer (150 mM NaCl, 50 mM Tris HCl, 1 mM EGTA, 0.24% sodium deoxycholate, 1% Igepal, and pH 7.5) containing 25 mM NaF and 2 mM Na3VO4. Aprotinin, leupeptin, pepstatin, and phenylmethylsulfonyl fluoride were added before each lysis. Whole lysate proteins of 5–20 µg were loaded onto a 7%–10% polyacrylamide gel for chromatography and transferred to polyvinylidene difluoride membrane. After blocking, primary antibody was applied overnight at 4°C including antibodies against active β-catenin (clone 8E7; Upstate, Temecula, CA), total β-catenin (BD, Bedford, MA), phospho-GSK3β (ser9, clone 2D3, Cell Signaling, Beverly, MA, www.cellsignal.-com), total GSK3β (Cell Signaling), vinculin (Sigma-Aldrich), total Akt (Cell Signaling), adipocyte protein 2 (aP2) (ProSci, Poway, CA, www.prosci-inc.com), adiponectin, and tubulin. Secondary antibody conjugated with horseradish peroxidase was detected with ECL plus chemiluminescence kit (Amersham Biosciences, www.gelifesciences.com). The images were acquired with an HPScanjet and densitometry determined using NIH ImageJ, 1.37v.
RhoA Assay
Purification of recombinant proteins. Construction of the pGEX4T-1 prokaryotic expression constructs containing the Rho-binding domain (RBD) of Rhotekin has been described [22]. Briefly, expression of the fusion proteins in Escherichia coli was induced with 100 µM isopropyl β-d-1-thiogalactopyranoside for 12–16 hours at room temperature. Bacterial cells were lysed in buffer containing 50 mM Tris (pH 7.6), 150 mM NaCl, 5 mM MgCl2, 1 mM dithiothreitol, 10 µg/ml each of aprotinin and leupeptin, and 1 mM phenylmethylsulfonyl fluoride, and the proteins are purified by incubation with glutathione-sepharose 4B beads (GE Healthcare) at 4°C. For glutathione S-transferase–RBD (GST-RBD) pull down, active RhoA pull down experiments were performed as described [23]. The cells were lysed in 50 mM Tris (pH 7.6), 500 mM NaCl, 1% Triton X-100, 0.1% SDS, 0.5% deoxycholate, 10 mM MgCl2, 200 µM orthovanadate, and protease inhibitors. Lysates were clarified by centrifugation, equalized for total volume and protein concentration and rotated for 30 minutes with 30 µg of purified GST-RBD bound to glutathione-sepharose beads. The bead pellets were washed in 50 mM Tris (pH 7.6), 150 mM NaCl, 1% Triton X-100, 10 mM MgCl2, and 200 µM orthovanadate, with protease inhibitors, and subsequently processed for SDS-PAGE.
Immunofluorescence
After the treatment, the cells were fixed with 4% paraformaldehyde for 20 minutes and then permeabilized in 0.1% Triton X-100 for 5 minutes, blocked in 0.2 M glycine for 10 minutes, and blocked in 5% donkey serum for 30 minutes. The cells were washed three times for 10 minutes each with 1× phosphate-buffered saline (PBS). The membranes were detached from plates with scalpel blade and transfer to six-well plate surface. The primary antibody, anti-vinculin (Sigma-Aldrich), was added with the concentration at 5 µg/ml and the plates were incubated at 4°C overnight. After the cells were washed with 1× PBS for three times of 10 minutes each, the secondary antibody, DyLight 649 AffiniPure donkey anti-mouse IgG (Jackson ImmunoResearch Laboratory, Inc.), was added at 1:500 and the cells were incubated for 30 minutes under low light at room temperature. To visualize actin stress fibers, Alexa Fluor 488 phalloidin (Invitrogen, www.invitrogen.com) was added at 1:100 and the cells were incubated for 30 minutes at room temperature. After the final wash of three times for 10 minutes each, the membranes were set on glass slides, covered, and sealed with mounting medium.
Microscopy and Image Analysis
Fixed and immunolabeled cells were imaged on an inverted microscope system Olympus BX61 using 20 or 40× objective lens with 649 or 488 nm illumination provided by Cy5-4040A mercury arc lamp. The adhesions were manually segmented by blinded experts. A fully automated algorithm based on connected components analysis was then used to extract the number of adhesions.
Statistical Analysis
Results are expressed as the means ±SE. Statistical significance was evaluated by one-way analysis of variance or t test (Prism [GraphPad, La Jolla CA, www.graphpad.com]). All the experiments were replicated at least once to assure reproducibility. Densitometry data, where given, were compiled from at least three separate experiments.
