Abstract
Nitric oxide (NO) is involved in various physiological functions and its role in tumorigenesis has been well studied. A large majority of human and experimental tumors appear to progress owing to NO resulting from iNOS, further stimulated by proinflammatory cytokines. Conversely, in some cases, NO is associated with induction of apoptosis and tumor regression. This dichotomy of NO is largely explained by the complexity of signaling pathways in tumor cells, which respond to NO very differently depending on its concentration. In addition, NO alters many signaling pathways through chemical modifications, such as the addition of S-nitrosothiols and nitrosotyrosine to target proteins altering various biological pathways. Hence, iNOS inhibitors are designed and developed to inhibit various organ site cancers including the colon. Here, we review iNOS expression, generation of NO, involvement of NO in altering signaling pathways, and iNOS select inhibitors and their possible use for the prevention and treatment of various cancers.
Nitric oxide (NO) is one of the smallest signaling molecules that can diffuse into the cell [1]. It is present in almost all cells in the body, synthesized through several enzymatic and non-enzymatic pathways. As a free radical with complex redox chemistry, NO can modify all biological molecules and is, therefore, implicated in all biological functions in living systems. These pleiotropic biological actions are reflected in the publication of more than 117,000 articles on NO in the past 20 years. Out of this number, over 16,000 have been published on the role of NO in cancer. In spite of this extensive research in cancer, the role of NO in cancer is still ambiguous because NO is reported to be both anti- and pro-tumorigenic in animals and humans. The reason for these seemingly opposite effects is that: different types of cells respond differently to NO exposure the presence of varied levels of different forms of NOS in different tissues; and exogenous NO coming from the diet adds to the complexity of NO signaling [2].
NO is an important bioregulatory mediator involved in a variety of biological processes in both normal and pathophysiological conditions. NOS, particularly inducible NOS (iNOS or NOSII) and endothelial forms are overexpressed in various cancers in both humans and rodents (Table 1) [3–6]. NO can exert its effects directly by forming reactive nitrogen–oxygen species and indirectly by post-translational modifications of proteins via S-nitrosylation or tyrosine nitration [7,8]. Nitrosylation of proteins in key signaling pathways causes dysregulation of these pathways leading to disease formation [7,9]. Significant efforts have been made to address the iNOS/NO in tumor production and its effect on tumor biology. Before logically designing an effective preventive and therapeutic strategy to target tumor-associated iNOS/NO, one must clearly understand the mechanisms of iNOS expression and NO production and their actions in the tumor microenvironment, which have yet to be fully defined. There is growing interest in the cancer field to understand and target the effects of NO that play a key role in mediating tumorigenesis and metastases. In this review, the authors present some recent evidence on both the pro- and anti-tumor activities through direct and indirect effects of NO and will also discuss the implications of these data on the use of iNOS inhibitors in prevention of colorectal and other cancers.
Table 1.
Inducible NOS expression in various cancers.
| Organ | Expression of inducible NOS in | Ref. |
|---|---|---|
| Colon | Aberrant crypt foci | [5,6,32] |
| Adenoma | ||
| Adenocarcinoma | ||
|
| ||
| Breast | Invasive lesions | [38,39] |
| Tumors | ||
|
| ||
| Prostate | High-grade prostatic intraepithelial neoplasia | [40–44,50] |
| Cancer | ||
|
| ||
| Bladder | Dysplastic lesions | [45–48] |
| Carcinoma | ||
|
| ||
| Skin | Preneoplastic | [50] |
| Papillilomatous lesions | ||
|
| ||
| Esophageal | Malignant melanoma | [51] |
|
| ||
| Head and neck | Carcinoma | [49] |
NO generation & synthesis
NO is a free diatomic molecule formed from the nitrogen in the guanidine present in l-arginine, under the catalytic action of the NOS enzymes, generating equimolar concentrations of l-citrulline [10–12]. NO synthesis occurs from the activation of NOS, which exists as two isoforms: the constitutive and the inducible isoforms [13–15]. The constitutive isoforms (cNOS) were originally found in the endothelium and in neurons, thus, they were initially called endothelial NOS (eNOS) and neuronal NOS (nNOS), respectively. Both isoforms (eNOS and nNOS) are stimulated by a complex signaling pathway that is dependent or independent of Ca2+. Both eNOS and nNOS require an electron donor, the NADP+ and co-factors such as flavine-adenine-dinucleotide (FAD), flavine mononucleotide (FMN) and tetrahydrobiopterin (BH4) [15–17]. In humans and, presumably, in most other species, these isoforms are encoded by three different genes located in three different chromosomes [18]. iNOS is induced by pathological stimuli, such as bacterial lipopolysaccharide (LPS), cytokines, including IL-1, endotoxins and TNF, which is Ca2+-independent, this isoform can be expressed in a large variety of cell types, including monocytes, macrophages, lymphocytes, neutrophils, eosinophils, Kupffer cells, hepatocytes and epithelial cells. One of the main differences between cNOS and iNOS is that during inflammatory response iNOS is able to release large amounts of NO for relatively long periods of time in a sustained manner, which can generate some exaggerated effects, producing toxic, tumorigenic responses in the body, whereas cNOS produces small amounts of NO within seconds and is short acting [19–21]. Irrespective of these differences in NOS isoforms, all of them act as catalysts in the oxidation of the terminal nitrogen atom in l-arginine forming equimolar amounts of NO and l-citrulline.
Non-enzymatic generation of NO
Dietary sources of nitrate and nitrite are physio-logically recycled in blood and tissues forming NO and other bioactive nitrogen oxides. Therefore, they are now viewed as storage pools for NO-like bioactivity, thereby complementing the NOS-dependent pathway. Nitrate is further reduced to NO by various pathways under hypoxic and acidic conditions. Nitrate from dietary sources is reduced to nitrite with the help of commensal bacteria present in the intestines. It is absorbed by into the bloodstream and picked up by the salivary glands. It is then released into mouth through saliva and oral bacteria use it as a food source to generate nitrite [22]. Nitrite is reabsorbed into the bloodstream [23].
Some of the animal studies suggested that nitrate itself is not carcinogenic. Maekawa et al. administered sodium nitrite in the drinking-water for 2 years at levels of 0.125 or 0.25% and sodium nitrate in the diet at levels 2.5 or 5% to F344 rats and observed no carcinogenic effect of nitrate or nitrite [24]. However, it has been reported that exposure to higher levels of nitrates and nitrites has been associated with increased incidence of cancer in adults [25–31]. Thus, the evidence that nitrate can cause diseases is controversial. There is even an emerging evidence of a possible benefit of nitrate in cardiovascular health. Although nitrates as such may not be carcinogenic, in processed meat foods, due to the formation of N-nitroso compounds, they are carcinogenic. Individuals with increased rates of endogenous formation of carcinogenic N-nitroso compounds are likely to be susceptible to the development of cancers in the digestive system.
Association of iNOS with various cancers
Studies from humans and laboratory rodents show that iNOS has been associated with the development of cancers. Table 1 summarizes evidence in support of iNOS association during tumor development in humans and animals. Most of the studies emphasize the positive association with aggressive tumor promotional activities in almost all epithelial cancers.
Colon
Studies in both carcinogen-induced and genetic models support a role for iNOS in the promotion of colon carcinogenesis [5,6]. Increased iNOS expression and activity were observed in carcinogen-induced rat dysplastic aberrant crypt foci (ACF), adenomas and adenocarcinomas, but not in hyperplastic ACF [32]. The tumor-enhancing effects of iNOS in the colon may be associated with the ability of NO to increase the expression/activity of the enzyme COX-2 [33], likely via cross-talk between the iNOS and COX-2 signaling pathways [6]. In support of a role for iNOS in colon tumor promotion, mice from a ApcMin/+-iNOS-knockout genetic background showed decreased intestinal tumor formation [34]. In humans, iNOS expression is up-regulated in carcinomas compared with patient's normal-appearing colonic mucosa (Table 1) [35,36].
Breast
Studies largely support a role for iNOS in the promotion of breast neoplasia. Vakkala et al. reported that iNOS expression increases with ductal carcinoma in situ (DCIS) grade and further increases in invasive lesions; increased expression also correlates with increased tumor vascularization and apoptotic index (Table 1) [37–39].
Prostate
Upregulation of iNOS expression has consistently been reported in cancerous prostatic tissue compared with normal and adjacent normal-appearing tissue [40–43]. Expression in precancerous high-grade prostate intraepithelial neoplasia (PIN) and cancerous is also more intense than that in low-grade PIN and benign lesions, suggesting that up-regulation is associated with progression to malignancy (Table 1) [44].
