Abstract
The intake of dietary fat above energy needs has contributed to the growing rates of obesity worldwide. The concept of disease development occurring in the fed state now has much support and dysregulation of substrate flux may occur due to poor handling of dietary fat in the immediate postprandial period. The present paper will review recent observations implicating cephalic phase events in the control of enterocyte lipid transport, the impact of varying the composition of meals on subsequent fat metabolism, and the means by which dietary lipid carried in chylomicrons can lead to elevated postprandial non-esterified fatty acid concentrations. This discussion is followed by an evaluation of the data on quantitative meal fat oxidation at the whole body level and an examination of dietary fat clearance to peripheral tissues — with particular attention paid to skeletal muscle and liver given the role of ectopic lipid deposition in insulin resistance. Estimates derived from data of dietary-TG clearance show good agreement with clearance to the liver equaling 8–12% of meal fat in lean subjects and this number appears higher (10–16%) in subjects with diabetes and fatty liver disease. Finally, we discuss new methods with which to study dietary fatty acid partitioning in vivo. Future research is needed to include a more comprehensive understanding of 1) the potential for differential oxidation of saturated versus unsaturated fatty acids which might lead to meaningful energy deficit and whether this parameter varies based on insulin sensitivity, 2) whether compartmentalization exists for diet-derived fatty acids within tissues vs. intracellular pools, and 3) the role of reduced peripheral fatty acid clearance in the development of fatty liver disease. Further advancements in the quantitation of dietary fat absorption and disposal will be central to the development of therapies designed to treat diet-induced obesity. This article is part of a Special Issue entitled Triglyceride Metabolism and Disease.
Keywords: Dietary fatty acid, Postprandial, Triglyceride, table isotope, Obesity
1. Introduction
Traditionally, cardiovascular disease risk factors have been determined and measured in the fasting state. However in recent years there has been an increasing awareness on the importance of fed-state events in the development and exacerbation of disease. Epidemiological studies have demonstrated that the presence of postprandial hypertriglyceridemia poses an independent risk for coronary atherosclerosis [1–7]. Furthermore, the efficiency with which the body manages incoming dietary lipid can modulate disease risk in other chronic conditions such as obesity [8], type 2 diabetes [9,10], and non-alcoholic fatty liver disease, NAFLD [11]. Indeed, if poor metabolism of dietary triglyceride (TG) leads to ectopic lipid deposition, postprandial events may contribute more to disease development than currently appreciated. The goal of the present paper is to review current knowledge on the absorption and metabolic fate of dietary-TG in humans. Beginning with recent observations of a delay in the complete absorption of meal-TG, the paper will describe the fates of chylomicron fatty acids, from clearance into adipose and skeletal muscle, to the latest data on the contribution of dietary-TG to the development of NAFLD. A better understanding of the process of dietary-TG partitioning is central to the future development of therapies designed to improve dietary fat handling. The long history of excellent papers published in this research area precludes mention of all significant work; we have focused on recent studies and highlighted technical developments that are supporting the rapidly expanding knowledge in this field.
2. Absorption of dietary fat
2.1. Intestinal metabolism and chylomicron clearance
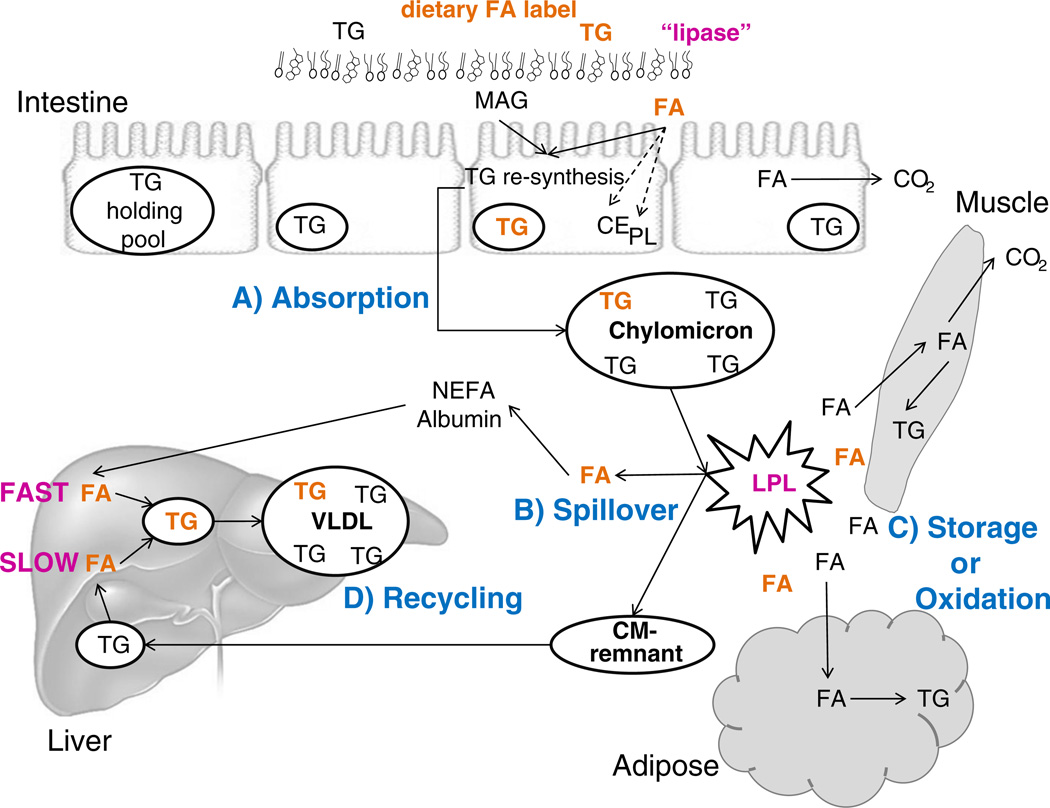
As shown in Fig. 1A dietary fat absorbed into the enterocyte can be 1) repackaged into chylomicron (CM) lipoprotein particles for distribution to the body tissues, 2) stored within the enterocyte in a lipid droplet or TG storage pool, 3) partitioned into other lipids including cholesteryl ester (CE) or phospholipid (PL), and 4) oxidized. Incorporation of dietary fatty acids into TG or other lipids within the enterocyte may depend on chain length and structure (saturation). For example, myristate is preferentially packaged into TG (over 95%), whereas a portion of palmitate is incorporated into PL (18%) and CE (7%) [12]. Stearate appears better incorporated into PL (estimated 33%) compared to palmitate, and less ends up in TG and CE [12,13]. Polyunsaturated fatty acids (PUFA) including 18:2n6 and 18:3n3 are also proportionally incorporated more into CE and PL compared to 16:0 and 18:1 [14,15]. Since the majority of dietary fatty acids are processed as CM-TG, the fate of this lipid fraction will be the focus of the remaining portion of this review.
