Abstract
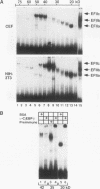
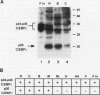
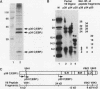
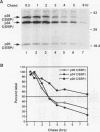
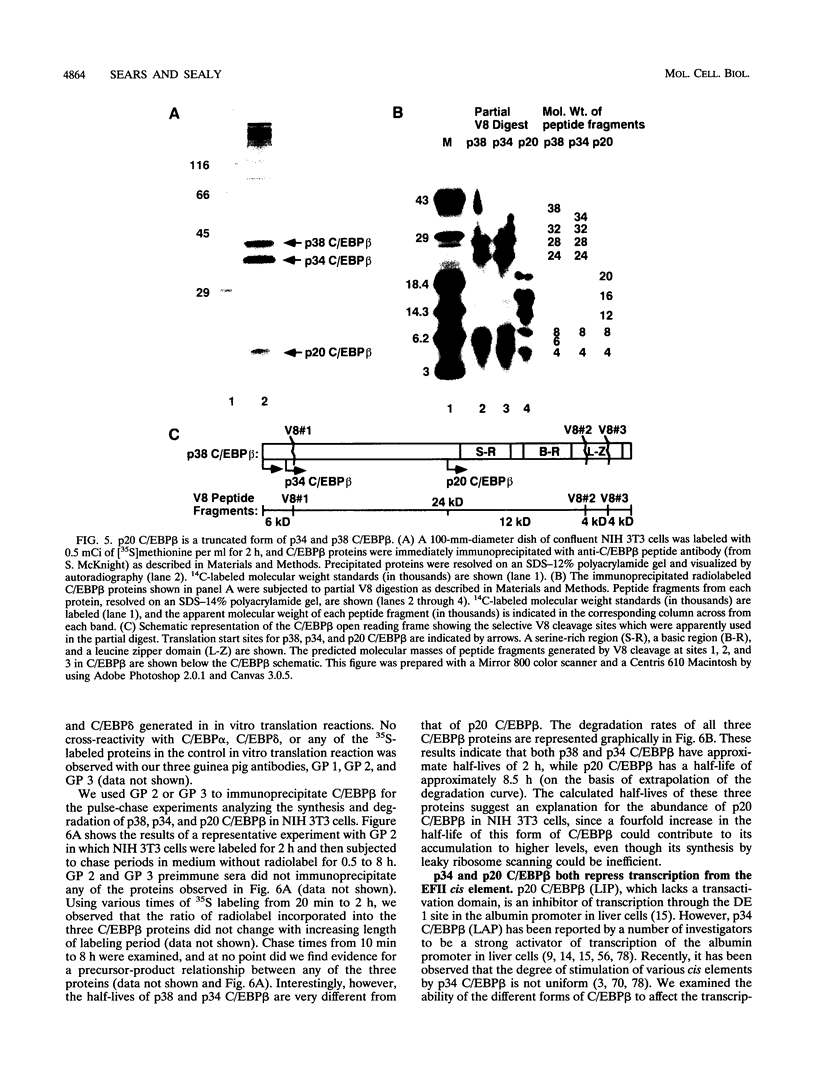
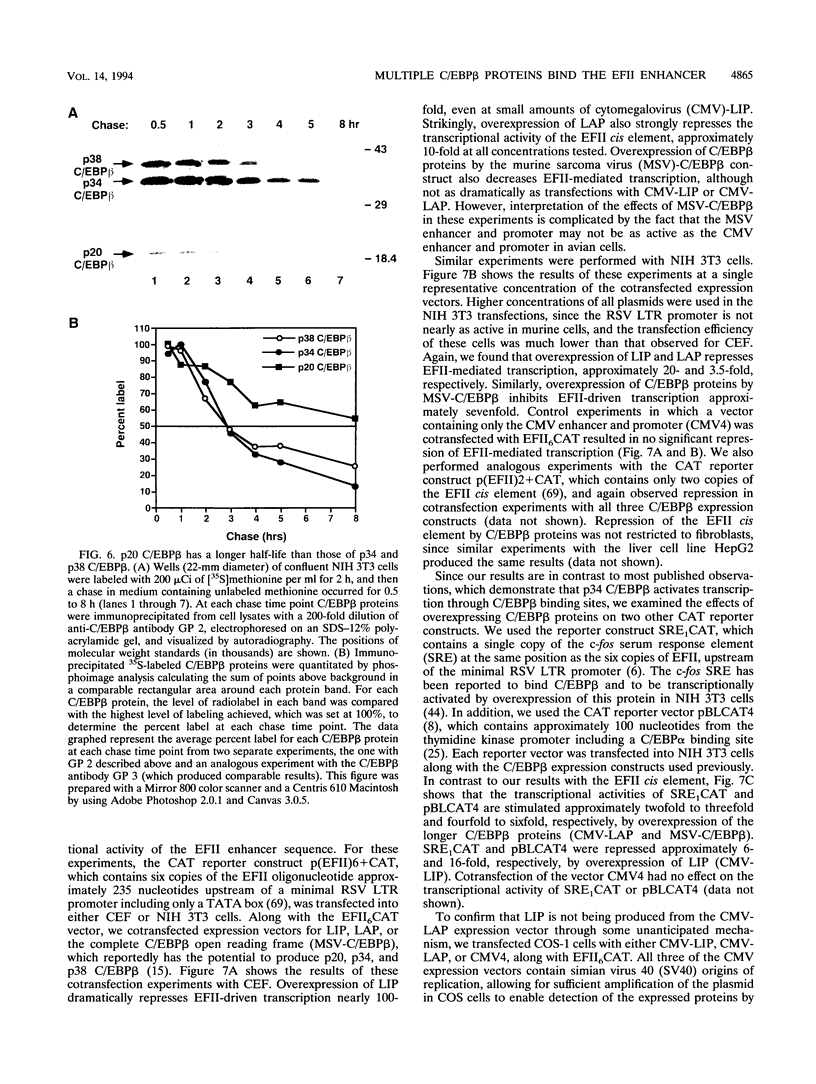
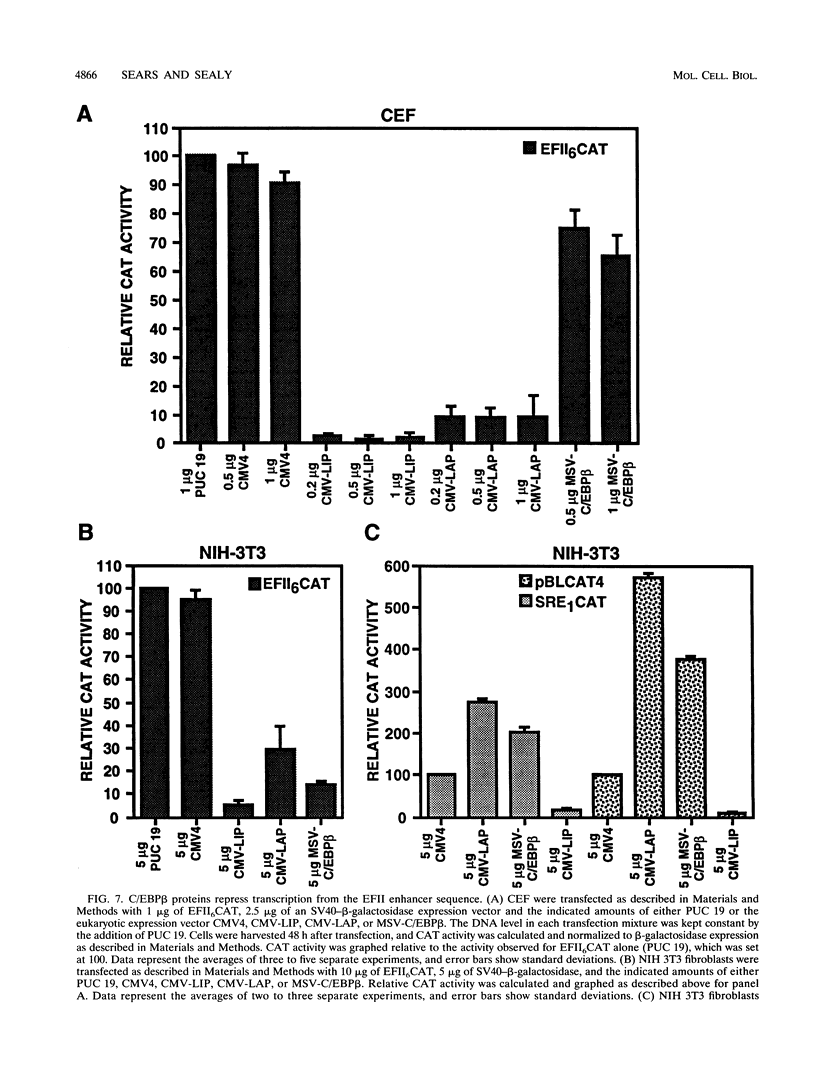
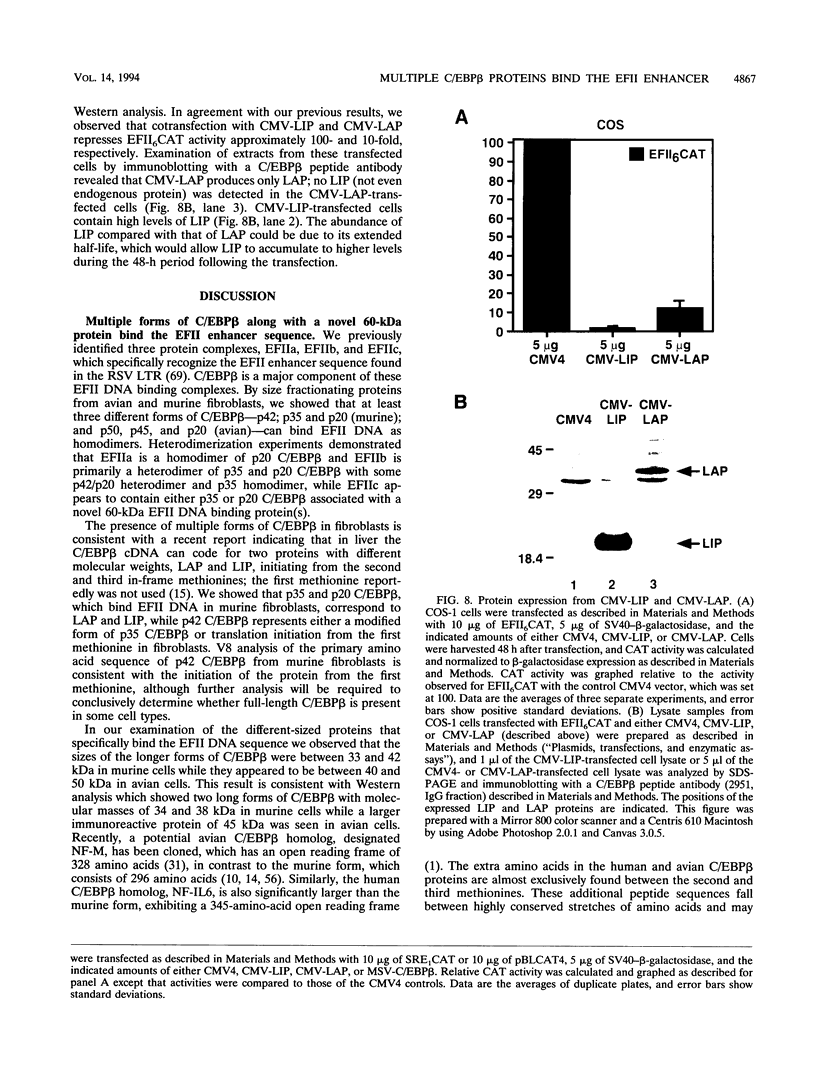
In this report we demonstrate that C/EBP beta is a major component of three EFII DNA binding complexes, EFIIa, EFIIb, and EFIIc, which we have previously shown to specifically recognize a C/EBP consensus binding site found in the EFII enhancer sequence from the Rous sarcoma virus long terminal repeat (R. C. Sears and L. Sealy, J. Virol. 66:6338-6352, 1992). Three different forms of C/EBP beta, p42, p35, and p20, can bind the EFII DNA sequence as homodimers, and dimerization experiments show that EFIIa is a homodimer of p20 C/EBP beta, EFIIb is primarily composed of a p20/p35 heterodimer with minor amounts of p20/p42 heterodimer and p35 homodimer, and EFIIc is composed of p20 and/or p35 heterodimerized with a novel 60-kDa protein. p20 C/EBP beta is likely equivalent to the internally initiated translation product of C/EBP beta, LIP (liver inhibitor protein), described by P. Descombes and U. Schibler (Cell 67:569-579, 1991). In contrast to the low level of LIP expressed in liver, postulated to occur because of leaky ribosome scanning, we found high levels of expression of p20 C/EBP beta in fibroblasts and lymphocytes. In murine fibroblasts, p20 C/EBP beta has an extended half-life, four times longer than those of p42 and p35 C/EBP beta, which could contribute to its abundant accumulation in this cell type, even though its synthesis by leaky ribosome scanning might be inefficient. Interestingly, overexpression of either the long or short form of C/EBP beta represses EFII-mediated transcription, suggesting that another unidentified EFII transactivator(s) exists, which may be dominantly inhibited by C/EBP beta proteins, and/or that transactivation by C/EBP beta proteins requires posttranslational modifications that were lacking in the transient overexpression experiments.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Isshiki H., Sugita T., Tanabe O., Kinoshita S., Nishio Y., Nakajima T., Hirano T., Kishimoto T. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 1990 Jun;9(6):1897–1906. doi: 10.1002/j.1460-2075.1990.tb08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam T., An M. R., Papaconstantinou J. Differential expression of three C/EBP isoforms in multiple tissues during the acute phase response. J Biol Chem. 1992 Mar 15;267(8):5021–5024. [PubMed] [Google Scholar]