Bladder
Increased iNOS expression also has been found consistently in bladder cancers [45–48]. In one study, all 94 transitional cell carcinomas examined exhibited some immunostaining for iNOS. All dysplastic lesions adjacent to carcinomas exhibited staining patterns similar to malignant tissue, suggesting that upregulation of iNOS is an early event during bladder carcinogenesis (Table 1) [46].
Skin
iNOS also is upregulated during progression of malignant melanoma. Although iNOS is absent from benign melanocytic nevi, its expression increases during progression from cutaneous melanoma in situ, to invasive melanomas, to subcutaneous metastases. Also, iNOS overexpression is reported in human skin squamous cell carcinoma [49].
Similarly, overexpression of iNOS was reported in head and neck [50], esophageal [51] and other cancers. Overall, there is strong evidence that most cancers overexpress iNOS and upregulate iNOS activity, justifying it as valid target for chemoprevention (Table 1).
NO-induced S-nitrosylation & its tumorigenic effects
NO diffuses easily into surrounding tissues and affects targets at different locations through covalent binding, resulting in direct chemical modifications such as addition of S-nitrosothiols and nitrosotyrosine residues. S-nitrosylation regulates numerous signaling pathways in intact cellular systems, and recent genetic evidence supports a diversity of regulatory roles for this protein-modification reaction [52–54]. Ions (Ca2+, Mg2+, H+) and O2-based modifications, which can cause changes in protein structure, have been shown to promote S-nitrosylation and/or denitrosylation [55–58], and they also play a significant role in specifying the sites of S-nitrosylation. S-nitrosylation has been reported to regulate the activity of a number of metabolic enzymes, oxidoreductases, proteases, protein kinases and phosphatases, both in vivo and in vitro, as well as respiratory proteins, receptors, ion channels and transporters, cytoskeletal and structural components, transcription factors and regulatory elements, including G proteins [59].
NO induces S-nitrosylation of the active site cysteines in caspases and other related proteins leading to inhibition of apoptosis and the formation of S-nitrosothiols leading to the oxidation of thiol proteins, which may act as switches in cells survival and apoptotic signaling pathways [60–62]. Nitrosylation of nucleic acid bases leads to conversion of cytosine to uracil and guanine [61]. NO also can inhibit DNA repair by nitrosylation of DNA repair enzymes [63–66]. This might be one of the key elements favoring the carcinogenesis process during inflammatory conditions. NO is known to stimulate the anti-apoptotic Akt pathway via activation of Ras [67]. NO can protect human colon cancer cells from apoptosis in vitro by scavenging superoxide radicals in mitochondria [68]. This results in the formation of peroxynitrate (ONOO-), which can inactivate iron–sulphur proteins and cause DNA damage. Furthermore, the oxidant products of peroxide can initiate NF-κB and AP1 activation leading to tumor cell proliferation and development. NO in nanomolar concentrations has been reported to activate Ras post-translational modification via S-nitrosylation of the critical CYS118 residue, which stimulates guanine nucleotide exchange [69]. Ras is overexpressed in many cancers including pancreas and colon cancers. It is possible that low concentrations of NO could amplify Ras signaling by inducing conformational changes of membrane-bound Ras proteins. The authors have previously shown that by using iNOS and COX-2 inhibitors, there is molecular synergism between NO and COX-2 pathways in colon cancer [5–6,70]. Furthermore, Kim et al. reported that an increase in PGE2 formation by iNOS activation is due to binding of iNOS to COX-2 to deliver NO in appropriate proximity for S-nitrosylation of COX-2, which, in turn, activates COX-2 formation of PGE2 [71]. This may create favorable inflammatory conditions for tumor development.
Estrogen stimulation of endothelial NO production is well established [72–74]. Estrogens are reported to stimulate dynamic endothelial protein S-NO via mechanisms linked to specific estrogen receptors (ERs; β/α), possibly on the plasma membrane, and endogenous production of NO. The authors have observed more iNOS expression in human colon cancer cell lines expressing ER-β [Janakiram NB, Rao CV et al., Unpublished Data]. Estrogen induced S-NO of proteins was blocked by both ERβ and ERα antagonists, suggests a potential role of estrogens in enhancing S-nitrosylation of proteins in cancer [75]. This observation of the authors and others suggest that estrogen may play a role in altering signal mechanisms through NO. These studies open a novel avenue for investigations on the effects of estrogens and their receptors on nitrosylation and the effects on colon cancer cell proliferation.
Anti-tumorigenic properties of NO
Several reports suggest that NO can exert pro-apoptotic effects. A positive role of S-nitration in killing the cancer cells was reported by Leon-Bollotte et al. [76], post-translational modifications (S-nitrosylation of cysteine residues 199 and 304) in the cytoplasmic domain of Fas occurs in colon and mammary cancer cells and this leads to enhanced apoptosis; S-nitrosylation at CYS-304 promotes redistribution of Fas to lipid rafts, formation of the death-inducing signal complex, and induction of cell death in these cancer cells. Hussain et al. provided genetic and mechanistic evidence that NO can suppress tumorigenesis in transgenic mice [77].
Hussain et al. reported that p53, a tumor suppressor protein, accumulates upon NO mediated DNA damage and growth arrest [78]. NO inhibits the activity of NF-κB by stabilizing its inhibitor, Iκ-Bα. NF-κB is a constituent of one of the anti-apoptotic pathways observed to be overactivated during tumorigenesis [79]. NO is also reported to inhibit NF-κB by nitrosylating its p50 subunit, thus, inhibiting its binding to DNA [80]. In the ApcMin/+ colon cancer mouse model, deletion of iNOS (iNOS−/− mice) has been found to promote intestinal tumorigenesis, thereby substantiating a role for iNOS in host defense mechanisms [81]. This report is contradictory to the finding by Ahn et al. [34]. The reasons for opposite results in these animal models may be due to the type of diets given and the conditions in which the mice were harbored. These results simulate the conditions seen with human patients and further confirm the dual role of NO in a given situation. Evidence also suggests that NO regulates cell proliferation at the translational level. NO causes a cell cycle arrest through inhibition of cyclin D1 protein. Ambs et al. reported an increased concentration of p21 in tumors of iNOS expressing LoVo cells [82]. Ropponen et al. suggest that high iNOS staining intensity and percentage distribution in tumor epithelium may have a protective role against colorectal cancer progression in humans [83]. They reported that iNOS expression correlated positively with cell cycle regulators p21 and AP-2.
Concentration-dependent effects of NO on tumor growth
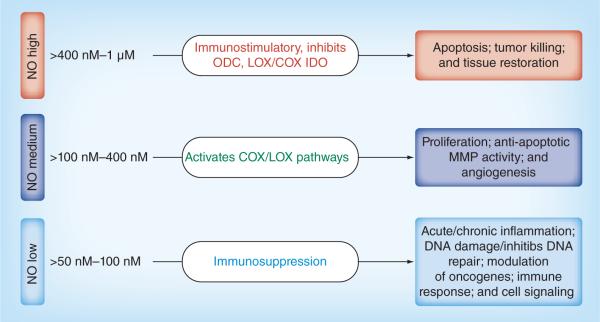
NO affects diverse signaling pathways and has opposing biological effects under different contexts and at concentrations (Figure 1). Low physio logical concentrations of NO are implicated in various different processes of tumorigenesis. Low concentrations of NO can stimulate cell growth and protect many cell types from apoptosis, whereas high concentrations of NO can inhibit cell growth and induce apoptosis [84]. Clinical and preclinical reports by the authors and others have shown a positive relationship between high expression of iNOS and tumorigenesis in colon tumors [5,6]. Solid tumors exhibited sustained levels of NO that may be produced by infiltrating cells such as monocytes, macrophages and fibroblasts. Some clinical studies have shown that iNOS increases significantly in colon adenoma and carcinoma with little or no expression in normal colon tissue [3,35,36], whereas other studies report that iNOS expression is decreased in colon cancer compared with high expression in normal colon tissue [85–88]. In animal models, induction of iNOS is correlated with colorectal cancer regression (both in situ and metastatic) [89,90], whereas the authors found that NOS inhibitors prevent colonic aberrant crypt foci formation and adenocarcinomas in azoxy methane (AOM)-induced rats (Table 2) [5,6,70,91]. NO also is shown either, to promote or to inhibit growth of colorectal cancer cells [92,93] and the effect was suggested to be concentration dependent [94]. Two separate groups recently reported opposite effects of iNOS gene knockouts in mice on intestinal carcinogenesis [3,95].
Figure 1. Pleiotropic effects of different levels of nitric oxide on inflammation and tissue response.
NO: Nitric oxide.
Table 2.