Results
Inhibition of Adipogenesis Is Amplified by Repetitive Dosing
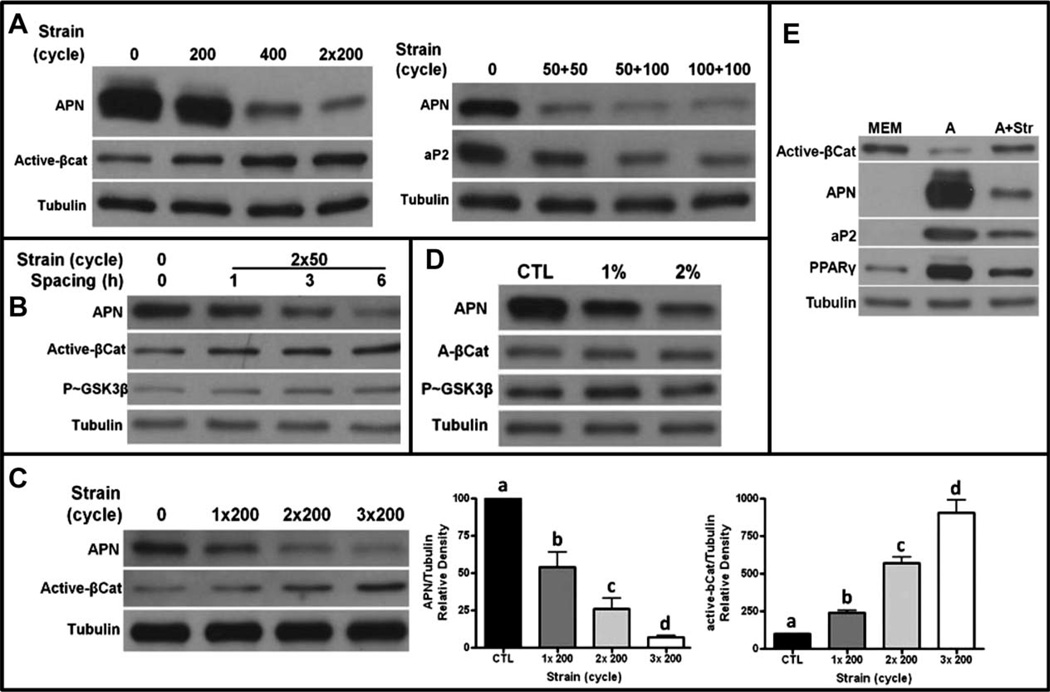
To understand how mechanical signals successfully suppress fat formation in MSCs, we set out to ascertain the minimal number of strain cycles necessary for inhibition of adipogenesis. Using adiponectin as a marker of adipogenesis, it was apparent that 200 cycles of dynamic mechanical stretch, delivered over 10 minutes as a single daily bout, were less effective in inhibiting adipogenesis than 400 cycles. Dividing 400 cycles of stretch into two bouts of 200 cycles and separating these bouts with a rest period of 3 hours, markedly increased the antiadipogenic efficacy above that realized by 400 cycles delivered in a single bout (Fig. 1A). The level of active β-catenin, critical to preventing adipogenesis [8] also increased following two bouts of 200 cycles to a level greater than that measured after 400 uninterrupted cycles. Mechanical inhibition of adipogenesis, as indicated by reduced adiponectin and aP2, was effective even when the mechanical application was reduced to 50 cycles, but only when delivered in two distinct bouts separated by at least 3 hours (50 + 50, right panel). The “recovery” time before the second mechanical application was important for efficacy, as reintroducing the mechanical challenge within 1 hour had essentially no influence on adipogenesis, whereas a 3- or 6-hour recovery period was effective using 50 cycles at each bout (Fig. 1B).
Figure 1.
Inhibition of adipogenesis is amplified by repetitive dosing. (A): Left panel: Mechanical treatment (200–400 daily strain cycles) reduces adipogenesis as measured by expression of adiponectin and increases in active β-catenin proteins; equal loading indicated by tubulin. 400 cycles is more effective than 200 cycles delivered as one or two bouts. Right panel: Strain cycles can be further reduced to 50 cycles when delivered in two bouts, 100 cycles is not more effective than 50 cycles. (B): A recovery period of more than 1 hour is required for the second mechanical bout to effectively reduce adiponectin due to GSK3β serine-9 phosphorylation and accumulation of active β-catenin. (C): Increasing the number of daily mechanical applications increases the antiadipogenic effect. Densitometry of three separate experiments shows both that adiponectin is significantly repressed by repetitive dosing and more active β-catenin is accumulated. a ≠ b ≠ c ≠ d, p < 0.05. (D): 200 cycles delivered at either 1% or 2% strain magnitude effectively suppress adipogenesis and preserve β-catenin when dosed as two daily mechanical bouts with an intervening 3 hours recovery period. (E): Comparison of mesenchymal stem cell phenotype between grown medium (MEM) and adipogenic (A) medium in the presence or absence of strain (200 cycles delivered twice daily) for 4 days. Adipogenic characteristics of decreased active β-catenin increased PPARγ, APN, and aP2 and are inhibited by cyclic strain. Abbreviations: MEM, minimal essential media; APN, adiponectin.
Increasing the number of mechanical bouts from two to three repetitions, each separated by a 3-hour recovery period, further increased the inhibition of adipogenesis, suggesting that the cell system was able to reestablish mechanosensitivity following each rest period, allowing an amplification of the cell’s response to mechanical input as demonstrated by decreased adiponectin and increased β-catenin with each additional daily bout (Fig. 1C). Although decreasing the strain magnitude to 1% failed to inhibit adipogenesis, if delivered in single daily bout of 200 cycles, this lower magnitude became efficacious if split into two bouts over the course of the day, emphasizing that inserting a recovery period enables the magnitude of a challenge to be reduced, albeit with a lower effect (Fig. 1D). The enhanced sensitivity of MSCs to mechanical signals enabled by these recovery periods suggested that the receptiveness of cell was “primed” by an adaptive—and transient—ability of the cell to perceive and respond to dynamic physical signals.
To highlight the importance of β-catenin, downstream from Akt/GSK3β events, in transmitting the antiadipogenic effect of mechanical strain [8], 200 strain cycles delivered twice daily with a 3-hour recovery period between treatments prevented the fall in β-catenin. The fall in β-catenin was associated the adipogenic phenotype, including expression of PPARγ nuclear receptor, adiponectin, and aP2 as shown in Figure 1E.