- Baumann H., Morella K. K., Campos S. P., Cao Z., Jahreis G. P. Role of CAAT-enhancer binding protein isoforms in the cytokine regulation of acute-phase plasma protein genes. J Biol Chem. 1992 Sep 25;267(27):19744–19751. [PubMed] [Google Scholar]
- Boulden A. M., Sealy L. J. Maximal serum stimulation of the c-fos serum response element requires both the serum response factor and a novel binding factor, SRE-binding protein. Mol Cell Biol. 1992 Oct;12(10):4769–4783. doi: 10.1128/mcb.12.10.4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulden A., Sealy L. Identification of a third protein factor which binds to the Rous sarcoma virus LTR enhancer: possible homology with the serum response factor. Virology. 1990 Jan;174(1):204–216. doi: 10.1016/0042-6822(90)90069-4. [DOI] [PubMed] [Google Scholar]
- Bowers W. J., Ruddell A. a1/EBP: a leucine zipper protein that binds CCAAT/enhancer elements in the avian leukosis virus long terminal repeat enhancer. J Virol. 1992 Nov;66(11):6578–6586. doi: 10.1128/jvi.66.11.6578-6586.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büscher M., Rahmsdorf H. J., Litfin M., Karin M., Herrlich P. Activation of the c-fos gene by UV and phorbol ester: different signal transduction pathways converge to the same enhancer element. Oncogene. 1988 Sep;3(3):301–311. [PubMed] [Google Scholar]
- Cao Z., Umek R. M., McKnight S. L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991 Sep;5(9):1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- Chang C. J., Chen T. T., Lei H. Y., Chen D. S., Lee S. C. Molecular cloning of a transcription factor, AGP/EBP, that belongs to members of the C/EBP family. Mol Cell Biol. 1990 Dec;10(12):6642–6653. doi: 10.1128/mcb.10.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C., Johnson D., Roman C., Avitahl N., Tucker P., Calame K. The C/EBP family of transcriptional activators is functionally important for Ig VH promoter activity in vivo and in vitro. J Immunol. 1992 Nov 15;149(10):3225–3231. [PubMed] [Google Scholar]
- Cullen B. R., Raymond K., Ju G. Functional analysis of the transcription control region located within the avian retroviral long terminal repeat. Mol Cell Biol. 1985 Mar;5(3):438–447. doi: 10.1128/mcb.5.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descombes P., Chojkier M., Lichtsteiner S., Falvey E., Schibler U. LAP, a novel member of the C/EBP gene family, encodes a liver-enriched transcriptional activator protein. Genes Dev. 1990 Sep;4(9):1541–1551. doi: 10.1101/gad.4.9.1541. [DOI] [PubMed] [Google Scholar]