Fig. 1.
Meal TG absorption and metabolism. After eating a meal, dietary fat (triglyceride, TG) is hydrolyzed by lipase to yield fatty acids (FA) and monoacylglycerol (MAG) which are then absorbed (A) into the enterocyte. Within the enterocyte, FA have several fates: 1) partitioning into cholesteryl ester (CE) or phospholipid (PL), 2) oxidation, or reesterification to form TG for 3) incorporation into chylomicrons (CM) or 4) storage in an intracellular TG storage or holding pool. Chylomicron-TG is metabolized at the tissue level by lipoprotein lipase (LPL), which hydrolyzes CM-TG to release FA for tissue uptake. Some of these released FA will not be taken up by tissues but will rather “spillover” (B) into the plasma non-esterified fatty acid (NEFA) pool and can then be taken up by the liver or other tissues. Within the major tissues (muscle or adipose), dietary-derived FA can be stored or oxidized (C) depending on tissue needs. After hydrolysis of chylomicron-TG the particle becomes smaller, forming a CM-remnant. This remnant particle is taken up by the liver and the TG remaining in the particle can be repackaged into VLDL (D) thereby recycling the dietary FA. This pathway is relatively slower for incorporation into VLDL-TG compared to the incorporation of FA from the plasma free fatty acid pool, which occurs rapidly.
Over the past 10 years, two striking characteristics of enterocyte-TG processing have emerged — 1) that lipids secreted at the very onset of a meal are those that were consumed in an earlier meal, suggesting the presence of an enterocyte storage pool for TG, and 2) a cephalic phase release of CM tied to oral stimulation by food intake. The first phenomenon noted is an early rise in blood-TG concentrations within minutes of the consumption of a meal containing fat [16]. This rise in TG occurring 10–30 min after the onset of the meal is denoted “the early peak” to delineate it from the primary postprandial peak of blood TG which occurs 3–4 h after meal initiation. The timing of the early peak observed in the blood occurs before the absorption of the meal fat into the enterocyte could have occurred [17] and is more likely to occur when the previous evening meal was high in fat [17,18]. We have recently shown via utilization of stable isotopes that 10–12% of TG consumed in the previous evening meal appears in new CM, first occuring 15–20 min after the onset of morning food consumption. This observation indicates that the timing of meal-TG storage in the intra-enterocyte pool can extend to at least 16 h [18] and supports a taste for fat. The second phenomenon is connected to the overall cephalic phase response [19–22]. The early meal-induced rise in CMTG concentration can occur when fat is only tasted and not consumed [23], and also when only glucose is consumed [24]. The existence of an oral taste sensor for lipids is intriguing and has led to the generation of hypotheses on a taste–gut–brain axis which is currently an active area of investigation [25].
Potential questions arising from these findings:
Does the pre-release of CM-TG before influx of meal fat provide some physiological benefit? Fat-sensitive hormone release may “prime” the body by stimulating upregulation of lipolytic enzymes and fatty acid transport proteins in preparation for the incoming lipid load.
Does glucose-induced release of CM-TG also occur with other sweet tastes (i.e. fructose or artificial sweeteners)? This would have implications for individuals who rely on artificially-sweetened foods and may already have impairments in postprandial lipid metabolism.
2.2. The second meal effect
Plasma concentrations of TG rise progressively over the day due to repeated consumption of fat-containing meals. Peak TG levels occur between midnight and 2 AM [26,27]. This change in plasma-TG is a result of increases in both CM particles and TG content [28]. After a given meal, CM-TG concentrations rise and peak after 3–4 h, whereas TG concentrations in very low-density lipoproteins (VLDL) remain relatively constant or peak later at 4–6 h [28–30]. However, humans rarely consume a single meal during the day. Consumption of a subsequent meal causes a higher CM-TG concentration than that which occurs after the first meal, even if the two meals are identical. As a result of this “second meal effect,” the CM-TG released after this second meal can contain a significant amount of lipid from the previous meal [29,30].