Akt Activation Is Primed by Mechanical Input
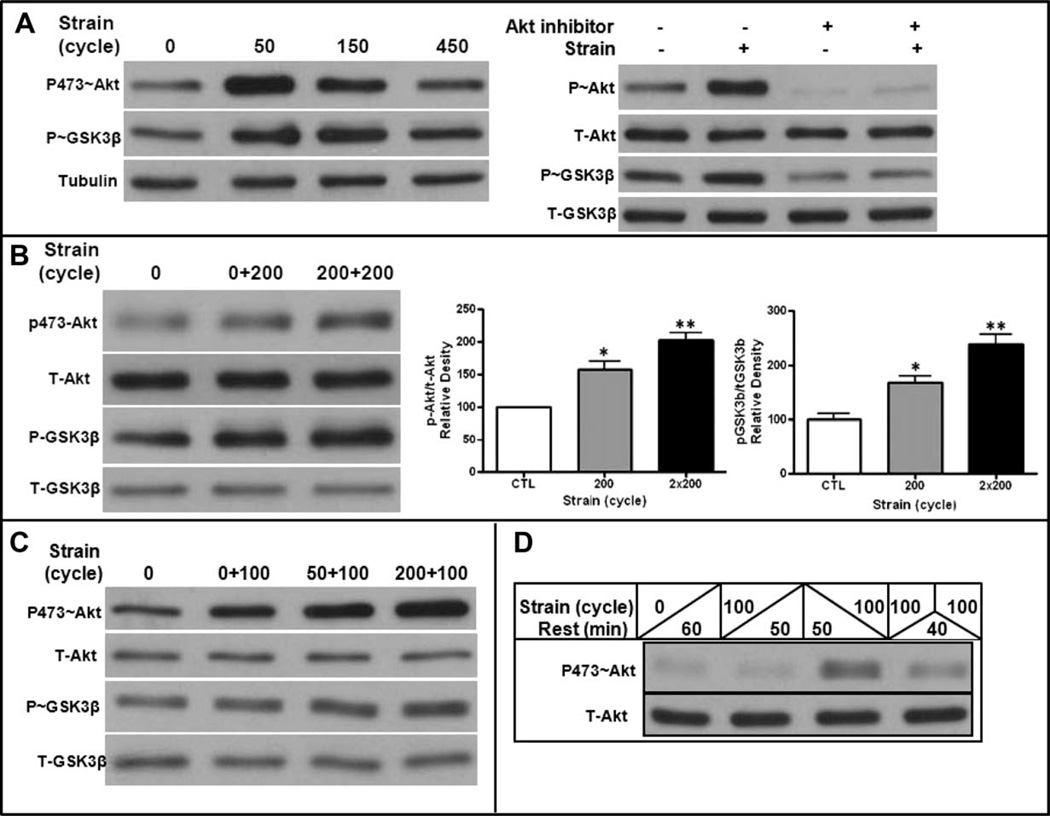
Multiple types of mechanical stimuli rapidly activate Akt [24, 25], which can target and phosphorylate GSK3β. We have previously shown that mechanical inhibition of GSK3β (by phosphorylation) is essential for mechanical inhibition of adipogenesis [7, 8] and here examined the response of Akt/GSK3β signaling on repeated mechanical treatments. Akt was demonstrated to be phosphorylated (Akt-S473) after 50 cycles of strain delivered over 5 minutes and rapidly returned to baseline phosphorylation levels 45 minutes after cessation of the mechanical stimulus (Fig. 2A, left). Akt activation was responsible for the consequent GSK3β phosphorylation, as GSK3β phosphorylation does not occur in the presence of an Akt inhibitor (Fig. 2A, right). GSK3β phosphorylation was also transient, consistent with the temporal nature of the mechanical effect on Akt activation.
Figure 2.
Akt activation is primed by mechanical input. (A): Left panel: Akt and GSK3β phosphorylation was measured after 0, 50, 150, and 450 cycles of 2% strain (e.g., from 5 to 45 minutes of continuous mechanical input). Akt activation and GSK3β inactivation are transient despite increasing cycle number. Tubulin reflects equal loading. Right panel: Akt activation is necessary for GSK3β phosphorylation, as it does not occur in the presence of an Akt inhibitor when measured after 50 strain cycles. (B): A second application of mechanical input delivered 3 hours after the first increases the amount of phosphorylated Akt, as well as downstream phosphorylation of GSK3β. Three experiments assessed for densitometry of both Akt and GSK3β phosphorylation, shown in graphs to the right, confirm a significant increase in phosphorylation with the second bout. *, p < .05 compared with control; **, p < .01. (C): Mechanical priming of Akt and GSK3β phosphorylation can be achieved with a reduction in mechanical cycles from 200 to 50. (D): Akt is refractory to mechanical reactivation at 60 minutes. Cells were ± strained at the beginning (left of upslanting bar) and/or end (right of downslanting bar) of a 60-minute period. Lane 1, nonstrained cells; Lane 2, 100 cycles followed by 50 minutes rest; Lane 3, 50 minutes rest followed by 100 cycles; Lane 4, 100 cycles followed by 40 minutes rest and reapplication of 100 cycles. Mechanically, activation of Akt has returned to baseline by 50 minutes and is refractory to retreatment 40 minutes later.