- Descombes P., Schibler U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell. 1991 Nov 1;67(3):569–579. doi: 10.1016/0092-8674(91)90531-3. [DOI] [PubMed] [Google Scholar]
- Ellenberger T. E., Brandl C. J., Struhl K., Harrison S. C. The GCN4 basic region leucine zipper binds DNA as a dimer of uninterrupted alpha helices: crystal structure of the protein-DNA complex. Cell. 1992 Dec 24;71(7):1223–1237. doi: 10.1016/s0092-8674(05)80070-4. [DOI] [PubMed] [Google Scholar]
- Faber M., Sealy L. Rous sarcoma virus enhancer factor I is a ubiquitous CCAAT transcription factor highly related to CBF and NF-Y. J Biol Chem. 1990 Dec 25;265(36):22243–22254. [PubMed] [Google Scholar]
- Friedman A. D., Landschulz W. H., McKnight S. L. CCAAT/enhancer binding protein activates the promoter of the serum albumin gene in cultured hepatoma cells. Genes Dev. 1989 Sep;3(9):1314–1322. doi: 10.1101/gad.3.9.1314. [DOI] [PubMed] [Google Scholar]
- Garlatti M., Tchesnokov V., Daheshia M., Feilleux-Duché S., Hanoune J., Aggerbeck M., Barouki R. CCAAT/enhancer-binding protein-related proteins bind to the unusual promoter of the aspartate aminotransferase housekeeping gene. J Biol Chem. 1993 Mar 25;268(9):6567–6574. [PubMed] [Google Scholar]
- Gentz R., Rauscher F. J., 3rd, Abate C., Curran T. Parallel association of Fos and Jun leucine zippers juxtaposes DNA binding domains. Science. 1989 Mar 31;243(4899):1695–1699. doi: 10.1126/science.2494702. [DOI] [PubMed] [Google Scholar]
- Goodwin G. H. Identification of three sequence-specific DNA-binding proteins which interact with the Rous sarcoma virus enhancer and upstream promoter elements. J Virol. 1988 Jun;62(6):2186–2190. doi: 10.1128/jvi.62.6.2186-2190.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowda S., Rao A. S., Kim Y. W., Guntaka R. V. Identification of sequences in the long terminal repeat of avian sarcoma virus required for efficient transcription. Virology. 1988 Jan;162(1):243–247. doi: 10.1016/0042-6822(88)90415-1. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Graves B. J., Johnson P. F., McKnight S. L. Homologous recognition of a promoter domain common to the MSV LTR and the HSV tk gene. Cell. 1986 Feb 28;44(4):565–576. doi: 10.1016/0092-8674(86)90266-7. [DOI] [PubMed] [Google Scholar]
- Greuel B. T., Sealy L., Majors J. E. Transcriptional activity of the Rous sarcoma virus long terminal repeat correlates with binding of a factor to an upstream CCAAT box in vitro. Virology. 1990 Jul;177(1):33–43. doi: 10.1016/0042-6822(90)90457-3. [DOI] [PubMed] [Google Scholar]