The term “second meal effect” has also been used to refer to the impact of previous macronutrient consumption on the metabolism of meal-TG. Robertson et al. demonstrated that carbohydrate and fat can negatively influence each other's metabolism after a meal. Specifically, plasma glucose concentrations during an oral glucose tolerance test appeared higher after a high-fat evening meal had been consumed the night before, compared to after a high-carbohydrate evening meal. Similarly, plasma-TG levels during an oral fat tolerance test were higher after a high-carbohydrate evening meal compared to a high-fat evening meal [17]. These data suggest that the fuel source making up the bulk of the calories in the previous meal (either consumed on the same day or even consumed the night before) impairs metabolism of the alternate fuel source. In other words, a high-fat evening meal may impair glucose metabolism the next day and vice versa. The negative impact of fatty acid oxidation on glucose oxidation has been explained by Randle [31]. However, it is unclear how recent carbohydrate intake and processing would elevate TG concentrations because glucose-induced hyperinsulinemia would presumably stimulate LPL activity, resulting in better TG clearance from plasma into tissues. The observation of the competition between macronutrient oxidation not only encompasses the negative interaction between these macronutrients, but may also extend to feed-forward mechanisms such that the body best metabolizes the primary fuel it has recently consumed (i.e., a high-carbohydrate breakfast will lead to better carbohydrate processing after lunch). Ruge et al. have shown that the efficiency of clearance of meal fatty acids into adipose rises from breakfast through dinner [32]. It is tempting to speculate that this improvement in TG clearance over the day may result from continued insulinization of adipose tissue leading to progressive up-regulation of enzymes for TG synthesis and storage (see discussion of lipoprotein lipase, LPL, below). Regardless of the mechanism, these findings have clear implications for the design of postprandial studies and underscore the importance of standardizing food consumption of human subjects before test meals are administered.
2.3. Lipoprotein lipase and the spillover pathway of lipoprotein-TG clearance
Once absorbed and released into the blood, dietary fatty acids carried in CM deliver lipids to the rest of the body to be metabolized. The release of fatty acids from CM-TG occurs through the activity of LPL which is bound to the capillary endothelium of major organs in the body. The hormonal control and tissue localization of LPL have been the topics of comprehensive studies in humans [33–37]. In general, LPL activity is higher in the postprandial state compared to fasting and can be upregulated with increased physical activity [38]. Insulin and glucose infusion upregulate LPL activity in adipose tissue [39], whereas LPL activity at skeletal muscle can remain consistently high throughout the day [32]. More than 20 years ago, it was evident that not all fatty acids liberated from CM by LPL are taken up into tissues [40] but rather are released into the blood as non-esterified fatty acids (NEFA) in a process referred to as “spillover” [41]. This process is depicted in Fig. 1B. Factors regulating the quantity of meal fatty acids entering the circulation through the spillover pathway have not yet been identified. Using a fat-containing, stable isotope-labeled liquid meal, Barrows et al. measured the magnitude of spillover in healthy men undergoing either slow duodenal infusion of the formula or a meal-feeding paradigm in which the formula was fed as a bolus orally [42]. Surprisingly, the time course and quantity of spillover fatty acids moving into the albumin pool over 9 h were similar between the two feeding regimens, suggesting that the extent of spillover depends on the quantity of lipid being fed.
Bickerton and colleagues used a multi-isotope labeling scheme to determine whether lipolysis of CM or VLDL was more likely to generate albumin-bound fatty acids [43]. U-13C-palmitate was fed in a test meal to label CM-TG, whereas 2H2-palmitate was infused as NEFA to label VLDL-TG. Extraction rates of fatty acids from CM and VLDL across subcutaneous abdominal adipose tissue and forearm muscle were estimated by calculating the arterio-venous differences. Estimates of the extraction of fatty acids derived from CM-TG lipolysis took into account the arterio-venous differences in U-13C-palmitate in TG and free U-13C-palmitate in plasma (i.e. non-esterified fatty acid spillover), and the calculations were the same for muscle and adipose tissue. Since a single marker, 2H2-palmitate, was used to trace the plasma NEFA pool as well as VLDL-TG, it was not possible to unequivocally identify 2H2-palmitate arising in the NEFA pool from VLDL-TG spillover. The authors assumed that in muscle, all fatty acids released from VLDL-TG by LPL would be taken up, whereas at adipose tissue various mathematical models were developed based on different degrees of LPL-mediated spillover to estimate uptake of VLDL-TG fatty acids released from LPL. The authors concluded that compared to plasma NEFA, greater fractional extraction of lipoprotein-derived fatty acids occurs at muscle and adipose tissue [43]. This fractional extraction of lipoprotein-derived fatty acids in preference to plasma NEFA at adipose tissue appeared greatest in the early postprandial period (87% of palmitate liberated from CM-TG) and then fell (48% by 6 h). In the early (0–2 h), mid (2–4 h), and late (4–6 h) postprandial periods, the authors concluded that CM-derived fatty acids are taken up by muscle (23%, 10%, and 6%, respectively) and adipose (22%, 11%, and 12%, respectively) tissue to a greater extent than VLDL-TG fatty acids at any of these time points. This preferential uptake of CM as opposed to VLDL fatty acids appears strongest in the earlier postprandial period, and then wanes in the later postprandial period [43].
In summary, many scientists still assume that NEFA concentration depends solely on adipose output. Yet spillover of dietary fatty acids is becoming recognized as a significant event in the postprandial period, contributing 5–35% of fatty acids to the plasma NEFA pool after a meal. Lastly, it is unknown whether evidence of dietary fatty acid spillover is an indicator of beneficial or detrimental events occurring in the postprandial state. Does spillover indicate elevated insulin concentrations and exaggerated LPL activity that outpaces tissue uptake? Does greater spillover of fatty acids mean that less is taken up by muscle and adipose, exposing more fatty acids to other tissues such as the liver and the heart? Do subjects who demonstrate a high level of spillover after a meal have high LPL activity and greater clearance of fatty acids by tissues? Spillover is reduced with weight loss which may prevent lipid deposition in other tissues such as liver and heart, but may encourage weight regain by improving fatty acid uptake by adipose tissue [44]. Current studies are underway to determine the role of muscle and liver in the clearance of these fatty acids.