We next investigated the phosphorylation response of Akt and GSK3β to a second mechanical treatment within a single day that was delivered after a rest period. As shown in Figure 2B, 200 cycles of mechanical loading induced phosphorylation of both Akt and GSK3β. When the same loading challenge was repeated 3 hours later, a time when Akt phosphorylation has returned to baseline, there was an enhanced to the second mechanical treatment. Densitometry revealed that the second mechanical treatment induced a significant enhancement in the phosphorylation of both Akt and GSK3β (2B, right graphs). Priming of Akt and GSK3β phosphorylation was still evident when the first mechanical stimulation was reduced from 200 to 50 cycles (Fig. 2C), as a first bout of 50 cycles successfully and equivalently enhanced the repeat Akt activation. The increased phosphorylation of Akt observed after the second mechanical bout caused enhanced phosphorylation/inactivation of GSK3β, corresponding to the enhanced effect of two daily mechanical bouts to inhibit adipogenesis. Importantly, the mechanical priming of Akt was not fully effective when a second bout was delivered after a rest period of only 1 hour: repeating the mechanical application within an hour after the first treatment failed to fully reactivate Akt, an effect consistent with signal downregulation (Fig. 2D). As demonstrated in this figure, the intensity of the response to the second bout is not due to a prolonged phosphorylation due to the first bout; not only is Akt phosphorylation transient but also it is in fact refractory to activation, when strain is applied after a limited 1 hour rest period.
Mechanical Input Induces FA Assembly
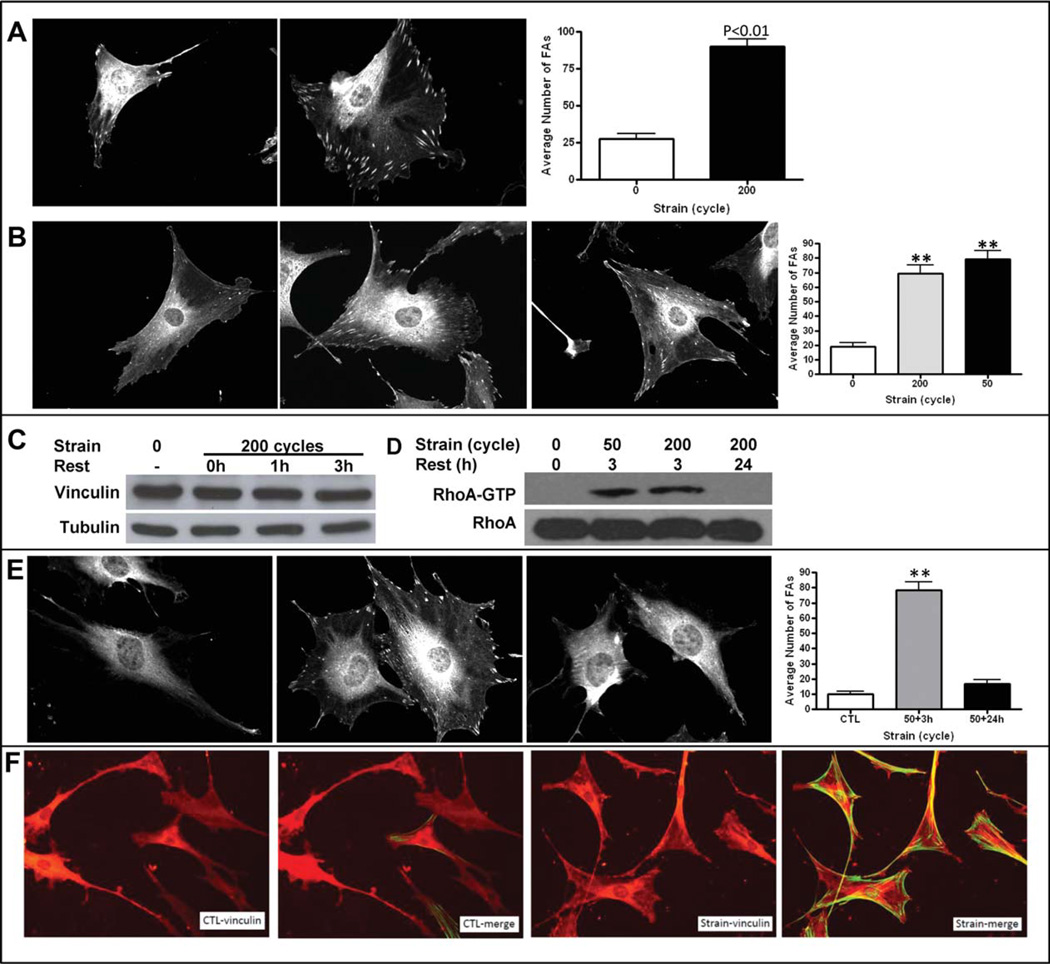
We next investigated whether a dynamic restructuring of cytoskeletal elements at the cell/surface interface was involved in the signal amplification that was evident following multiple mechanical treatments. As FA assembly is both transient [16] and can enhance mechanical forces transmitted through the substrate [17, 18] we hypothesized that FAs might be involved in the adaptive cellular mechanoresponse. Three hours after 200 cycles of mechanical load to MSC, a time when signal amplification was measured (Fig. 2), FAs were identified by staining for vinculin, an F-actin binding protein that connects integrins to actin filaments and transmits mechanical tension [26]. After staining for vinculin, FAs were counted in at least 30 randomly chosen cells by an operator blinded to mechanical treatment. One hour following loading, increased FA number was evident around the cell periphery, and continued to increase over the next several hours by more than fivefold (Fig. 3A, 3B). The increase in FAs was not due to an increase in vinculin protein, as shown in by equivalent vinculin analyzed by Western blot 1 and 3 hours after 200 strain cycles (Fig. 3C). Consistent with the equivalent efficacy of either 50 or 200 cycles to prime for both antiadipogenesis and Akt/GSK3β phosphorylation, induction of FA formation was induced equivalently by 50 and 200 cycles within a given mechanical session (Fig. 3B, right graph).