Effect of inducible NOS inhibitors on aberrant crypt foci/tumors inhibition in different animal cancer models.
| Organ site | iNOS inhibitor | % inhibition | Ref. |
|---|---|---|---|
| Colon cancer | |||
| Rat (AOM)- aberrant crypt foci | PBIT | 78 (4/ > crypts Foci) | [5] |
| PBIT-Se | 47 (4/ > crypts Foci) | [125] | |
| AG1(high dose) | 48 (4/ > crypts Foci) | [6] | |
| SC51 (high dose) | 52 (4/ > crypts Foci) | [6] | |
| SC51+celecoxib (low-dose combination) | 41 (4/ > crypts Foci) | [6] | |
|
| |||
| Rat (AOM) adenocarcinoma | PBIT | 43 – incidence | [126] |
| 60 – multiplicity | |||
| 70 – incidence | [126] | ||
| 83 – multiplicity | |||
| PBIT and celecoxib | 40 – incidence | [126] | |
| 46 – multiplicity | |||
| NILT | 40 – incidence | [70] | |
| Adenocarcinoma | 60 – multiplicity | [70] | |
| Invasive adenocarcinoma | 67 – multiplicity | [70] | |
| NILT + Celecoxib | 70 – incidence | [70] | |
| Adenocarcinomas | 77 – multiplicity | ||
|
| |||
| Mouse (ApcMin/+-iNOS−/−) | AG | 29 – incidence | [34] |
| 7 – multiplicity | |||
|
| |||
| Mouse (ApcMin/+) high-fat diet | PBIT | >80 | [127] |
|
| |||
| Mouse (xenograft with iNOS-expressing human colon tumor) | 1400W | Promoting effect | [128] |
|
| |||
| Mouse (iNOS-expressing intra-tumoral-macrophages) | 1400W | No effect | [128] |
| Gastric cancer | |||
| Mice (Xenografts) | Aminoguanidine | 35–47 | [129] |
| Mammary cancer | |||
| Mouse (iNOS-expressing adenocarcinoma) | 1400W | 49–59 | [128] |
| Liver cancer | |||
| Mice (HepG2 tumors) | Cavtratin | 52 | [119] |
| Lung cancer | |||
| Mice (Lewis lung cancer tumors) | Cavtratin | 38 | [119] |
| Esophageal cancer | |||
| Rat (nitrosomethylbenzylamine) | PBIT | 77–83 – incidence | [124] |
| 50–59 – multiplicity | |||
| Head and neck | |||
| Rabbit (human tumor specimens, A-431 cells) | l-NMMA | Reduced angiogenesis/invasion | [129] |
| Melanoma | |||
| Mice (xenografts with B16F1 and B16F10) | l-NMMA | Reduced angiogenesis/invasion | [122] |
AG: Aminoguanidine; AOM: Azoxymethane; iNOS: Inducible NOS; NILT: N6-iminoethyl-lysine tetrazoleamide; NMMA: N-Monomethyl-l-arginine; PBIT: S,S'-1,4-phenylenebis(1,2-ethanediyl)bis-isothiourea.
Liu et al. observed growth inhibitory activity at higher concentrations of GSNO, an NO-releasing compound. At low doses, NO exposure for 8 days with drug replenishment every other day caused cytostasis of Caco-2 cells by inhibition of ODC activity [97]. ODC is an important enzyme in polyamine synthesis, which is expressed highly in colon cancer and polyamines help in increased proliferation of tumor cells. This report suggests that a low dose of NO is an inhibitor of ODC and proliferation of tumor cells. In non-tumorigenic, non-transformed colon cells, the NO donors SNAP and NOR-1 increased both COX-2 mRNA transcription and protein synthesis [98]. Liu et al. reported that GSNO induced both COX-1 and -2 protein expression and stimulated PGE2 production in a dose- and time-dependent manner in three colon cancer cell lines [96]. Jenkins et al. reported that NO slowed down the growth of DLD-1, a colon adeno carcinoma cell line, in vitro but accelerated its cell growth in vivo [99]. However, micro encapsulated iNOS-expressing cells (human fetal kidney cell line EcR293) could inhibit DLD-1 cell growth in vivo (xenografts) and this was ascribed to the difference in iNOS activity in the cells [94,93]. It is possible that high levels of iNOS expression may be cytostatic/cytotoxic for tumor cells; lower activity can have the opposite effect, promoting tumor growth and neovascularization (Figure 1).
iNOS selective inhibitors & NO-releasing donors in cancer
NOS activity was increased in AOM-induced colonic tumors in rats [5,6,100] and NO and NOS are increased in Crohn's disease [101] and ulcerative colitis [102]. NO is overexpressed in preneoplastic lesions of the colon [103] and in human adenocarcinomas of the colon [104–106]. Wan et al. found high iNOS levels in the colon when animals were fed a high-fat diet and it is well known that a high-fat diet is associated with colon cancer in humans [107]. The many reports indicating high NOS activity and a positive role of NO in colon cancer support the testing of structural analogues of iNOS substrates and iNOS inhibitors to suppress/inhibit the induction of iNOS and to reduce its tumorigenic effects.
l-arginine structural analogues (Table 3) have been in use to inhibit tumor growth and proliferation. l-nitroarginine methyl ester (l-NAME) is an irreversible inhibitor of brain constitutive NOS (both nNOS and eNOS) with reversible effects on macrophage iNOS [108]. Kawamori et al. examined its influence on the development of ACF induced by AOM at a dose of 15 mg/kg once a week for 2 weeks in rats [91]. Dietary administration of 100 ppm of l-NAME for 11 weeks inhibited the development of ACF by 32% in terms of multiplicity (Table 2) [91]. The results are in line with the authors' study, which showed that S,S-1,4-phenylene-bis(1,2-ethanediyl)bis-isothiourea, a selective iNOS inhibitor, suppressed the development of ACF induced by AOM in rats and reduced protein levels of COX-2 and iNOS in colonic mucosa (Table 2) [5]. A similar observation was observed with Se-PBIT on ACFs induced by AOM in rats (Table 2). We have extensively studied the role of iNOS in molecular mechanisms of colon carcinogenesis [6,109].
Table 3.
List of induced NOS inhibitors and their selectivity at a particular concentration for different NOS.
| Arginine analogues | Neuronal NOS | Found in |
|---|---|---|
| l-arginine | 1.6 μM | Rat |
|
| ||
| l-canavanine | 60 μM | Murine |
|
| ||
| l-NAMA | 0.18 μM | Rat |
|
| ||
| l-NNA | 15 nM | Bovine |
| N6, NG-dimethyl-l-arginine | ||
| l-NIL | 35 μM | Human |
| 92 μM | Rat | |
|
| ||
| NILT | 1850 μM | Human |
|
| ||
| l-NIO | 1.7 μM | Rat |
|
| ||
| Vinyl-l-NIO | 100 nM | Rat |
|
| ||
| AMT | 34 μM | Rat |
|
| ||
| l-thiocitrulline | 0.06 μM | Rat |
|
| ||
| S-methyl-l-thiocitrulline | 50 nM | Rat |
| 1.2 nM | Human | |
|
| ||
| N-propyl-l-arginine | 57 nM | Bovine |
|
| ||
| 1400W | 2 μM | Human |
| Urea/guanidine derivatives | ||
| A-guanidinoglutaric acid | 2.7 μM | Rat |
|
| ||
| S-(2-aminoethyl)isothiourea | 1.8 μM | Human |
| EIT hydrobromide | ||
| AMT | 34 nM | Rat |
|
| ||
| Mercaptoethylguanidine | 60 μm | Rat |
|
| ||
| S-ethylisothiourea | 29 nM | Human |
| 250 nM | Rat | |
|
| ||
| S-ethyl-N-[4-(trifuromethyl)phenyl] isothiourea | 0.32 μM | Human |
|
| ||
| S-isopropylisothiourea | 37 nM | Human |
|
| ||
| S-methylisothiourea | 0.16 μM | Human |
|
| ||
| 1,3-PBIT | 0.25 μM | Human |
|
| ||
| 1,4-PBIT | 16 nM | Human |
|
| ||
| Aminoguanidine | 160 μM | Rat |
| Other induced NOS inhibitors | ||
| 2-imi no-4-methylpiperidine | 0.2 μM | |
|
| ||
| TRIM | 28.2 μM | Murine |
|
| ||
| 7-nitroindazole | 0.7 μM | Murine |
|
| ||
| 3-bromo-7-nitroindazole | 0.17 μM | Rat |
|
| ||
| BYK 191023 dihydrochloride | 17000 nM | |
AMT: 2-amino-5,6-dihydro-6-methyl-4H-l,3-thiazine; l-NIL: l-N6-(1-iminoethyl)lysine dihydrochloride; l-NIO: N(5)-(-iminoethyl)-l-ornithine; l-NNA: Nω-nitro-l-arginine; NILT: N6-iminoethyl-lysine tetrazoleamide; PBIT: S,S'-1,4-phenylenebis(1,2-ethanediyl)bis-isothiourea; TRIM: 1-[2-trifluoromethylphenyl] imidazole.