3. Uptake of fatty acids and tissue oxidation versus storage
3.1. How much meal fat is oxidized during the day?
Obesity-associated insulin resistance has been characterized as a condition in which fatty acid oxidation is reduced [45] and thus, numerous studies have focused on understanding factors that impact postprandial fatty acid oxidation (denoted pathway C in Fig. 1). Research investigating the fate of dietary fatty acids has utilized labeling of meal lipid with both radioactive and stably-labeled substrates; data from the latter are summarized in Table 1. Both Jones et al. [46] and DeLany et al. [47] fed 13C-labeled fatty acids in breakfast meals to determine the impact of fatty acid chain-length and unsaturation on postprandial oxidation rate. In both studies following breakfast, subjects were supplied with an unlabeled lunch which would have facilitated the absorption of the breakfast (see ‘second meal effect’ above) during the 9 h sampling period. The pattern of meal-fat oxidation was strikingly similar between the studies showing an exponential increase of the label in breath CO2 over the day. When 18:0 (a saturated fatty acid, SFA) was fed as a stable isotope in capsule form, approximately 2–3% of the meal dose was recovered in breath CO2 over 9 h [46], whereas when 18:0 was fed in a mixed meal, cumulative oxidation rates reached 10–12% within 9 h [47]. Both of these studies compared the oxidation of 18:0 with that of 18:1 and 18:2 and found that 10–20% of the mono unsaturated fatty acids (MUFA) and 14–22% of the PUFA were oxidized over the 9 h time frame.
Table 1.
Studies demonstrating the extent of meal fatty acid oxidation and effects of fatty acid chain length and saturation
| Reference | Vehicle for meal fatty acid delivery | Time frame of measurement | Tracer/Fatty Acid | % fatty acid oxidized within that time frame |
|---|---|---|---|---|
| Votruba et al. [50] | Fed in a breakfast | 2h | 13C1-16:0 (breath) | 2% |
| D31-16:0 (urine) | 4% | |||
| 6h | 13C1-16:0 (breath) | 8% | ||
| D31-16:0 (urine) | 10% | |||
| 10h | 13C1-16:0 (breath) | 13% | ||
| D31-16:0 (urine) | 13% | |||
| Jones et al. [46] | Fed in capsule | 9h | 13C1-18:0 | 2–3% |
| 13C1-18:1 | ~10% | |||
| DeLany et al. [47] | Fed with mixed meal | 9h | 13C1-12:0 | 41% |
| 13C1-16:0 | 16% | |||
| 13C1-18:0 | 13% | |||
| 13C1-18:1n9-cis | 18% | |||
| 13C1-18:1n9-trans | 21% | |||
| 13C1-18:2n6 | 20% | |||
| 13C1-18:3n3 | 27% | |||
| MacDougall et al. [55] | Oral bolus with test | 9h | 13C1-14:0 | 7–9% |
| 13C1-16:0 | 2.5–3% | |||
| Raman et al. [48] | Fed in a breakfast drink | 9h | 13C1-16:0 (breath) | 7% |
| D31-16:0 (urine) | 11% | |||
| Jones et al. [56] | Emulsified into shake | 9h | 13C1-16:0 | 16% |
| 13C1-18:0 | 15% | |||
| 13C1-18:1 | 19% | |||
| 24h | 13C1-16:0 | 26% | ||
| 13C1-18:0 | 24% | |||
| 13C1-18:1 | 27% | |||
| Burdge et al. [81] | Emulsified into shake | 24h | U13C-18:3n3 | 32–36% |
An early demonstration of the utility of measuring oxidation of dietary fatty acids was presented by Schoeller and colleagues who fed deuterated lipid added to meals [48,49]. In this method, urinary water is collected for measurement of deuterium instead of breath CO2 to assess oxidation [50]. Using this method, Cooper et al. found that 80–100% of dietary 18:1 was oxidized within 24 h of feeding the labeled meal, while 10–25% of dietary 16:0 was oxidized over the same time frame [51]. Similarly, greater oleate oxidation has been observed in overweight, post-menopausal women [52]. Contradictory studies exist that show lower whole-body fat oxidation on a MUFA-rich diet compared to a diet rich in trans-fatty acids [53] and no difference in fat oxidation rates on MUFA- vs. SFA-rich diets [54]. Thus, although the beneficial effects of oleate on meal fatty acid oxidation are controversial, the data represent a promising area of research. As shown in Table 1 overall, the order of preference for oxidation appears to be PUFA>MUFA>SFA. A secondary hierarchy may exist between fatty acids within the same class of saturation; for example, shorter chain saturated fatty acids may be oxidized to a greater extent than longer chain fatty acids [47,55], but significant differences in oxidation between fatty acids of different chain lengths are not consistent across studies [56]. However, within a high-fat diet, the fatty acid composition of the diet seems to play less of a factor than quantity of fat [51,55]. A final parameter that deserves mention as a focus of much research is the effect of meal carbohydrate content on fat oxidation. One study found that oxidation of meal fat was reduced almost 30% when carbohydrate was present in the diet compared to a high fat diet [57]. This may be due to fatty acids being funneled away from oxidation and towards esterification in muscle [57], and/or because in the context of high-carbohydrate feeding, glucose is preferentially oxidized.
3.2. Dietary-TG clearance to adipose and muscle
In a series of elegant experiments using radioactive fatty acids in meals (3H-triolein), gas exchange measurements, and adipose tissue biopsies, Jensen and colleagues have investigated adipose depot-specific clearance of meal lipid [58–60]. In one study of lean individuals, over a 24-h period, 49% of the fatty acids are oxidized in agreement with some, but not all of the whole-body oxidation studies described above. Of the remainder, ~29% of FA cleared from CM-TG were stored in upper-body subcutaneous depots, roughly 9% were stored in lower-body subcutaneous fat and 9% in intra-abdominal depots [58]. In overweight but non-diabetic humans, storage of meal fat increased from 6 h to 24 h into upper (17% and 21%, respectively) and lower (13% and 19%, respectively) subcutaneous fat depots [61]. The lower meal fat storage in upper fat depots and greater storage in lower fat depots noted in overweight individuals support potential differences in storage depending on adiposity. Furthermore, differences between men and women exist, as one study demonstrated greater storage of meal fatty acids in adipose tissue in women (24% and 12%, respectively) compared to men (16% and 7%, respectively) [62]. Indeed, in healthy subjects, adipose clearance and storage [63], along with short-term fatty acid oxidation, represent the primary fates of meal fat but alterations in these modes of fatty acid handling have been proposed in obesity and insulin resistance and may impact metabolic risk [64].