- Hsu W., Chen-Kiang S. Convergent regulation of NF-IL6 and Oct-1 synthesis by interleukin-6 and retinoic acid signaling in embryonal carcinoma cells. Mol Cell Biol. 1993 Apr;13(4):2515–2523. doi: 10.1128/mcb.13.4.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. F., Landschulz W. H., Graves B. J., McKnight S. L. Identification of a rat liver nuclear protein that binds to the enhancer core element of three animal viruses. Genes Dev. 1987 Apr;1(2):133–146. doi: 10.1101/gad.1.2.133. [DOI] [PubMed] [Google Scholar]
- Kageyama R., Sasai Y., Nakanishi S. Molecular characterization of transcription factors that bind to the cAMP responsive region of the substance P precursor gene. cDNA cloning of a novel C/EBP-related factor. J Biol Chem. 1991 Aug 15;266(23):15525–15531. [PubMed] [Google Scholar]
- Kapiloff M. S., Mathis J. M., Nelson C. A., Lin C. R., Rosenfeld M. G. Calcium/calmodulin-dependent protein kinase mediates a pathway for transcriptional regulation. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3710–3714. doi: 10.1073/pnas.88.9.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz S., Kowenz-Leutz E., Müller C., Meese K., Ness S. A., Leutz A. The NF-M transcription factor is related to C/EBP beta and plays a role in signal transduction, differentiation and leukemogenesis of avian myelomonocytic cells. EMBO J. 1993 Apr;12(4):1321–1332. doi: 10.1002/j.1460-2075.1993.tb05777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny S., Guntaka R. V. Localization by mutational analysis of transcription factor binding sequences in the U3 region of Rous sarcoma virus LTR. Virology. 1990 Jun;176(2):483–493. doi: 10.1016/0042-6822(90)90018-m. [DOI] [PubMed] [Google Scholar]
- Kinoshita S., Akira S., Kishimoto T. A member of the C/EBP family, NF-IL6 beta, forms a heterodimer and transcriptionally synergizes with NF-IL6. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1473–1476. doi: 10.1073/pnas.89.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Tsichlis P., Khoury G. Multiple enhancer domains in the 3' terminus of the Prague strain of Rous sarcoma virus. Nucleic Acids Res. 1984 Aug 24;12(16):6427–6442. doi: 10.1093/nar/12.16.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., Adashi E. Y., Graves B. J., McKnight S. L. Isolation of a recombinant copy of the gene encoding C/EBP. Genes Dev. 1988 Jul;2(7):786–800. doi: 10.1101/gad.2.7.786. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The DNA binding domain of the rat liver nuclear protein C/EBP is bipartite. Science. 