Figure 3.
Mechanical input transiently induces focal adhesions (FAs). (A): FAs, shown by vinculin staining, are increased 3 hours after application of 200 strain cycles. The left micrograph (40×) is an example of an unstrained and the right a strained mesenchymal stem cells (MSCs). FA numbers quantified in 30 cells per treatment show a significant increase in number after strain, shown in the graph to the right. **, p < .01 compared with control. Single channel images are displayed in black and white. (B): FA induction is equivalent in cells treated with 200 or 50 cycles of mechanical strain. The left micrograph (40×) shows an unstrained cell, the middle an example of a cell 3 hours after 200 strain cycles, and the rightmost a MSC subject to 50 strain cycles. Quantification of FAs shows that induction of FA assembly was equivalent after 50 or 200 strain cycles. **, p < .01 compared with control. (C): Protein expression of vinculin is not induced 3 hours after mechanical strain. MSC were 6 treated with 200 strain cycles and protein collected immediately (0 hours) and 1 and 3 hours after strain. (D): RhoA activation (GTP association) increases equivalently after 50 or 200 cycles of strain and returns to baseline 24 hours after strain. Cell lysates for GTP-bound RhoA were collected 3 or 24 hours after 50 or 200 strain cycles as shown. (E): FA assembly after 50 strain cycles is not present 24 hours after strain. MSC treated with 0 (left) or 50 strain cycles (middle and right panels) were examined for FA staining after 3 hours (middle) or 24 hours (right) after strain cessation. Quantification of FA shows assembly at 3 hours, which has returned to baseline after 24 hours; **, p < .01 compared with control. (F): MSCs were stained for FAs (vinculin) and for stress fibers (phalloidin). Micrographs (20×) demonstrate that 3 hours after 100 strain cycles, FA formation is associated with actin stress fiber formation (merge vinculin + phalloidin stain). Abbreviation: GTP, guanosine triphosphate.
RhoA activation, required during FA assembly [27], was also increased 3 hours after mechanical challenge, but was not influenced by increasing cycle number from 50 or 200 (Fig. 3D). Importantly, the FAs induced by mechanical stimuli disassembled with a 24-hour period, as shown both by a return of RhoA activation to baseline (Fig. 3D) and a decrease in vinculin staining of recognizable peripheral FAs (Fig. 3E). This appearance and disappearance of FA could underlie the heightened, but transient, signaling response leading to the antiadipogenic effect of mechanical stimuli.
Unstrained control cells have scattered FA staining, shown by vinculin, but very few cells possess stress fibers (Fig. 3F). In contrast, 3 hours after application of 100 strain cycles, not only is there an increase in FAs, shown by vinculin staining, but also virtually every observed cell contains stress fibers.
FAs Are Necessary for Mechanical, But Not insulin, Activation of Akt
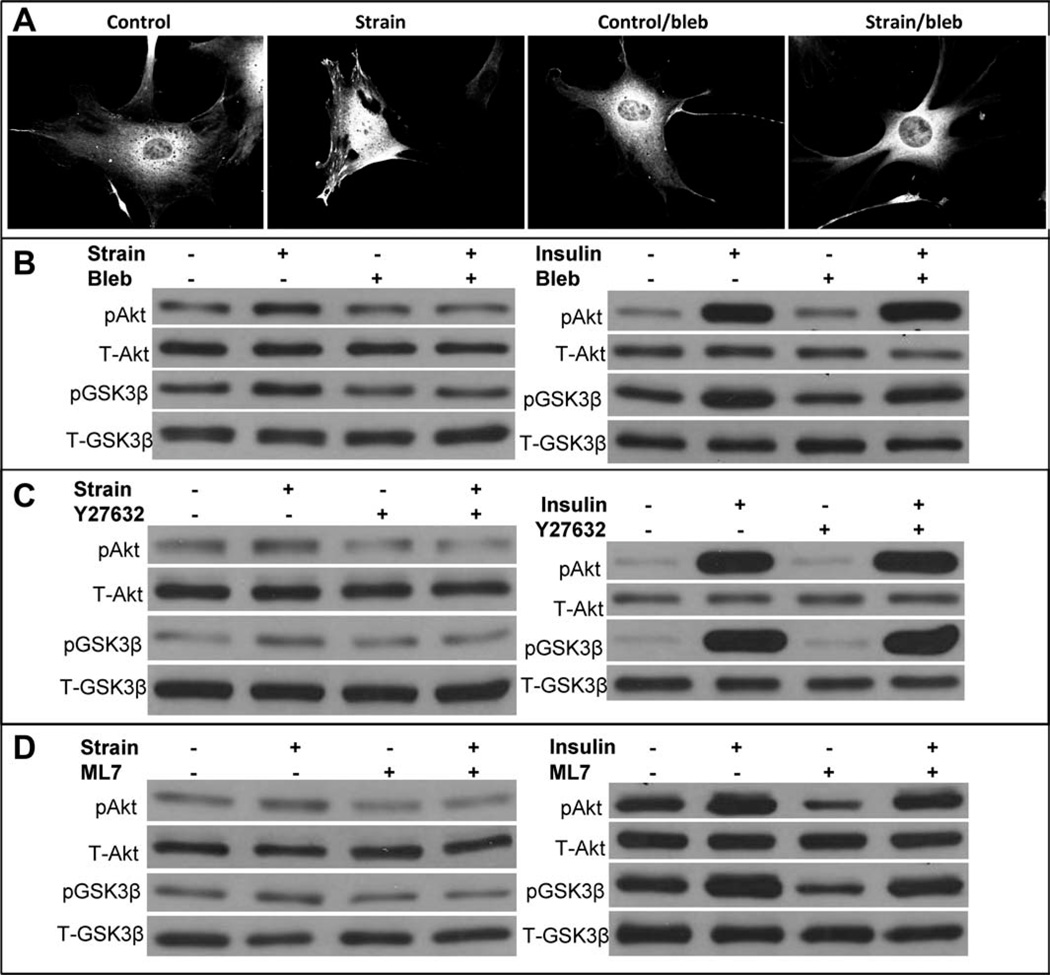
To identify FA assembly as a critical link in the proximal AKT activation by which mechanical loading suppresses adipogenesis, we sought to associate FA assembly with mechanically mediated phosphorylation of Akt. In the presence of the nonmuscle myosin II inhibitor, blebbistatin [28], FA failed to form after mechanical stimuli (Fig. 4A), and Akt was not phosphorylated, indicating that mechanical activation of the Akt/GSK3β signaling cascade requires force sensing by FAs (Fig. 4B). As insulin binding of its cognate receptor does not require cell adhesion, we considered whether insulin Akt activation required FAs. Insulin activation of PI3-kinase generates exposure of the pleckstrin domain of PIP3, thus causing recruitment of Akt to the membrane where it is phosphorylated and activated [29]. Also shown in Figure 4B (right panel), in the presence of blebbistatin, insulin fully activated Akt followed by GSK3β phosphorylation. Inhibition of RhoA action, which is necessary for the formation of FA formation [27], also blocks mechanical activation of Akt but not Akt activation by insulin (Fig. 4C).
Figure 4.