With regard to dietary fatty acid clearance to muscle, numerous studies have used chronic dietary strategies to determine the impact of fatty acid composition on muscle metabolism [65–67]. Kien et al. investigated the fatty acid composition of lipids in muscle biopsies after healthy subjects consumed diets high in either palmitic or oleic acid for 7 d [65]. A strong congruence in the fatty acid pattern was observed between the diet, muscle-TG, muscle-diacylglycerol, and the acylcarnitines present in serum. These data suggested that the clearance and turnover of muscle fatty acids were nearly complete within the 7 d of feeding [65] — an observation echoed in data from a 7 d, high-fat feeding study by Schrauwen-Hinderling et al. [67]. A recently-developed method to assess dietary fatty acid clearance to tissues combines positron emission tomography and computed tomography along with administration of a fatty acid analog, the positron-emitting 14(R, S)-[18F]fluoro-6-thia-heptadecanoic acid (18FTHA). Subjects consumed the isotope with fat in capsules and the label was shown to accumulate in the liver, heart, skeletal muscles, and subcutaneous and abdominal adipose tissues over the next 6 h [69]. In a subsequent study, healthy and diabetic subjects were studied with intravenous infusion of 18FTHA simultaneously with continuous oral intake of a liquid meal high in PUFA. Plasma NEFA uptake into tissues was similar in the controls and diabetics, although reduced fractional plasma extraction of NEFA at muscle was demonstrated in the diabetics secondary to reduced muscle blood flow in the postprandial state [70]. The lower fractional extraction of TG-fatty acids in diabetics is in contrast to the higher extraction at 2 h postprandially in insulin resistant subjects [68]. More studies will be needed to understand the dynamics of dietary fatty acid uptake into skeletal muscle and how insulin action and resistance affects this process. Lastly, although not reviewed here, the topic of fatty acid clearance to the heart represents a new and significant area of clinical research [71]. New techniques, including the novel PET/CT methods described above, will allow better assessment of the impact of high-fat meals on lipid clearance, utilization and ectopic lipid accrual in the heart.
3.3. Dietary-TG clearance to liver
Diets high in fat content have been shown to be strongly associated with the incidence of obesity and its co-morbidities such as diabetes and atherosclerosis [72–74]. The complexity of this concept with regard to types of dietary fat and incidence of various diseases has been recently discussed [75] and it is clear that individual responses to high-fat diets may be the key to understanding variability in risk to diet-induced obesity. One of the growing side effects of obesity is non-alcoholic fatty liver disease (NAFLD) and data are emerging to show that a significant source of liver-TG can be derived from meal fat. Using a multiple stable isotope approach followed by liver biopsies in patients with suspected NAFLD, we have calculated that 10–16% of dietary-TG passes through and/or gets stored in liver-TG throughout the day. This estimate is based on the amount of spillover of dietary fatty acids after a labeled meal and fatty acids evident in VLDL-TG (Fig. 1D) over 10 h. The amount of dietary FA found in VLDL-TG was greater than that which could be accounted for by spillover and fatty acid flux and which presumably entered the liver via CM remnant uptake [11]. This information was combined with VLDL-TG secretion rates measured in these subjects (Donnelly and Parks, unpublished data), and the liver-TG labeled with the dietary stable isotope after 5 days of feeding [11]. The 10–16% estimate is similar to the liver clearance value of dietary-TG that we have calculated from the data of Ravikumar et al. who fed low-fat meals to 8 healthy obese and 12 diabetic subjects and assessed liver-TG accrual with magnetic resonance spectroscopy over a 24 h period [76]. Based on the peak label detection in the liver within 12 h after the meal, 9% of meal fat was stored in the liver of the healthy subjects compared to 13% in the diabetics. Lastly, using VLDL secretion rates in 6 healthy males studied by Timlin and Barrows [42,77,78] bolus-feeding of a liquid formula orally resulted in 12% of the meal-TG being recycled through the liver and re-secreted back into plasma in VLDL, while slowly infusing the same quantity of formula through a duodenal feeding tube resulted in only 8% of the meal-TG being recycled through the liver.
These data can be summarized as follows: meal-TG can be delivered to the liver through the spillover pathway after liberation via LPL and also through direct hepatic chylomicron uptake (Fig. 1D). Slower rates of CM synthesis may support better peripheral handling of dietary fatty acids compared to large meals containing greater amounts of fat. Data from healthy subjects suggest that 8–12% of meal-TG is taken up by the liver after a single meal and that in diabetics and individuals with NAFLD, a greater burden of meal fatty acids (10–16%) may be handled by the liver in the postprandial state. These observations are consistent with lower peripheral clearance of meal lipid in obesity and insulin resistance [79] and increased message for fatty acid transport proteins (FATP and CD36) in the liver in NAFLD [80].