1989 Mar 31;243(4899):1681–1688. doi: 10.1126/science.2494700. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Li X., Liao W. S. Cooperative effects of C/EBP-like and NF kappa B-like binding sites on rat serum amyloid A1 gene expression in liver cells. Nucleic Acids Res. 1992 Sep 25;20(18):4765–4772. doi: 10.1093/nar/20.18.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie J. H., Wootton J. A., MacCallum D. K., McKelvey S. W., Minor R. R. Electrophoretic isolation and peptide mapping of collagen types from microsamples of tissue. Methods Enzymol. 1987;145:171–183. doi: 10.1016/0076-6879(87)45008-8. [DOI] [PubMed] [Google Scholar]
- Luciw P. A., Bishop J. M., Varmus H. E., Capecchi M. R. Location and function of retroviral and SV40 sequences that enhance biochemical transformation after microinjection of DNA. Cell. 1983 Jul;33(3):705–716. doi: 10.1016/0092-8674(83)90013-2. [DOI] [PubMed] [Google Scholar]
- López-Cabrera M., Letovsky J., Hu K. Q., Siddiqui A. Multiple liver-specific factors bind to the hepatitis B virus core/pregenomic promoter: trans-activation and repression by CCAAT/enhancer binding protein. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5069–5073. doi: 10.1073/pnas.87.13.5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor G. R., Caskey C. T. Construction of plasmids that express E. coli beta-galactosidase in mammalian cells. Nucleic Acids Res. 1989 Mar 25;17(6):2365–2365. doi: 10.1093/nar/17.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majors J. The structure and function of retroviral long terminal repeats. Curr Top Microbiol Immunol. 1990;157:49–92. doi: 10.1007/978-3-642-75218-6_3. [DOI] [PubMed] [Google Scholar]
- Metz R., Ziff E. The helix-loop-helix protein rE12 and the C/EBP-related factor rNFIL-6 bind to neighboring sites within the c-fos serum response element. Oncogene. 1991 Dec;6(12):2165–2178. [PubMed] [Google Scholar]
- Milos P. M., Zaret K. S. A ubiquitous factor is required for C/EBP-related proteins to form stable transcription complexes on an albumin promoter segment in vitro. Genes Dev. 1992 Jun;6(6):991–1004. doi: 10.1101/gad.6.6.991. [DOI] [PubMed] [Google Scholar]