Previously, we reported that low-dose combination of a COX-2 inhibitor (celecoxib) and an iNOS inhibitor (SC-51) inhibited AOM-induced crypt formation in rats (Table2) [6]. Cianchi et al. also reported that iNOS and COX-2 are co-expressed within same cancer cells and iNOS levels and PGE2 production correlative [110]. These data indicate that iNOS expression may correlate with increased COX-2 expression in cancer cells, which is similar to the observation in inflammatory conditions, hence, this effect may play a pivotal role in colon tumorigenesis [111]. It is likely that iNOS is associated with the modulation of COX-2 activity in colon cancer. NO enhances the activity and expression of COX-2 in a variety of cell types [112]. Overall, these data suggest that the rate of development of adenoma, adenocarcinoma [91,113] and adenomatous polyps [34] is significantly decreased with l-NAME and iNOS-specific inhibitors (Table 2).
NO has been reported to be involved in invasion and metastatic spread of tumors. The signaling cascades involved in the initiation of migratory behavior have been noted to be NO dependent. For example, colon tumors that are exposed to an iNOS inhibitor showed decreased potential for invasion, with decreased expression of VEGF [82]. Ridnour et al. reported the MMP9 is a key physiologic mediator of the effects of NO. MMPs are associated with cancer progression and usually high expressions of MMPs are observed in cancers [114]. Table 3 summarises various iNOS inhibitors and their IC50 values in human and animal cells [115]. Conversely, as discussed above, NO can also be anti-tumorigenic. Hence, chemopreventive interventions were created to develop NO-release compounds in colon cancer. The NO-releasing agent DETONONOate sensitized SW620 metastatic colon cancer cells to pro-apoptotic treatments [116]. The NO donor sodium nitroprusside induced caspases and reduced Bcl2 expression. Hence, these NO donors are being developed to sensitize or enhance apoptosis of cancer cells. NO drug molecules tagged to known nonsteroidal anti-inflammatory drugs (NSAIDs) as NO–NSAIDs, such as NO–aspirin, NO–sulindac, NO–naproxen, NO–ibuprofen are well studied. These agents are being tested in vitro and in vivo for growth inhibition in colon cancer [117]. The mode of action of some of these agents is reported to be through S-nitrosylation and tyrosine nitration of important signaling proteins, such as p53, NF-κB and Wnt signaling proteins in human colon cancer cells [118].
iNOS inhibitors are studied in detail in colon cancer, whereas very little studies are available on use of iNOS inhibitors in other cancers. The following available studies have suggested the usefulness of iNOS inhibitors in other cancers. NOS inhibitors (l-NAME, l-NMMA, cavtratin) showed decreased angiogenesis and tumor growth:
-
■
In murine melanomas;
-
■
By inhibition of eNOS in murine lungs;
-
■
By inhibition of iNOS in breast tumors;
-
■
By NOS inhibition in human head and neck tumors;
-
■
By inhibition of e-NOS and iNOS in hepatoma by xenografts (Table 2) [119–121].
In another mouse study, iNOS deficiency decreased pulmonary metastases, impaired angiogenesis, and suppressed pleural effusion of injected murine melanoma cells [122]. Aminoguanidine caused statistically significant reduced proliferation and increased apoptosis in a xenograft model with injected gastric cancer cells compared with untreated animals [123]. S,S′-1,4-phenylenebis(1,2-ethanediyl)bis-isothiourea PBIT reduced the production of NO in nitrosomethylbenzylamine (NMBA)-induced preneoplastic and papillomatous esophageal lesions when compared with comparable lesions in rats treated with NMBA only [124]. PBIT caused a statistically significant reduction in tumor incidence and multiplicity in rats fed PBIT in an NMBA-induced rat model of esophageal cancer (Table 2) [124].
Conclusion & future perspective
NO plays an important role in growth, immunity and development. The role of NO in cancer is multifactorial based on its temporal and spatial concentrations. Various iNOS inhibitors have been under development for colon cancer, individually or in combination with COX-2 inhibitors for better efficacy. Simultaneous inhibition of external and internal sources of NO can prove beneficial in suppressing tumor formation. The number of factors affecting NO generation, including types of NOS present and the complex concentration-dependent actions, make the design of specific inhibitors for use in colon cancer challenging. Post-translational modification of proteins by NO, such as protein nitrosylation, has emerged as an important mechanism for regulation of the activity and function of key proteins in various signaling pathways. Identification and targeting of specific nitrosylated proteins in key pathways affecting tumor initiation, growth and metastasis may provide a better approach to tackle in colon and other cancers. Although the most desirable animal models in colon cancer, such as AOM-induced colon cancer in F344 rats, ApcMin/+ mice are available, opt preclinical models that simulate initiation and development of other human cancers, as well as methods to evaluate the role of NO and its inhibition during initiation, or development of cancer are needed. Continuous investigation into the biology of NO at initiation or early development of cancers will improve our knowledge on its signaling functions and may help in development of more appropriate agents to prevent colon and other cancers.
Executive summary.
Background
-
■
NO is synthesized by NOS. Specifically, inducible NOS (iNOS) is implicated in various cancers. NO can cause protein post-translational modifications leading to deregulation of signaling pathways causing diseases. Both pro- and anti-tumorigenic effects of NO are reported.
NO generation & synthesis
-
■
NO is generated by the action of NOS enzymes on l-arginine.
Non-enzymatic generation of NO
-
■
Inorganic nitrate from dietary sources is another major source of NO, independent of the arginine-dependent NOS pathway.
Association of iNOS with various cancers
-
■
High iNOS expression is reported in almost all epithelial cancers, suggesting its tumorigenic potential. iNOS expressions were observed at preneoplastic or low grade lesion stages in the mentioned epithelial cancers justifying it as a valid target for chemoprevention.
NO induced S-nitrosylation & its tumorigenic effects
-
■
NO can produce reactive nitrogen species that cause post-translational modification of proteins, such as enzymes, receptors, respiratory proteins and ion channels, altering their functions. This leads to anti-apoptotic signaling and helps in tumor development.
Anti-tumorigenic properties of NO
-
■
NO mediates growth arrest on tumor cells. Also, S-nitrosylation of proteins causes pro-apoptotic effects of tumor cells.
Concentration-dependent effects of NO on tumor growth
-
■
NO is observed to possess both pro- and anti-tumorigenic properties. This biphasic nature of NO is attributed to its concentrations effects on tumor growth or inhibition.
iNOS-selective inhibitors & NO-releasing donors in cancer
-
■
As it is evident that iNOS is expressed at preneoplastic lesion stages, use of specific iNOS inhibitors at early stages in epithelial cancers such as colon cancer inhibited these early lesions and also invasive adenocarcinomas. Also, NO-releasing donors have shown anti-tumorigenic efficacy in in vivo and in vitro cancer models.
Conclusion & future perspective
-
■
Expression of iNOS, concentrations of NO, time of NO exposure, region of exposure, are important factors that determine NO functions. Also, understanding the above effects of NO and role of nitrosylated proteins in a tumor cell or in tumor environment may help in better designing of preventive inhibitors for colon and other epithelial cancers.
Acknowledgements
The authors wish to thank J Sando for her valuable suggestions and editorial help.
Funds are partly derived from NIH/NCI-R01-CA-109247 and Kerley-Cade chair Endowment. No writing assistance was utilized in the production of this manuscript.
Footnotes
Financial & competing interests disclosure The authors have other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as:
■ of interest
- 1.Moller MN, Lancaster JR, Denicola A., Jr The interaction of reactive oxygen and nitrogen species with membranes. Curr. Top. Membr. 2008;61:23–42. [Google Scholar]
- 2.Fukumura D, Kashiwagi S, Jain RK. The role of nitric oxide in tumour progression. Nat. Rev. Cancer. 2006;6:521–534. doi: 10.1038/nrc1910. [DOI] [PubMed] [Google Scholar]
- 3.Ambs S, Merriam WG, Bennett WP, et al. Frequent nitric oxide synthase-2 expression in human colon adenomas: implication for tumor angiogenesis and colon cancer progression. Cancer Res. 1998;58:334–341. [PubMed] [Google Scholar]; ■ Demonstrates expression of VEGF and inducible NOS (iNOS) expression in human colon adenomas and may contribute to colon cancer progression from adenoma to carcinoma.