Focal adhesions (FAs) are necessary for mechanical but not insulin activation of Akt. (A): Mechanically induced FA assembly does not occur in the presence of blebbistatin (50 µM). Representative mesenchymal stem cells (MSCs) show control, strain (200 cycles), control + blebbistatin, and strain + blebbistatin. (B): pAkt and phosphorylated GSK3β were assayed 30 minutes after 200 strain cycles. Mechanical effects on Akt and GSK3β were not seen in the presence of blebbistatin. The panel on the right shows insulin (100 ng/ml) induced Akt and GSK3β phosphorylation, which was unaffected by blebbistatin. (C): Mechanical phosphorylation of Akt and GSK3β does not occur in MSCs pretreated with ROCK inhibitor Y27632 (10 µM). Insulin induced phosphorylation of Akt and GSK3β phosphorylation occurs in the presence of ROCK inhibitor Y27632 (10 µM) (right panel). (D): Mechanical phosphorylation of Akt and GSK3β does not occur in MSCs pretreated with myosin light-chain kinase inhibitor (ML7, 10 µM). Insulin activation is unaffected (right panel).
To further associate the force generated during stress with mechanical Akt phosphorylation, but not Akt-phosphorylation due to insulin, cells were treated with ML7, an inhibitor to myosin light chain kinase [30]. As shown in Figure 4D, interfering with force generation prevented Akt phosphorylation due to cyclic strain, but not to insulin, an effect similar to both inhibition of myosin II and RhoA. This indicates that development of tension via FAs is critical to mechanical challenge and suggests that increasing cytoskeletal elements will amplify the response.
Discussion
Exogenous mechanical information provides critical regulatory signals to stem cells, biasing the ultimate fate selection of these progenitors [2, 31–33]. In the case of bone marrow MSCs, commitment toward adipose or osteoblast lineage appears to be reciprocally regulated [34]. The bias toward adipocyte differentiation from MSCs, which accompanies conditions such as aging [35] and anorexia [36], not only represses osteoblast commitment but also by itself may impair hematopoietic stem cell development [37]. As such, control of bone marrow adipogenesis is important in determining stem cell decisions of multiple lineages. We have shown that multiple types of mechanical signals limit adipogenesis from MSCs through a β-catenin dependent process [8, 20]. Understanding how best to activate this inhibitory mechanical signal cascade not only to optimize stem cell fate but also translate the ability of physical signals to suppress obesity as considered in the clinic.
Little is known about the specific features of the mechanical environment, which serve as critical determinants of stem cell fate (e.g., duration, amplitude, and recovery time), or how these signals are ultimately perceived and transduced by the cell. The data reported here support a conclusion that mechanical influences on stem cell decisions saturate quickly, such that the necessary signaling cascade is initiated after only a few mechanical cycles. However, while accumulation of mechanical information is not necessarily critical to defining stem cell outcomes, we found that when the mechanical stimulus is repeated after a rest period of greater than 1 hour, the cell system has not only reestablished its ability to respond but also experiences an amplification of the incoming mechanical signal. To understand this amplification mechanism, we studied those cell processes that are critical in the protection of β-catenin by mechanical signals [8]. As the mechanical information required is delivered in a manner of minutes, we focused on GSK3β and the kinase that inactivates it, which we have shown here to be Akt, a pleiomorphic kinase, known to be involved in mechanotransduction [25]. In MSCs, Akt is rapidly activated by mechanical loading, but is refractory to reactivation until a rest period has occurred. Supporting the hypothesis that this signal cascade is crucial to the antiadipogenic potential of mechanical signals, mechanical information delivered following the rest period was amplified, such that Akt activation and subsequent GSK3β phosphorylation were enhanced.