- Nakajima T., Kinoshita S., Sasagawa T., Sasaki K., Naruto M., Kishimoto T., Akira S. Phosphorylation at threonine-235 by a ras-dependent mitogen-activated protein kinase cascade is essential for transcription factor NF-IL6. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2207–2211. doi: 10.1073/pnas.90.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio Y., Isshiki H., Kishimoto T., Akira S. A nuclear factor for interleukin-6 expression (NF-IL6) and the glucocorticoid receptor synergistically activate transcription of the rat alpha 1-acid glycoprotein gene via direct protein-protein interaction. Mol Cell Biol. 1993 Mar;13(3):1854–1862. doi: 10.1128/mcb.13.3.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordeen S. K., Green P. P., 3rd, Fowlkes D. M. A rapid, sensitive, and inexpensive assay for chloramphenicol acetyltransferase. DNA. 1987 Apr;6(2):173–178. doi: 10.1089/dna.1987.6.173. [DOI] [PubMed] [Google Scholar]
- Norton P. A., Coffin J. M. Bacterial beta-galactosidase as a marker of Rous sarcoma virus gene expression and replication. Mol Cell Biol. 1985 Feb;5(2):281–290. doi: 10.1128/mcb.5.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton P. A., Coffin J. M. Characterization of Rous sarcoma virus sequences essential for viral gene expression. J Virol. 1987 Apr;61(4):1171–1179. doi: 10.1128/jvi.61.4.1171-1179.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea E. K., Rutkowski R., Kim P. S. Evidence that the leucine zipper is a coiled coil. Science. 1989 Jan 27;243(4890):538–542. doi: 10.1126/science.2911757. [DOI] [PubMed] [Google Scholar]
- O'Shea E. K., Rutkowski R., Kim P. S. Mechanism of specificity in the Fos-Jun oncoprotein heterodimer. Cell. 1992 Feb 21;68(4):699–708. doi: 10.1016/0092-8674(92)90145-3. [DOI] [PubMed] [Google Scholar]
- Park E. A., Gurney A. L., Nizielski S. E., Hakimi P., Cao Z., Moorman A., Hanson R. W. Relative roles of CCAAT/enhancer-binding protein beta and cAMP regulatory element-binding protein in controlling transcription of the gene for phosphoenolpyruvate carboxykinase (GTP). J Biol Chem. 1993 Jan 5;268(1):613–619. [PubMed] [Google Scholar]
- Pei D. Q., Shih C. H. Transcriptional activation and repression by cellular DNA-binding protein C/EBP. J Virol. 1990 Apr;64(4):1517–1522. doi: 10.1128/jvi.64.4.1517-1522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli V., Mancini F. P., Cortese R. IL-6DBP, a nuclear protein involved in interleukin-6 signal transduction, defines a new family of leucine zipper proteins related to C/EBP. Cell. 1990 Nov 2;63(3):643–653. doi: 10.1016/0092-8674(90)90459-r. [DOI] [PubMed] [Google Scholar]
- Ramji D. P., Vitelli A., Tronche F., Cortese R., Ciliberto G. The two C/EBP isoforms, IL-6DBP/NF-IL6 and C/EBP delta/NF-IL6 beta, are induced by IL-6 to promote acute phase gene transcription via different mechanisms. Nucleic Acids Res. 1993 Jan 25;21(2):289–294. doi: 10.1093/nar/21.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehnmark S., Antonson P., Xanthopoulos K. G., Jacobsson A. Differential adrenergic regulation of C/EBP alpha and C/EBP beta in brown adipose tissue. FEBS Lett. 1993 Mar 8;318(3):235–241. doi: 10.1016/0014-5793(93)80519-z. [DOI] [PubMed] [Google Scholar]