- 4.Takahashi M, Fukuda K, Ohata T, Sugimura T, Wakabayashi K. Increased expression of inducible and endothelial nitric oxide synthases in rat colon tumors induced by azoxymethane. Cancer Res. 1997;57:1233–1237. [PubMed] [Google Scholar]
- 5.Rao CV, Kawamori T, Hamid R, Reddy BS. Chemoprevention of colonic aberrant crypt foci by an inducible nitric oxide synthase-selective inhibitor. Carcinogenesis. 1999;20:641–644. doi: 10.1093/carcin/20.4.641. [DOI] [PubMed] [Google Scholar]
- 6.Rao CV, Cooma I, Simi B, Manning PT, Connor JR, Reddy BS. Chemopreventive properties of a selective inducible nitric oxide synthase inhibitor in colon carcinogenesis, administered alone or in combination with celecoxib, a selective cyclooxygenase-2 inhibitor. Cancer Res. 2002;62:165–170. [PubMed] [Google Scholar]; ■ Demonstration of decreased azoxy methane-induced preneoplastic lesions in F344 rats by low dose combination of iNOS-specific inhibitor SC-51 and COX-2 inhibitor celecoxib.
- 7.Gow AJ, Chen Q, Hess DT, Day BJ, Ischiropoulos H, Stamler JS. Basal and stimulated protein S-nitrosylation in multiple cell types and tissues. J. Biol. Chem. 2002;277:9637–9640. doi: 10.1074/jbc.C100746200. [DOI] [PubMed] [Google Scholar]
- 8.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat. Rev. Mol. Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 9.Foster MW, McMahon TJ, Stamler JS. S-nitrosylation in health and disease. Trends Mol. Med. 2003;9:160–168. doi: 10.1016/s1471-4914(03)00028-5. [DOI] [PubMed] [Google Scholar]
- 10.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology pathophysiology and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 11.Cohen RA, Vanhoutte PM. Endothelium-dependent hyperpolarization – beyond nitric oxide and cyclic GMP. Circulation. 1995;92:3337–3349. doi: 10.1161/01.cir.92.11.3337. [DOI] [PubMed] [Google Scholar]
- 12.Lehninger A. Principles of Biochemistry (3rd Edition) Sarvier; São Paulo, Brazil: 2002. [Google Scholar]
- 13.Michel T, Feron O. Nitric oxide synthases: which, where, how, and why? J. Clin. Invest. 1997;100(9):2146–2152. doi: 10.1172/JCI119750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmer RMJ, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 15.Bredt DS, Hwang PM, Glatt CE, Lowenstein C, Reed RR, Snyder SH. Cloned and expression nitric oxide synthase structurally resembles cytocrome P-450 reductase. Nature. 1991;351:714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- 16.Crane BR, Arvai AS, Gachhui R, et al. The structure of nitric oxide synthase oxygenase domain and inhibitor complexes. Science. 1997;278(5337):425–431. doi: 10.1126/science.278.5337.425. [DOI] [PubMed] [Google Scholar]
- 17.Crane BR, Arvai AS, Ghosh DK, et al. Structure of nitric oxide synthase oxygenase dimer with pterin and substrate. Science. 1998;279(5359):2121–2126. doi: 10.1126/science.279.5359.2121. [DOI] [PubMed] [Google Scholar]
- 18.Bruckdorfer R. The basics about nitric oxide. Mol. Aspects Med. 2005;26:3–31. doi: 10.1016/j.mam.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Schulz R, Triggle CR. Role of NO in vascular smooth muscle and cardiac muscle function. Trends Pharmacol. Sci. 1994;15:255–259. doi: 10.1016/0165-6147(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 20.Dominiczack AF, Bohr DF. Nitric oxide and its putative role in hypertension. Hypertension. 1995;25:1202–1211. doi: 10.1161/01.hyp.25.6.1202. [DOI] [PubMed] [Google Scholar]
- 21.Tibiriça E. Fisiopatologia em Medicina Cardiovascular. Revinter; Rio de Janeiro, Brazil: 2001. [Google Scholar]
- 22.Govoni M, Jansson EA, Weitzberg E, Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide. 2008;19(4):333–337. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]; ■ Demonstrates non-enzymatic generation of nitric oxide (NO) through dietary sources.
- 23.Cosby K, Partovi KS, Crawford JH, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003;9(12):1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 24.Maekawa A, Ogiu T, Onodera H, et al. Carcinogenicity studies of sodium nitrite andsodium nitrate in F-344 rats. Food Chem. Toxicol. 1982;20(1):25–33. doi: 10.1016/s0278-6915(82)80005-7. [DOI] [PubMed] [Google Scholar]
- 25.Preston-Martin S. N-Nitroso compounds and childhood brain tumors: a case-control study. Cancer Res. 1982;42(12):5240–5245. [PubMed] [Google Scholar]
- 26.Preston-Martin S. Maternal consumption of cured meats and vitamins in relation to pediatric brain tumors. Cancer Epidemiol. Biomarkers Prev. 1996;5(8):599–605. [PubMed] [Google Scholar]
- 27.Ward MH. Dietary exposure to nitrite and nitrosamines and risk of nasopharyngeal carcinoma in Taiwan. Int. J. Cancer. 2000;86(5):603–609. doi: 10.1002/(sici)1097-0215(20000601)86:5<603::aid-ijc1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 28.Pogoda JM, Preston-Martin S. Maternal cured meat consumption during pregnancy and risk of paediatric brain tumour in offspring: potentially harmful levels of intake. Public Health Nutr. 2001;4(2):183–189. doi: 10.1079/phn200060. [DOI] [PubMed] [Google Scholar]
- 29.Sarasua S, Savitz DA. Cured and broiled meat consumption in relation to childhood cancer: Denver, Colorado (United States) Cancer Causes Control. 1994;5(2):141–148. doi: 10.1007/BF01830260. [DOI] [PubMed] [Google Scholar]
- 30.McCredie M. Antenatal risk factors for malignant brain tumors in New South Wales children. Int. J. Cancer. 1994;56(1):6–10. doi: 10.1002/ijc.2910560103. [DOI] [PubMed] [Google Scholar]
- 31.Volkmer BG. Influence of nitrate levels in drinking water on urological malignancies: a community-based cohort study. Br. J. Urol. Int. 2005;95(7):972–976. doi: 10.1111/j.1464-410X.2005.05450.x. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi M, Mutoh M, Kawamori T, Sugimura T, Wakabayashi K. Altered expression of β-catenin, inducible nitric oxide synthase and cyclooxygenase-2 in azoxymethane-induced rat colon carcinogenesis. Carcinogenesis. 2000;21:1319–1327. [PubMed] [Google Scholar]
- 33.Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J. Natl Cancer Inst. 2002;94:252–266. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- 34.Ahn B, Ohshima H. Suppression of intestinal polyposis in Apc(Min/+) mice by inhibiting nitric oxide production. Cancer Res. 2001;61:8357–8360. [PubMed] [Google Scholar]; ■ Demonstrates the tumorigenic role of nitric oxide in transgenic ApcMin/+ mice model.