We considered what cellular adaptation induced by mechanical input might enhance Akt-GSK3β signal transduction. FAs represent an intracellular connection between the cell substrate surface and the cytoskeleton and are known to develop tension in response to mechanical perturbation [26]. These focal contacts can grow in response to applied mechanical force [18, 19], a process that not only contributes to the enhanced structure of the cell but also influences lineage specification through cytoskeletal structure [14]. Furthermore, MSCs responsiveness to mechanical signals has been suggested to function on a micrometer scale suggesting interactions between FAs [38]. In response to the first session of loading, we found that FAs assembled at the edges of the MSCs increased by nearly fivefold in strained cells. Not surprisingly, the increase in FAs was paralleled by an increase in RhoA activity, as RhoA activation promotes adhesion maturation [39], and RhoA, through Rock-mediated myosin II activation, increases cellular contractility. The resulting tension on integrin triggers protein recruitment and signaling pathway activation leading to strengthening and growth of the adhesion [40] emphasizing that the cytoskeletal adaptations, while certainly contributing to cell structure, also proactively influence biological processes. In the absence of RhoA activation, FAs failed to form after mechanical loading.
The mechanically mediated assembly of FAs is a striking confirmation that the MSC both recognizes and adapts to exogenous physical stimuli and that these changes influence lineage selection of the precursor. Importantly, the degree to which MSCs perceive and respond to mechanical signals is strongly dependent on the temporal nature of the signal. The cell system, following an initial burst of mechanical signal, must incorporate a rest period before the mechanosensitivity is fully recovered. It appears that this refractory period allows a restructuring of cytoskeletal elements—indicating a transient cellular adaptation— and that this cytoskeletal rearrangement enables downstream amplification of incoming physical signals. As such, it is clear that signal timing, particularly in the context of a 24-hour cycle, is as important as is the magnitude of the signal to optimizing outcomes. As importantly, the temporary nature of the assembled FAs emphasizes yet another dynamic component of the cell response to mechanical signals and may underlie the established benefits of repetitive daily loading [7].
Our data emphasize the importance of the cell cytoskeleton in enabling mechanotransduction pathways and that mechanically mediated restructuring of the architecture of the cell allows for amplification of these biological pathways. We believe these data also indicate a role for the cytoskeleton as an active transducer rather than passive scaffold in cellular responsiveness to physical signals. The transient nature of these cytoskeletal adaptations then serves as a short term memory of the physical environment and temporarily facilitates the amplification of further cell responses to downstream challenges. As such, the dynamic cytoskeletal restructuring is not only a means of enhancing signal sensitivity and responsiveness of the cell but also the transient nature of this response indicates that the absence of mechanical signals would ultimately be permissive to the dissolution of mechanosensitivity of the cell system.
Conclusion
Mechanical activation of Akt in MSC, resulting in GSK3β inhibition with activation of β-catenin, limits adipogenesis. The ability of mechanical signals to suppress adipogenesis is further enhanced, enabling a reduction in magnitude and cycles of mechanical input, by delivering subsequent mechanical bouts within a given 24 hours period when a rest period of at least 3 hours is allowed before the next challenge ensues. It appears that the amplification Akt and the suppression of adipogenesis are achieved via the mechanical induction of FAs and associated cytoskeletal enhancements, a transient architectural adaptation that sensitizes the cell to physical stimuli. This information sheds light on biological and morphological processes within the cell that enable and augment mechanotransduction and suggest ways in which mechanical signals might be used more effectively to yield a range of benefits from mechanical challenges.
Acknowledgments
This work was supported by NIH Grants AR042360, AR043498, AR056655, and GM024860. C.G. is supported by a Marie Curie Outgoing International Fellowship from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 254747.
Footnotes
Author contributions: B.S. and C.G.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; Z.X.: conception and design, collection and/or assembly of data, data analysis and interpretation; N.C.: conception and design, data analysis and interpretation, manuscript writing; MS: data analysis and interpretation, manuscript writing; J.T.: data analysis and interpretation; I.O.: collection and/or assembly of data, data analysis and interpretation; C.R.: conception and design, data analysis and interpretation, final approval of manuscript; K.B.: conception and design, data analysis and interpretation, manuscript writing; J.R.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
C.R. is a founder of Marodyne Medical, Inc., and holds several patents related to the importance of mechanical signals in determining cell fate. All other authors indicate no potential conflicts interest.