4. Summary and future directions
The present paper has summarized recent developments in the study of dietary fatty acid absorption, clearance to tissues, storage, and recycling. Over the past 20 years it has become clear that dietary fatty acids carried in CM clear into many peripheral tissues in quantities not previously appreciated, and that this clearance likely contributes to the development of ectopic lipids that occurs in over-nutrition. A number of scientific questions are in need of further research and include, “What are the factors that control CM-TG clearance to tissues versus the uptake of albumin-bound NEFA?” “How does the spillover of dietary fatty acids into the NEFA pool impact glucose utilization?” “What is the role of lipid transport proteins in ectopic lipid deposition?” Furthermore, it is unknown if channeling occurs to target particular intracellular compartments with fatty acids derived from extracellular CM hydrolysis. Recent advancements in methodology to track the fate of dietary fatty acids in humans will support research to answer these questions and spur the development of therapies designed to improve dietary fatty acid handling in obesity and other chronic diseases.
Abbreviations
- CE
cholesterol ester
- CM
chylomicrons
- 18FTHA
[18F]fluoro-6-thiaheptadecanoic acid
- NAFLD
non-alcoholic fatty liver disease
- LPL
lipoprotein lipase
- MUFA
monounsaturated fatty acids
- NEFA
non-esterified fatty acids
- PET/CT
positron emission tomography and computed tomography
- PL
phospholipid
- PUFA
polyunsaturated fatty acids
- SFA
saturated fatty acids
- TG
triglycerides
- VLDL
very low-density lipoprotein
Footnotes
Supported by: 5RL1DK081187.
This article is part of a Special Issue entitled Triglyceride Metabolism and Disease.
References
- 1.Ginsberg HN. Is hypertriglyceridemia a risk factor for atherosclerotic vascular disease? A simple question with a complicated answer. Ann. Intern. Med. 1997;126(11):912–914. doi: 10.7326/0003-4819-126-11-199706010-00012. [DOI] [PubMed] [Google Scholar]
- 2.Austin MA, Holkanson JE, Edwards KL. Hypertriglyceridemia as a cardiovascular risk factor. Am. J. Cardiol. 1998;81(4A):7B–12B. doi: 10.1016/s0002-9149(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 3.Miller M. Is hypertriglyceridaemia an independent risk factor for coronary heart disease? Eur. Heart J. 1998;19(suppl H):H18–H22. [PubMed] [Google Scholar]
- 4.Hyson D, Rutledge JC, Berglund L. Postprandial lipemia and cardiovascular disease. Curr. Atheroscler. Rep. 2003;5(6):437–444. doi: 10.1007/s11883-003-0033-y. [DOI] [PubMed] [Google Scholar]
- 5.Bansal S, et al. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298(3):309–319. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 6.Boullart AC, de Graaf J, Stalenhoef AF. Serumtriglycerides and risk of cardiovascular disease. Biochim. Biophys. Acta. 2011 Oct 8; doi: 10.1016/j.bbalip.2011.10.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Jackson KG, Poppitt SD, Minihane AM. Postprandial lipemia and cardiovascular disease risk: interrelationships between dietary, physiological and genetic determinant. Atherosclerosis. 2011 Sep 9; doi: 10.1016/j.atherosclerosis.2011.08.012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.McQuaid SE, et al. Downregulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition? Diabetes. 2011;60(1):47–55. doi: 10.2337/db10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodson L, et al. The contribution of splanchnic fat to VLDL triglyceride is greater in insulin-resistant than insulin-sensitivemen and women: studies in the postprandial state. Diabetes. 2007;56(10):2433–2441. doi: 10.2337/db07-0654. [DOI] [PubMed] [Google Scholar]
- 10.Masding MG, et al. The benefits of oestrogens on postprandial lipid metabolism are lost in post-menopausal women with Type 2 diabetes. Diabet. Med. 2006;23(7):768–774. doi: 10.1111/j.1464-5491.2006.01867.x. [DOI] [PubMed] [Google Scholar]
- 11.Donnelly KL, et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patientswith nonalcoholic fatty liver disease. J. Clin. Invest. 2005;115(5):1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes TA, et al. Comparative lipoprotein metabolism of myristate, palmitate, and stearate in normolipidemic men. Metabolism. 1996;45(9):1108–1118. doi: 10.1016/s0026-0495(96)90010-4. [DOI] [PubMed] [Google Scholar]
- 13.Emken EA. Metabolism of dietary stearic acid relative to other fatty acids in human subjects. Am. J. Clin. Nutr. 1994;60(6 Suppl):1023S–1028S. doi: 10.1093/ajcn/60.6.1023S. [DOI] [PubMed] [Google Scholar]
- 14.Hodson L, et al. Differences in partitioning ofmeal fatty acids into blood lipid fractions: a comparison of linoleate, oleate and palmitate. Am. J. Physiol. Endocrinol. Metab. 2009;296(1):E64–E71. doi: 10.1152/ajpendo.90730.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burdge GC, Jones AE, Wootton SA. Eicosapentaenoic and docosapentaenoic acids are the principal products of a-linolenic acid metabolism in young men. Br. J. Nutr. 2002;88:355–363. doi: 10.1079/BJN2002662. [DOI] [PubMed] [Google Scholar]
- 16.Mattes RD. Oral fat exposure increases the first phase triacylglycerol concentration due to release of stored lipid in humans. J. Nutr. 2002;132:3656–3662. doi: 10.1093/jn/132.12.3656. [DOI] [PubMed] [Google Scholar]