- 35.Kojima M, Morisaki T, Tsukahara Y, et al. Nitric oxide synthase expression and nitric oxide production in human colon carcinoma tissue. J. Surg. Oncol. 1999;70:222–229. doi: 10.1002/(sici)1096-9098(199904)70:4<222::aid-jso5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 36.Yagihashi N, Kasajima H, Sugai S, et al. Increased in situ expression of nitric oxide synthase in human colorectal cancer. Virch. Arch. 2000;436:109–114. doi: 10.1007/pl00008208. [DOI] [PubMed] [Google Scholar]
- 37.Vakkala M, Kahlos K, Lakari E, Paakko P, Kinnula V, Soini Y. Inducible nitric oxide synthase expression, apoptosis, and angiogenesis in in situ and invasive breast carcinomas. Clin. Cancer Res. 2000;6:2408–2416. [PubMed] [Google Scholar]
- 38.Tschugguel W, Schneeberger C, Unfried G, et al. Expression of inducible nitric oxide synthase in human breast cancer depends on tumor grade. Breast Cancer Res. Treat. 1999;56:145–151. doi: 10.1023/a:1006288526311. [DOI] [PubMed] [Google Scholar]
- 39.De Paepe B, Verstraeten VLRM, De Potter CR, Bullock GR. Increased angiotensin II type-2 receptor density in hyperplasia, DCIS and invasive carcinoma of the breast is paralleled with increased iNOS expression. Histochem. Cell Biol. 2002;117:13–19. doi: 10.1007/s00418-001-0356-0. [DOI] [PubMed] [Google Scholar]
- 40.Aaltomaa SH, Lipponen PK, Kosma V-M. Inducible nitric oxide synthase (iNOS) expression and its prognostic value in prostate cancer. Anticancer Res. 2001;21:3101–3106. [PubMed] [Google Scholar]
- 41.Uotila P, Valve E, Martikainen P, Nevalainen M, Nurmi M, Harkonen P. Increased expression of cyclooxygenase-2 and nitric oxide synthase-2 in human prostate cancer. Urol. Res. 2001;29:23–28. doi: 10.1007/s002400000148. [DOI] [PubMed] [Google Scholar]
- 42.Aaltomaa SH, Lipponen PK, Viitanen J, Kankkunen J-P, Ala-Opas MY, Kosma V-M. The prognostic value of inducible nitric oxide synthase in local prostate cancer. Br. J. Urol. Int. 2000;86:234–239. doi: 10.1046/j.1464-410x.2000.00787.x. [DOI] [PubMed] [Google Scholar]
- 43.Klotz T, Bloch W, Volberg C, Engelmann U, Addicks K. Selective expression of inducible nitric oxide synthase in human prostate carcinoma. Cancer. 1998;82:1897–1903. [PubMed] [Google Scholar]
- 44.Baltaci S, Orhan D, Gogus C, Turkolmez K, Tulunay O, Gogus O. Inducible nitric oxide synthase expression in benign prostatic hyperplasia, low- and high-grade prostatic intraepithelial neoplasia and prostatic carcinoma. Br. J. Urol. Int. 2001;88:100–103. doi: 10.1046/j.1464-410x.2001.02231.x. [DOI] [PubMed] [Google Scholar]
- 45.Swana HS, Smith SD, Perrotta PL, Saito N, Wheeler MA, Weiss RM. Inducible nitric oxide synthase with transitional cell carcinoma of the bladder. J. Urol. 1999;161:630–634. [PubMed] [Google Scholar]
- 46.Hayashi H, Kuwahara M, Fujisaki N, Furihata M, Ohtsuki Y, Kagawa S. Immunohistochemical findings of nitric oxide synthase expression in urothelial transitional cell carcinoma including dysplasia. Oncol. Rep. 2001;8:1275–1279. doi: 10.3892/or.8.6.1275. [DOI] [PubMed] [Google Scholar]
- 47.Wolf H, Haeckel C, Roessner A. Inducible nitric oxide synthase expression in human urinary bladder cancer. Virch. Arch. 2000;437:662–666. doi: 10.1007/s004280000296. [DOI] [PubMed] [Google Scholar]
- 48.Klotz T, Bloch W, Jacobs G, Niggemann S, Engelmann U, Addicks K. Immunolocalization of inducible and constitutive nitric oxide synthases in human bladder cancer. Urology. 1999;54:416–419. doi: 10.1016/s0090-4295(99)00212-5. [DOI] [PubMed] [Google Scholar]
- 49.Brennan PA, Conroy B, Spedding AV. Expression of inducible nitric oxide synthase and p53 in oral epithelial dysplasia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2000;90:624–629. doi: 10.1067/moe.2000.108800. [DOI] [PubMed] [Google Scholar]
- 50.Brennan PA, Umar T, Smith GI, Lo CH, Tant S. Expression of nitric oxide synthase-2 in cutaneous squamous cell carcinoma of the head and neck. Br. J. Oral Maxillofac. Surg. 2002;40:191–194. doi: 10.1054/bjom.2001.0680. [DOI] [PubMed] [Google Scholar]
- 51.Wilson KT, Fu S, Ramanujam KS, Meltzer SJ. Increased expression of inducible nitric oxide synthase and cyclooxygenase-2 in Barrett's esophagus and associated adenocarcinomas. Cancer Res. 1998;58:2929–2934. [PubMed] [Google Scholar]; ■ References [32–51] show increased expression of iNOS in early stage lesions and in cancer.
- 52.Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat. Cell Biol. 2001;3:193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- 53.Jesus-Berrios MD, Liu L, Nussbaum JC, Cox GM, Stamler JS, Heitman J. Enzymes that counteract nitrosative stress promote fungal virulence. Curr. Biol. 2003;13:1963–1968. doi: 10.1016/j.cub.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 54.Liu L. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 55.Stamler JS, Jia L, Eu JP, et al. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276:2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- 56.Eu JP, Sun J, Xu L, Stamler JS, Meissner G. The skeletal muscle calcium release channel: coupled O2 sensor and NO signaling functions. Cell. 2000;102:499–509. doi: 10.1016/s0092-8674(00)00054-4. [DOI] [PubMed] [Google Scholar]
- 57.Lai TS, Hausladen A, Slaughter TF, Eu JP, Stamler JS, Greenberg CS. Calcium regulates S-nitrosylation, denitrosylation, and activity of tissue transglutaminase. Biochemistry. 2001;40:4904–4910. doi: 10.1021/bi002321t. [DOI] [PubMed] [Google Scholar]
- 58.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: role of nitric oxide and S-nitrosohemoglobin. Annu. Rev. Physiol. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 59.Stamler JS, Lamas S, Fang FC. Nitrosylation: the prototypic redox-based signaling mechanism. Cell. 2001;106:675–683. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]; ■ Demonstrates nitrosylation regulates various proteins and alter signal mechanisms.
- 60.Mannick JB, Schonhoff C, Papeta N, et al. Nitrosylation of mitochondrial caspases. J. Cell Biol. 2001;20(3):245–248. doi: 10.1083/jcb.200104008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lechner M, Lirk P, Rieder J. Inducible nitric oxide synthase (iNOS) in tumor biology: the two sides of the same coin. Semi. Cancer Biol. 15(4):277–289. doi: 10.1016/j.semcancer.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 62.Rossig L, Fichtlscherer B, Breitschopf K, et al. Nitric oxide inhibits caspase-3 by S-nitrosation in vivo. J. Biol. Chem. 1999;274:6823–6828. doi: 10.1074/jbc.274.11.6823. [DOI] [PubMed] [Google Scholar]
- 63.Nguyen T, Brunson D, Crespi CL, Penman BW, Wishnok JS, Tannenbaum SR. DNA damage and mutation in human cells exposed to nitric oxide in vivo. Proc. Natl Acad. Sci. USA. 1992;89:3030–3034. doi: 10.1073/pnas.89.7.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wink DA, Vodovotz Y, Laval J, Laval F, Dewhirst MW, Mitchell JB. Multifaceted role of iNOS in cancer. Carcinogenesis. 1998;19:711–721. doi: 10.1093/carcin/19.5.711. [DOI] [PubMed] [Google Scholar]
- 65.Morita EH, Ohkulo T, Kuroka I, Shikawa M, Tanaka K, Morikawa K. Implications of the zinc finger motif found in the DNA binding domain of the human XPA protein genes. Cell. 1996;1(5):437–442. doi: 10.1046/j.1365-2443.1996.d01-252.x. [DOI] [PubMed] [Google Scholar]
- 66.Jiaswal M, Larusso NF, Nishioka N, nakabeppu Y, Gores GJ. Human ogg1, a protein involved in the repair of 8-oxoguanine is inhibited by nitric oxide. Cancer Res. 2001;61(17):6388–6393. [PubMed] [Google Scholar]
- 67.Parvin S, Singh R, Hernandez E, Wu G, Chaudhuri G. Nitric oxide in physiologic concentrations targets the translational machinery to increase the proliferation of human breast cancer cells: involvement of mammalian target of rapamycin/eIF4E pathway. Cancer Res. 2007;67(1):289–299. doi: 10.1158/0008-5472.CAN-05-4623. [DOI] [PubMed] [Google Scholar]
- 68.Wenzel U, Kuntz S, de Sansa UJ, Daneil H. Nitric oxide supresess apoptosis in human colon cancer cells by scavenging mitochondrial superoxide anions. Int. J. Cancercer. 2003;106(5):666–675. doi: 10.1002/ijc.11294. [DOI] [PubMed] [Google Scholar]
- 69.Gallo O, Masini E, Morbidelli L, et al. Role of nitric oxide in angiogenesis and tumor progression in head and neck cancer. J. Natl Cancer Inst. 1998;90:587–596. doi: 10.1093/jnci/90.8.587. [DOI] [PubMed] [Google Scholar]
- 70.Janakiram BJ, Malisetty VS, Patlolla JM, Guruswamy S, Rao CV. A selective iNOS inhibitor NILT, suppress invasive colonic cancers and improves preventive efficacy of low dose COX-2 inhibitor, celecoxib in F344 rats. American Association for Cancer Research 101st Annual meeting; Washington, DC, USA. 17–21 April 2010. [Google Scholar]
- 71.Kim SF, Huri DA, Snyder SH. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science. 2005;310(5756):1966–1970. doi: 10.1126/science.1119407. [DOI] [PubMed] [Google Scholar]
- 72.Caulin-Glaser T, García-Cardeña G, Sarrel P, Sessa WC, Bender JR. 17β-estradiol regulation of human endothelial cell basal nitric oxide release, independent of cytosolic Ca2+ mobilization. Circ. Res. 1997;81:885–892. doi: 10.1161/01.res.81.5.885. [DOI] [PubMed] [Google Scholar]
- 73.Gratton JP, Fontana J, O'Connor DS, Garcia-Cardena G, McCabe TJ, Sessa WC. Reconstitution of an endothelial nitric-oxide synthase (eNOS), hsp90, and caveolin-1 complex in vitro. Evidence that hsp90 facilitates calmodulin stimulated displacement of eNOS from caveolin-1. J. Biol. Chem. 2000;275:22268–22272. doi: 10.1074/jbc.M001644200. [DOI] [PubMed] [Google Scholar]
- 74.Chen DB, Bird IM, Zheng J, Magness RR. Membrane estrogen receptor-dependent extracellular signal-regulated kinase pathway mediates acute activation of endothelial nitric oxide synthase by estrogen in uterine artery endothelial cells. Endocrinology. 2004;145:113–125. doi: 10.1210/en.2003-0547. [DOI] [PubMed] [Google Scholar]
- 75.Zhang H, Feng L, Livnat I, et al. Estradiol-17β Stimulates specific receptor and endogenous nitric oxide-dependent dynamic endothelial protein S-nitrosylation: analysis of endothelial nitrosyl-proteome. Endocrinology. 2010;151(8):3874–3887. doi: 10.1210/en.2009-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leon-Bollotte L, Subramaniam S, Cauvard O, et al. S-nitrosylation of the death receptor Fas promotes Fas ligand–mediated apoptosis in cancer cells. Gastroenterology. 2011;140:e4. doi: 10.1053/j.gastro.2011.02.053. [DOI] [PubMed] [Google Scholar]
- 77.Hussain SP, Trivers GE, Hofseth LJ, et al. Nitric oxide, a mediator of inflammation, suppresses tumorigenesis. Cancer Res. 2004;64:6849–6853. doi: 10.1158/0008-5472.CAN-04-2201. [DOI] [PubMed] [Google Scholar]
- 78.Hussain SP, Amstad P, Raja K, et al. Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: a cancer-prone chronic inflammatory disease. Cancer Res. 2000;60:3333–3337. [PubMed] [Google Scholar]
- 79.Reynaert NL, Ckless K, Korn SH, et al. Nitric Oxide represess inhibitory kappaB Kinase through S-nitrosylation. Proc. Natl Acad. Sci. USA. 2004;101(24):8945–8950. doi: 10.1073/pnas.0400588101. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ References [76–79] demonstrate the anti-tumorigenic effects of nitric oxide.