References
- 1.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Engler AJ, Sen S, Sweeney HL, et al. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 3.Ozcivici E, Luu YK, Adler B, et al. Mechanical signals as anabolic agents in bone. Nat Rev Rheumatol. 2010;6:50–59. doi: 10.1038/nrrheum.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gurkan UA, Akkus O. The mechanical environment of bone marrow: A review. Ann Biomed Eng. 2008;36:1978–1991. doi: 10.1007/s10439-008-9577-x. [DOI] [PubMed] [Google Scholar]
- 5.Menuki K, Mori T, Sakai A, et al. Climbing exercise enhances osteoblast differentiation and inhibits adipogenic differentiation with high expression of PTH/PTHrP receptor in bone marrow cells. Bone. 2008;43:613–620. doi: 10.1016/j.bone.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 6.Minaire P, Edouard C, Arlot M, et al. Marrow changes in paraplegic patients. Calcif Tissue Int. 1984;36:338–340. doi: 10.1007/BF02405340. [DOI] [PubMed] [Google Scholar]
- 7.Sen B, Xie Z, Case N, et al. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology. 2008;149:6065–6075. doi: 10.1210/en.2008-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sen B, Styner M, Xie Z, et al. Mechanical loading regulates NFATc1 and beta-catenin signaling through a GSK3beta control node. J Biol Chem. 2009;284:34607–34617. doi: 10.1074/jbc.M109.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong Y, Slee RB, Fukai N, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 10.Qiu W, Andersen TE, Bollerslev J, et al. Patients with high bone mass phenotype exhibit enhanced osteoblast differentiation and inhibition of adipogenesis of human mesenchymal stem cells. J Bone Miner Res. 2007;22:1720–1731. doi: 10.1359/jbmr.070721. [DOI] [PubMed] [Google Scholar]
- 11.Taelman VF, Dobrowolski R, Plouhinec JL, et al. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell. 2010;143:1136–1148. doi: 10.1016/j.cell.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong VJ, Muzylak M, Sunters A, et al. Wnt/beta-catenin signaling is a component of osteoblastic bone cells’ early responses to load-bearing, and requires estrogen receptor alpha. J Biol Chem. 2007;282:20715–20727. doi: 10.1074/jbc.M703224200. [DOI] [PubMed] [Google Scholar]
- 13.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 14.McBeath R, Pirone DM, Nelson CM, et al. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 15.Salaita K, Nair PM, Petit RS, et al. Restriction of receptor movement alters cellular response: Physical force sensing by EphA2. Science. 2010;327:1380–1385. doi: 10.1126/science.1181729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galbraith CG, Yamada KM, Sheetz MP. The relationship between force and focal complex development. J Cell Biol. 2002;159:695–705. doi: 10.1083/jcb.200204153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Ann Rev Cell Dev Biol. 2003;19:677–695. doi: 10.1146/annurev.cellbio.19.111301.153011. [DOI] [PubMed] [Google Scholar]
- 18.Riveline D, Zamir E, Balaban NQ, et al. Focal contacts as mechanosensors: Externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol. 2001;153:1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shemesh T, Geiger B, Bershadsky AD, et al. Focal adhesions as mechanosensors: A physical mechanism. Proc Natl Acad Sci USA. 2005;102:12383–12388. doi: 10.1073/pnas.0500254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sen B, Xie Z, Case N, et al. Mechanical signal influence on mesenchymal stem cell fate is enhanced by incorporation of refractory periods into the loading regimen. J Biomech. 2011;44:593–599. doi: 10.1016/j.jbiomech.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Case N, Xie Z, Sen B, et al. Mechanical activation of beta-catenin regulates phenotype in adult murine marrow-derived mesenchymal stem cells. J Orthop Res. 2010;28:1531–1538. doi: 10.1002/jor.21156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu BP, Burridge K. Vav2 activates Rac1, Cdc42, and RhoA downstream from growth factor receptors but not beta1 integrins. Mol Cell Biol. 2000;20:7160–7169. doi: 10.1128/mcb.20.19.7160-7169.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arthur WT, Burridge K. RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol Biol Cell. 2001;12:2711–2720. doi: 10.1091/mbc.12.9.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Case N, Ma M, Sen B, et al. Beta-catenin levels influence rapid mechanical responses in osteoblasts. J Biol Chem. 2008;283:29196–29205. doi: 10.1074/jbc.M801907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sedding DG, Hermsen J, Seay U, et al. Caveolin-1 facilitates mechanosensitive protein kinase B (Akt) signaling in vitro and in vivo. Circ Res. 2005;96:635–642. doi: 10.1161/01.RES.0000160610.61306.0f. [DOI] [PubMed] [Google Scholar]
- 26.Grashoff C, Hoffman BD, Brenner MD, et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 2010;466:263–266. doi: 10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 28.Zhang M, Rao PV. Blebbistatin, a novel inhibitor of myosin II ATPase activity, increases aqueous humor outflow facility in perfused enucleated porcine eyes. Invest Ophthalmol Vis Sci. 2005;46:4130–4138. doi: 10.1167/iovs.05-0164. [DOI] [PubMed] [Google Scholar]
- 29.Brazil DP, Park J, Hemmings BA. PKB binding proteins. Getting in on the Akt. Cell. 2002;111:293–303. doi: 10.1016/s0092-8674(02)01083-8. [DOI] [PubMed] [Google Scholar]
- 30.Dumbauld DW, Shin H, Gallant ND, et al. Contractility modulates cell adhesion strengthening through focal adhesion kinase and assembly of vinculin-containing focal adhesions. J Cell Physiol. 2010;223:746–756. doi: 10.1002/jcp.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adamo L, Naveiras O, Wenzel PL, et al. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459:1131–1135. doi: 10.1038/nature08073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li D, Zhou J, Chowdhury F, et al. Role of mechanical factors in fate decisions of stem cells. Regen Med. 2011;6:229–240. doi: 10.2217/rme.11.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akune T, Ohba S, Kamekura S, et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113:846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shih TT, Chang CJ, Hsu CY, et al. Correlation of bone marrow lipid water content with bone mineral density on the lumbar spine. Spine. 2004;29:2844–2850. doi: 10.1097/01.brs.0000147803.01224.5b. [DOI] [PubMed] [Google Scholar]
- 36.Ecklund K, Vajapeyam S, Feldman HA, et al. Bone marrow changes in adolescent girls with anorexia nervosa. J Bone Miner Res. 2010;25:298–304. doi: 10.1359/jbmr.090805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naveiras O, Nardi V, Wenzel PL, et al. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu J, Wang YK, Yang MT, et al. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat Methods. 2010;7:733–736. doi: 10.1038/nmeth.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeMali KA, Wennerberg K, Burridge K. Integrin signaling to the actin cytoskeleton. Curr Opin Cell Biol. 2003;15:572–582. doi: 10.1016/s0955-0674(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 40.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]