- Roman C., Platero J. S., Shuman J., Calame K. Ig/EBP-1: a ubiquitously expressed immunoglobulin enhancer binding protein that is similar to C/EBP and heterodimerizes with C/EBP. Genes Dev. 1990 Aug;4(8):1404–1415. doi: 10.1101/gad.4.8.1404. [DOI] [PubMed] [Google Scholar]
- Ron D., Habener J. F. CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev. 1992 Mar;6(3):439–453. doi: 10.1101/gad.6.3.439. [DOI] [PubMed] [Google Scholar]
- Ruddell A., Linial M. L., Groudine M. Tissue-specific lability and expression of avian leukosis virus long terminal repeat enhancer-binding proteins. Mol Cell Biol. 1989 Dec;9(12):5660–5668. doi: 10.1128/mcb.9.12.5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddell A., Linial M., Schubach W., Groudine M. Lability of leukosis virus enhancer-binding proteins in avian hematopoeitic cells. J Virol. 1988 Aug;62(8):2728–2735. doi: 10.1128/jvi.62.8.2728-2735.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryden T. A., Beemon K. Avian retroviral long terminal repeats bind CCAAT/enhancer-binding protein. Mol Cell Biol. 1989 Mar;9(3):1155–1164. doi: 10.1128/mcb.9.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryden T. A., de Mars M., Beemon K. Mutation of the C/EBP binding sites in the Rous sarcoma virus long terminal repeat and gag enhancers. J Virol. 1993 May;67(5):2862–2870. doi: 10.1128/jvi.67.5.2862-2870.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
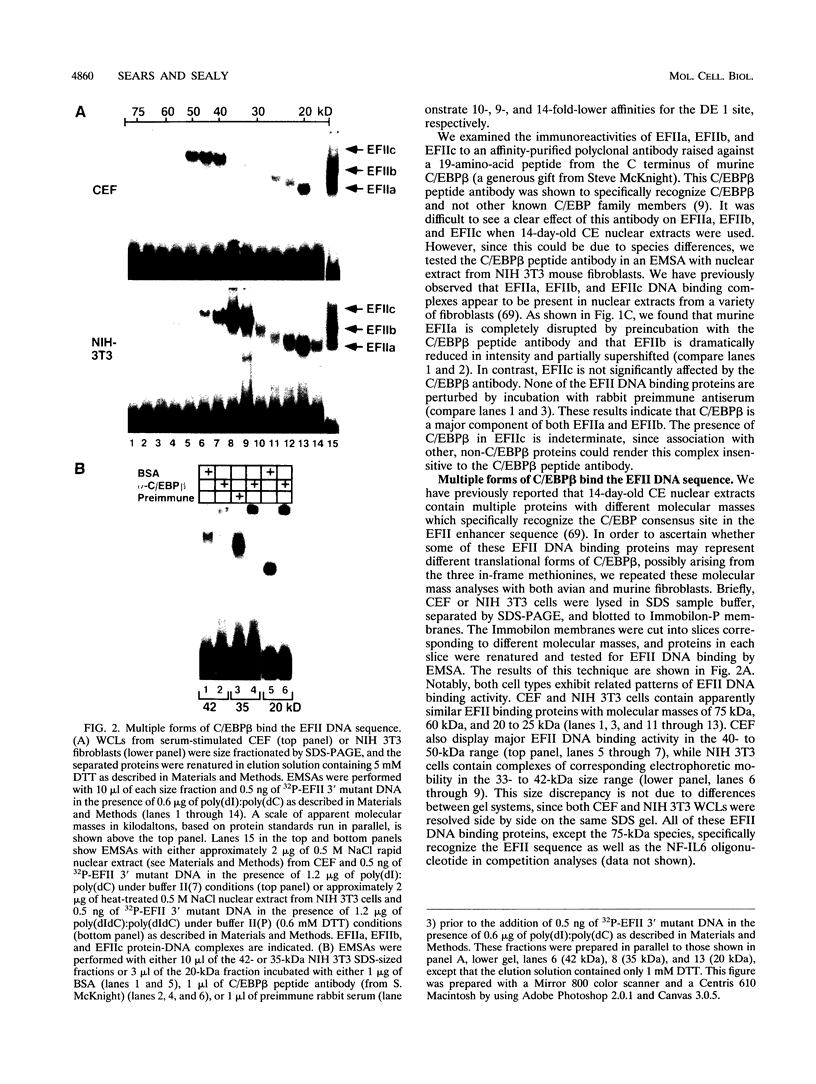
- Scott L. M., Civin C. I., Rorth P., Friedman A. D. A novel temporal expression pattern of three C/EBP family members in differentiating myelomonocytic cells. Blood. 1992 Oct 1;80(7):1725–1735. [PubMed] [Google Scholar]
- Sealey L., Chalkley R. At least two nuclear proteins bind specifically to the Rous sarcoma virus long terminal repeat enhancer. Mol Cell Biol. 1987 Feb;7(2):787–798. doi: 10.1128/mcb.7.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears R. C., Sealy L. Characterization of nuclear proteins that bind the EFII enhancer sequence in the Rous sarcoma virus long terminal repeat. J Virol. 1992 Nov;66(11):6338–6352. doi: 10.1128/jvi.66.11.6338-6352.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spergel J. M., Hsu W., Akira S., Thimmappaya B., Kishimoto T., Chen-Kiang S. NF-IL6, a member of the C/EBP family, regulates E1A-responsive promoters in the absence of E1A. J Virol. 