- 80.DelaTorre A, Schroeder RA, Kuo PC. Alteration of NF-kappa B p50 DNA binding kinetics by S-nitrosylation. Biochem. Biophys. Res. Commun. 1997;238(3):703–706. doi: 10.1006/bbrc.1997.7279. [DOI] [PubMed] [Google Scholar]
- 81.Scott DJ, Hull MA, Cartwright EJ, et al. Lack of inducible nitric oxide synthase promotes intestinal tumorigenesis in the Apc(Min/+) mouse. Gastroenterology. 2001;121(4):889–899. doi: 10.1053/gast.2001.27994. [DOI] [PubMed] [Google Scholar]; ■ Demonstrates anti-tumorigenic role of NO in a transgenic animal model of colon cancer.
- 82.Ambs S, Merriam WG, Ogunfusika MO, et al. p53 and vascular endothelial growth factor regulate tumor growth of NOS2-expressing human carcinoma cells. Nat. Med. 1998;4:1371–1376. doi: 10.1038/3957. [DOI] [PubMed] [Google Scholar]
- 83.Ropponen KM, Kellokoski JK, Lipponen PK, et al. Expression of inducible nitric oxide synthase in colorectal cancer and its association with prognosis. Scand. J. Gastroenterol. 2000;11:1204–1211. doi: 10.1080/003655200750056709. [DOI] [PubMed] [Google Scholar]
- 84.Kim SF, Huri DA, Snyder SH. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science. 2005;310(5756):1966–1970. doi: 10.1126/science.1119407. [DOI] [PubMed] [Google Scholar]
- 85.Hao XP, Pretlow TG, Rao JS, Pretlow TP. Inducible nitric oxide synthase is expressed similarly in multiple aberrant crypt foci and colorectal tumors from the same patients. Cancer Res. 2001;61:419–422. [PubMed] [Google Scholar]
- 86.Chhatwal VJ, Ngoi SS, Chan ST, Chia YW, Moochhala SM. Aberrant expression of nitric oxide synthase in human polyps, neoplastic colonic mucosa and surrounding peritumoral normal mucosa. Cancinogenesis. 1994;15:2081–2085. doi: 10.1093/carcin/15.10.2081. [DOI] [PubMed] [Google Scholar]
- 87.Bing RJ, Miyataka M, Rich KA, et al. Nitric oxide, prostanoids, cyclooxygenase and angiogenesis in colon and breast cancer. Clin. Cancer Res. 2001;7:3385–3392. [PubMed] [Google Scholar]
- 88.Roberts PJ, Riley GP, Morgan K, Miller R, Hunter JO, Middleton SJ. The physiological expression of inducible nitric oxide synthase in the human colon. J. Clin. Pathol. 2001;54:293–297. doi: 10.1136/jcp.54.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Onier N, Hilpert S, Reveneau S, et al. Expression of inducible nitric oxide synthase in tumors in relation with their regression induced by lipid S in rats. Int. J. Cancer. 1999;81:755–760. doi: 10.1002/(sici)1097-0215(19990531)81:5<755::aid-ijc15>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 90.Xie K, Huang S, Dong Z, Gutman M, Fidler IJ. Direct correlation between expression of endogenous inducible nitric oxide synthase and regression of M5076 reticulum cell sarcoma hepatic metastases in mice treated with liposomes containing lipopeptide CGP 31362. Cancer Res. 1995;55:3123–3131. [PubMed] [Google Scholar]
- 91.Kawamori T, Takahashi M, Watanabe K, et al. Suppression of azoxymethane-induced colonic aberrant crypt foci by a nitric oxide synthase inhibitor. Cancer Lett. 2000;148:33–37. doi: 10.1016/s0304-3835(99)00310-9. [DOI] [PubMed] [Google Scholar]
- 92.Siegert A, Rosenberg C, Schmitt WD, Denkert C, Hauptmann S. Nitric oxide of human colorectal adenocarcinoma cell lines promotes tumor cell invasion. Br. J. Cancer. 2002;86:1310–1315. doi: 10.1038/sj.bjc.6600224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jenkins DC, Charles IG, Thomsen LL, et al. Roles of nitric oxide in tumor growth. Proc. Natl Acad. Sci. USA. 1995;92:4392–4396. doi: 10.1073/pnas.92.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu W, Liu L, Charles IG. Microencapsulated iNOS-expressing cells cause tumor suppression in mice. FASEB J. 2002;16:213–215. doi: 10.1096/fj.01-0590fje. [DOI] [PubMed] [Google Scholar]
- 95.Scott DJ, Hull MA, Cartwright EJ, et al. Lack of inducible nitric oxide synthase promotes intestinal tumorigenesis in the Apc(Min/+) mouse. Gastroenterology. 2001;121:889–899. doi: 10.1053/gast.2001.27994. [DOI] [PubMed] [Google Scholar]
- 96.Liu Q, Chan STF, Mhendran R. Nitric oxide induces cyclooxygenase expression and inhibits cell growth in colon cancer cell lines. Carcinogenesis. 2003;24:637–642. doi: 10.1093/carcin/bgg014. [DOI] [PubMed] [Google Scholar]
- 97.Buga GM, Wei LH, Bauer PM, Fukuto JM, Ignarro LJ. NG-hydroxy-l-arginine and nitric oxide inhibit Caco-2 tumor cell proliferation by distinct mechanisms. Am. J. Physiol. 1998;275(4 Pt 2):R1256–R1264. doi: 10.1152/ajpregu.1998.275.4.R1256. [DOI] [PubMed] [Google Scholar]
- 98.Mei JM, Hord NG, Winterstein DF, Donald SP, Phang JM. Expression of prostaglandin endoperoxide H synthase-2 induced by nitric oxide in conditionally immortalized murine colonic epithelial cells. FASEB J. 2000;14:1188–1201. doi: 10.1096/fasebj.14.9.1188. [DOI] [PubMed] [Google Scholar]
- 99.Jenkins DC, Charles IG, Thomsen LL, et al. Roles of nitric oxide in tumor growth. Proc. Natl Acad. Sci. USA. 1995;92:4392–4396. doi: 10.1073/pnas.92.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Takahashi M, Fukuda K, Ohata T, Sugimura T, Wakabayashi K. Increased expression of inducible and endothelial constitutive nitric oxide synthases in rat colon tumors induced by azoxymethane. Cancer Res. 1997;57:1233–1237. [PubMed] [Google Scholar]
- 101.Rachmilewitz D, Stamler JS, Bachwich D, Karmeli F, Ackerman Z, Podolsky DK. Enhanced colonic nitric oxide generation and nitric oxide synthase activity in ulcerative colitis and Crohn's disease. Gut. 1995;36:718–723. doi: 10.1136/gut.36.5.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Colon AL, Menchen L, Lizasoain I, et al. Inducible nitric oxide synthase activity is expressed not only in inflamed but also in normal colonic mucosa in patients with ulcerative colitis: a potential prognostic marker. Am. J. Gastroenterol. 2000;95:1371–1373. doi: 10.1111/j.1572-0241.2000.02047.x. [DOI] [PubMed] [Google Scholar]
- 103.Hao XP, Pretlow TG, Rao JS, Pretlow TP. Inducible nitric oxide synthase (iNOS) is expressed similarly in multiple aberrant crypt foci and colorectal tumors from the same patients. Cancer Res. 2001;61:419–422. [PubMed] [Google Scholar]
- 104.Yagihashi N, Kasajima H, Sugai S, et al. Increased in situ expression of nitric oxide synthase in human colorectal cancer. Virchows Arch. 2000;436:109–114. doi: 10.1007/pl00008208. [DOI] [PubMed] [Google Scholar]