- 17.Robertson MD, et al. Extended effects of evening meal carbohydrate-to-fat ratio on fasting and postprandial substratemetabolism. Am. J. Clin. Nutr. 2002;75:505–510. doi: 10.1093/ajcn/75.3.505. [DOI] [PubMed] [Google Scholar]
- 18.Chavez-Jauregui RN, Mattes RD, Parks EJ. Dynamics of fat absorption and impact of sham feeding on postprandial lipema. Gastroenterology. 2010;139(5):1538–1548. doi: 10.1053/j.gastro.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiraoka T, et al. Effects of oral stimulationwith fats on the cephalic phase of pancreatic enzyme secretion in esophagostomized rats. Physiol. Behav. 2003;79:713–717. doi: 10.1016/s0031-9384(03)00201-4. [DOI] [PubMed] [Google Scholar]
- 20.Mattes RD. Is there a fatty acid taste? Annu. Rev. Nutr. 2009;29:7.1–7.23. doi: 10.1146/annurev-nutr-080508-141108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahren B, Holst JJ. The cephalic insulin response to meal ingestion in humans is dependent on both cholinergic and noncholinergic mechanisms and is important for postprandial glycemia. Diabetes. 2001;50(5):1030–1038. doi: 10.2337/diabetes.50.5.1030. [DOI] [PubMed] [Google Scholar]
- 22.Teff KL, Engelman K. Oral sensory stimulation improves glucose tolerance: effects on post-prandial glucose, insulin, C-peptide and glucagon. Am. J. Physiol. 1996;270:R1371–R1379. doi: 10.1152/ajpregu.1996.270.6.R1371. [DOI] [PubMed] [Google Scholar]
- 23.Mattes RD. Brief oral stimulation, but especially oral fat exposure, reliably elevates serumtriglycerides in humans. Am. J. Physiol. 2009;296:G365–G371. doi: 10.1152/ajpgi.90591.2008. (Am J Physiol Gastrointest Liver Physiol) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robertson MD, et al. Mobilisation of enterocyte fat stores by oral glucose in humans. Gut. 2003;52:834–839. doi: 10.1136/gut.52.6.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan NA, Besnard P. Oro-sensory perception of dietary lipids: new insights into the fat taste transduction. Biochim. Biophys. Acta. 2009:149–155. doi: 10.1016/j.bbalip.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 26.van Oostrom AJHHM, et al. Diurnal triglyceride profiles in healthy normolipidemic male subjects are associated to insulin sensitivity, body composition and diet. Eur. J. Clin. Invest. 2000;30:964–971. doi: 10.1046/j.1365-2362.2000.00732.x. [DOI] [PubMed] [Google Scholar]
- 27.Teff K, et al. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J. Clin. Endocrinol. Metab. 2004;89:2963–2972. doi: 10.1210/jc.2003-031855. [DOI] [PubMed] [Google Scholar]
- 28.Schneeman BO, et al. Relationships between the responses of triglyceride-rich lipoproteins in blood plasma containing apolipoproteins B-48 and B-100 to a fat containing meal in normolipidemic humans. Proc. Natl. Acad. Sci. U. S. A. 1993;90:2069–2073. doi: 10.1073/pnas.90.5.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heath RB, et al. Dietary fatty acids make a rapid and substantial contribution to VLDL-triacylglycerol in the fed state. Am. J. Physiol. Endocrinol. Metab. 2007;292(3):E732–E739. doi: 10.1152/ajpendo.00409.2006. [DOI] [PubMed] [Google Scholar]
- 30.Evans K, et al. Rapid chylomicron clearance following sequential meals: effects of second meal composition. Br. J. Nutr. 1998;79:425–429. doi: 10.1079/bjn19980072. [DOI] [PubMed] [Google Scholar]
- 31.Randle PJ, Garland PB, Newsholm EA. The glucose-fatty acid cycle: its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 32.Ruge T, et al. Fasted to fed trafficking of fatty acids in human adipose tissue reveals a novel regulatory step for enhanced fat storage. J. Clin. Endocrinol. Metab. 2009;94:1781–1788. doi: 10.1210/jc.2008-2090. [DOI] [PubMed] [Google Scholar]
- 33.Ferrano RT, et al. Relationship between skeletal muscle lipoprotein lipase activity and 24-hour macronutrient oxidation. J. Clin. Invest. 1993;92:441–445. doi: 10.1172/JCI116586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guy-Grand B, Rebuffe-Scrive M. lipase in human adipose tissue: nutritional control and pathological variations. In: Crepaldi G, Lefebvre PJ, Galton DJ, editors. Diabetes, Obesity, and Hyperlipidemias II. London: Academic Press; 1983. pp. 169–178. [Google Scholar]
- 35.Sadur CN, Yost TJ, Eckel RH. Fat feeding decreases insulin responsiveness of adipose tissue to lipoprotein lipase. Metabolism. 1984;33(11):1043–1047. doi: 10.1016/0026-0495(84)90235-x. [DOI] [PubMed] [Google Scholar]
- 36.Taskinen MR, Nikkila EA. Lipoprotein lipase of adipose tissue and skeletal muscle in human obesity: response to glucose and to semistarvation. Metabolism. 1981;30(8):810–817. doi: 10.1016/0026-0495(81)90028-7. [DOI] [PubMed] [Google Scholar]
- 37.Yost TJ, et al. Effect of dietary macronutrient composition on tissue-specific lipoprotein lipase activity and insulin action in normal-weight subjects. Am. J. Clin. Nutr. 1998;68(2):296–302. doi: 10.1093/ajcn/68.2.296. [DOI] [PubMed] [Google Scholar]