1992 Feb;66(2):1021–1030. doi: 10.1128/jvi.66.2.1021-1030.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman B. M., Frank M., Green H. Molecular cloning of mRNA from 3T3 adipocytes. Regulation of mRNA content for glycerophosphate dehydrogenase and other differentiation-dependent proteins during adipocyte development. J Biol Chem. 1983 Aug 25;258(16):10083–10089. [PubMed] [Google Scholar]
- Stein B., Cogswell P. C., Baldwin A. S., Jr Functional and physical associations between NF-kappa B and C/EBP family members: a Rel domain-bZIP interaction. Mol Cell Biol. 1993 Jul;13(7):3964–3974. doi: 10.1128/mcb.13.7.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomassin H., Hamel D., Bernier D., Guertin M., Belanger L. Molecular cloning of two C/EBP-related proteins that bind to the promoter and the enhancer of the alpha 1-fetoprotein gene. Further analysis of C/EBP beta and C/EBP gamma. Nucleic Acids Res. 1992 Jun 25;20(12):3091–3098. doi: 10.1093/nar/20.12.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao B. P., Wang X. F., Peterson C. L., Calame K. In vivo functional analysis of in vitro protein binding sites in the immunoglobulin heavy chain enhancer. Nucleic Acids Res. 1988 Apr 25;16(8):3239–3253. doi: 10.1093/nar/16.8.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson C. R., Sigler P. B., McKnight S. L. Scissors-grip model for DNA recognition by a family of leucine zipper proteins. Science. 1989 Nov 17;246(4932):911–916. doi: 10.1126/science.2683088. [DOI] [PubMed] [Google Scholar]
- Weber F., Schaffner W. Enhancer activity correlates with the oncogenic potential of avian retroviruses. EMBO J. 1985 Apr;4(4):949–956. doi: 10.1002/j.1460-2075.1985.tb03723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner M., Cao Z., Rosenfeld M. G. Calcium-regulated phosphorylation within the leucine zipper of C/EBP beta. Science. 1992 Apr 17;256(5055):370–373. doi: 10.1126/science.256.5055.370. [DOI] [PubMed] [Google Scholar]
- Williams S. C., Cantwell C. A., Johnson P. F. A family of C/EBP-related proteins capable of forming covalently linked leucine zipper dimers in vitro. Genes Dev. 1991 Sep;5(9):1553–1567. doi: 10.1101/gad.5.9.1553. [DOI] [PubMed] [Google Scholar]
- Zachow K. R., Conklin K. F. CArG, CCAAT, and CCAAT-like protein binding sites in avian retrovirus long terminal repeat enhancers. J Virol. 1992 Apr;66(4):1959–1970. doi: 10.1128/jvi.66.4.1959-1970.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]