- 105.Lagares-Garcia JA, Moore RA, Collier B, Heggere M, Diaz F, Qian F. Nitric oxide synthase as a marker in colorectal carcinoma. Am. J. Surg. 2001;67:709–713. [PubMed] [Google Scholar]
- 106.Szaleczky E, Pronai L, Nakazawa H, Tulassay Z. Evidence of in vivo peroxynitrite formation in patients with colorectal carcinoma, higher plasma nitrate/nitrite levels, and lower protection against oxygen free radicals. J. Clin. Gastroenterol. 2000;30:47–51. doi: 10.1097/00004836-200001000-00008. [DOI] [PubMed] [Google Scholar]
- 107.Wan G, Kato N, Watanabe H. High fat diet elevates the activity of inducible nitric oxide synthase and 1,2-dimethylhydrazine-induced aberrant crypt foci in colon of rats. Oncol. Rep. 2000;7:391–395. doi: 10.3892/or.7.2.391. [DOI] [PubMed] [Google Scholar]
- 108.Dwyer MA, Bredt DS, Snyder SH. Nitric oxide synthase: irreversible inhibition by l-NG-nitroarginine in brain in vitro and in vivo. Biochem. Biophys. Res. Commun. 1991;176:1136–1141. doi: 10.1016/0006-291x(91)90403-t. [DOI] [PubMed] [Google Scholar]
- 109.Rao CV. Nitric Oxide signaling in colon cancer chemoprevention. Mut. Res. 2004;555:107–119. doi: 10.1016/j.mrfmmm.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 110.Cianchi F, Cortesini C, Fantappie O, Messerini L. Inducible nitric oxide synthase expression in human colorectal cancer. Am. J. Pathol. 2003;162:793. doi: 10.1016/S0002-9440(10)63876-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Landino LM, Crews BC, Timmons MD, Morrow JD, Marnett LJ. Peroxynitrite, the coupling product of nitric oxide and superoxide, activates prostaglandin biosynthesis. Proc. Natl Acad. Sci. USA. 1996;93:15069–15074. doi: 10.1073/pnas.93.26.15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Salvemini D, Settle SL, Masferrer JL, Seibert K, Currie MG, Needleman P. Regulation of prostaglandin production by nitric oxide; an in vivo analysis. Br. J. Pharmacol. 1995;114:1171–1178. doi: 10.1111/j.1476-5381.1995.tb13330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schleiffer R, Duranton B, Gossé F, Bergmann C, Raul F. Nitric oxide synthase inhibition promotes carcinogen-induced preneoplastic changes in the colon of rats. Nitric Oxide. 2000;4(6):583–589. doi: 10.1006/niox.2000.0310. [DOI] [PubMed] [Google Scholar]
- 114.Ridnour LA, Windhausen AN, Isenberg JS, et al. Nitric oxide regulates matrix metalloproteinase-9 activity by guanylylcyclase-dependent and -independent pathways. Proc. Natl Acad. Sci. USA. 2007;104(43):16898–16903. doi: 10.1073/pnas.0702761104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brinckerhoff CE, Rutter JL, Benbow U. Interstitial collagenases as markers of tumor progression. Clin. Cancer Res. 2000;6(12):4823–4830. [PubMed] [Google Scholar]
- 116.Huerta S, Chilka S, Bonavida B. Nitric oxide donors: novel cancer therapeutics. Int. J. Oncol. 2008;33:909–927. [PubMed] [Google Scholar]
- 117.Rigas B, Kashfi K. Nitric-oxide-donating NSAIDs as agents for cancer prevention. Trends Mol. Med. 2004;10(7):324–330. doi: 10.1016/j.molmed.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 118.Williams JL, Ping J, Nengtai O, Levy K, Rigas B. Protein nitration and nitrosylation by NO-donating aspirin in colon cancer cells: relevance to its mechanism of action. Exp. Cell Res. 2011;317(10):1359–1367. doi: 10.1016/j.yexcr.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gratton JP, Lin MI, Yu J, et al. Selective inhibition of tumor microvascular permeability by cavtratin blocks tumor progression in mice. Cancer Cell. 2003;4:31–39. doi: 10.1016/s1535-6108(03)00168-5. [DOI] [PubMed] [Google Scholar]
- 120.Kashiwagi S, Izumi Y, Gohongi T, et al. NO mediates mural cell recruitment and vessel morphogenesis in murine melanomas and tissue-engineered blood vessels. J. Clin. Invest. 2005;115:1816–1827. doi: 10.1172/JCI24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jadfeski LC, Lala PK. Nitric oxide synthase inhibition by NG-nitro-l-arginine methyl ester inhibits tumor-induced angiogenesis in mammary tumors. Am. J. Pathol. 1999;155:1381–1390. doi: 10.1016/S0002-9440(10)65240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kashiwagi S, Izumi Y, Gohongi T, et al. NO mediates mural cell recruitment and vessel morphogenesis in murine melanomas and tissue-engineered blood vessels. J. Clin. Invest. 2005;115:1816–1827. doi: 10.1172/JCI24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang G-Y, Ji B, Wang X, Gu J-H. Anti-cancer effect of iNOS inhibitor and its correlation with angiogenesis in gastric cancer. World J. Gastroenterol. 2005;11(25):3830–3833. doi: 10.3748/wjg.v11.i25.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen T, Nines RG, Peschke SM, Kresty LA, Stoner GD. Chemopreventive effects of a selective nitric oxide synthase inhibitor on carcinogen-induced rat esophageal tumorigenesis. Cancer Res. 2004;64:3714–3717. doi: 10.1158/0008-5472.CAN-04-0302. [DOI] [PubMed] [Google Scholar]
- 125.Janakiram NB, Mohammed A, Ravillah D, et al. Chemoprevention of colon carcinogenesis in F344 rats by Se,Se′-1,4-phenylenebis(1,2-ethanediyl)bis-isoselenourea (PBISe) a novel analog of PBIT, an iNOS inhibitor. Cancer Prevent. Res. 2010;3(Suppl. 1):A35. [Google Scholar]
- 126.Rao CV, Guruswamy S, Patlolla J, Janakiram N, Malisetty V. Chemoprevention of colon cancer by PBIT, an iNOS-selective inhibitor, administered alone or in combination with low-doses of celecoxib, a COX-2 selective inhibitor. Cancer Prevent. Res. 2010;3(Suppl. 1):A53. [Google Scholar]
- 127.Rao CV, Malisetty SV, Cooma I, Reddy BS. Chemoprevention of familial adenomatous polyps and carcinomas by iNOS and COX-2 selective inhibitors administered individually, and in combination in the ApcMin-mice model. Proc. Am. Assoc. Cancer Res. 2002;43:670. [Google Scholar]
- 128.Thomsen LL, Scott JMJ, Topley P, Knowles RG, Keerie A-J, Frend AJ. Selective inhibition of inducible nitric oxide synthase inhibits tumor growth in vivo: studies with 1400W, a novel inhibitor. Cancer Res. 1997;57:3300–3304. [PubMed] [Google Scholar]
- 129.Ziche M, Morbidelli L, Choudhuri R, et al. Nitric oxide synthase lies downstream from vascular endothelial growth factor–induced but not basic fibroblast growth factor–induced angiogenesis. J. Clin. Invest. 1997;99:2625–2634. doi: 10.1172/JCI119451. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ References [125–129] show efficacy of specific iNOS inhibitors in different cancer models.