- 38.Kiens B, et al. Effects of insulin and exercise on muscle lipoprotein lipase activity in man and its relation to insulin action. J. Clin. Invest. 1989;84:1124–1129. doi: 10.1172/JCI114275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farese RV, Jr., Yost TJ, Eckel RH. Tissue-specific regulation of lipoprotein lipase activity by insulin/glucose in normal-weight humans. Metabolism. 1991;40(2):214–216. doi: 10.1016/0026-0495(91)90178-y. [DOI] [PubMed] [Google Scholar]
- 40.Peterson J, et al. Fatty acid control of lipoprotein lipase: a link between energy metabolism and lipid transport. Proc. Natl. Acad. Sci. U. S. A. 1990;87(3):909–913. doi: 10.1073/pnas.87.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miles JM, et al. Systemic and forearm triglyceride metabolism: fate of lipoprotein lipase-generated glycerol and free fatty acids. Diabetes. 2004;53:521–527. doi: 10.2337/diabetes.53.3.521. [DOI] [PubMed] [Google Scholar]
- 42.Barrows BR, Timlin MT, Parks EJ. Spillover of dietary fatty acids and use of serum nonesterified fatty acids for the synthesis of VLDL-triacylglycerol under two different feeding regimens. Diabetes. 2005;54:2668–2673. doi: 10.2337/diabetes.54.9.2668. [DOI] [PubMed] [Google Scholar]
- 43.Bickerton AST, et al. Preferential uptake of dietary fatty acids in adipose tissue and muscle in the postprandial period. Diabetes. 2007;56(1):168–176. doi: 10.2337/db06-0822. [DOI] [PubMed] [Google Scholar]
- 44.Faraj M, et al. Enhanced dietary fat clearance in postobese women. J. Lipid Res. 2001;42:571–580. [PubMed] [Google Scholar]
- 45.Ravussin E, Smith SR. Increased fat intake, impaired fat oxidation, and failure of fat cell proliferation result in ectopic fat storage, insulin resistance, and type 2 diabetes mellitus. Ann. N. Y. Acad. Sci. 2002 Jun;967:363–378. doi: 10.1111/j.1749-6632.2002.tb04292.x. [DOI] [PubMed] [Google Scholar]
- 46.Jones PJH, Pencharz PB, Clandinin MT. Whole body oxidation of dietary fatty acids: implications for energy utilization. Am. J. Clin. Nutr. 1985;42(5):769–777. doi: 10.1093/ajcn/42.5.769. [DOI] [PubMed] [Google Scholar]
- 47.DeLany JP, et al. Differential oxidation of individual dietary fatty acids in humans. Am. J. Clin. Nutr. 2000;72:905–911. doi: 10.1093/ajcn/72.4.905. [DOI] [PubMed] [Google Scholar]
- 48.Raman A, et al. Validation of deuterium-labeled fatty acids for the measurement of dietary fat oxidation during physical activity. J. Lipid Res. 2004;45(12):2339–2344. doi: 10.1194/jlr.M400289-JLR200. [DOI] [PubMed] [Google Scholar]
- 49.Bergouignan A, et al. Physical inactivity differentially alters dietary oleate and palmitate trafficking. Diabetes. 2009;58(2):367–376. doi: 10.2337/db08-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Votruba SB, Zeddun SM, Schoeler DA. Validation of deuterium labeled fatty acids for the measurement of dietary fat oxidation: a method formeasuring fat-oxidation in free-living subjects. Int. J. Obes. 2001;25:1240–1245. doi: 10.1038/sj.ijo.0801672. [DOI] [PubMed] [Google Scholar]
- 51.Cooper JA, et al. Influence of dietary fatty acid composition and exercise on changes in fat oxidation from a high-fat diet. J. Appl. Physiol. 2010;109(4):1011–1018. doi: 10.1152/japplphysiol.01025.2009. [DOI] [PubMed] [Google Scholar]
- 52.Soares MJ, et al. The acute effects of olive oil v. cream on postprandial thermogenesis and substrate oxidation in postmenopausalwomen. Br. J. Nutr. 2004;91(2):245–252. doi: 10.1079/BJN20031047. [DOI] [PubMed] [Google Scholar]
- 53.Lovejoy JC, et al. Effects of diets enriched in saturated (palmitic), monounsaturated (oleic), or trans (elaidic) fatty acids on insulin sensitivity and substrate oxidation in healthy adults. Diabetes. 2002;Care 25(8):1283–1288. doi: 10.2337/diacare.25.8.1283. [DOI] [PubMed] [Google Scholar]
- 54.Piers LS, et al. Substitution of saturated with monounsaturated fat in a 4-week diet affects body weight and composition of overweight and obese men. Br. J. Nutr. 2003;90(3):717–727. doi: 10.1079/bjn2003948. [DOI] [PubMed] [Google Scholar]
- 55.MacDougall DE, et al. Utilization ofmyristic and palmitic acid in humans fed different dietary fats. Eur. J. Clin. Invest. 1996;26:755–762. doi: 10.1046/j.1365-2362.1996.1980545.x. [DOI] [PubMed] [Google Scholar]
- 56.Jones AE, et al. Effect of fatty acid chain length and saturation on the gastrointestinal handling and metabolic disposal of dietary fatty acids in women. Br. J. Nutr. 1999;81(1):37–43. [PubMed] [Google Scholar]
- 57.Roberts R, et al. Reduced oxidation of dietary fat after a short termhigh-carbohydrate diet. Am. J. Clin. Nutr. 2008;87(4):824–831. doi: 10.1093/ajcn/87.4.824. [DOI] [PubMed] [Google Scholar]
- 58.Jensen MD, et al. Regional uptake of meal fatty acids in humans. Am. J. Phys. 2003;285(6):E1282–E1288. doi: 10.1152/ajpendo.00220.2003. [DOI] [PubMed] [Google Scholar]
- 59.Roust LR, Jensen MD. Postprandial free fatty acid kinetics are abnormal in upper body obesity. Diabetes. 1993;42:1567–1573. doi: 10.2337/diab.42.11.1567. [DOI] [PubMed] [Google Scholar]
- 60.Santosa S, et al. The influence of sex and obesity phenotype on meal fatty acid metabolism before and after weight loss. Am. J. Clin. Nutr. 2008;88:1134–1141. doi: 10.1093/ajcn/88.4.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Basu A, et al. Meal fat storage in subcutaneous adipose tissue: comparison of pioglitazone and glipizide treatment of type 2 diabetes. Obesity. 2010;18:2058–2060. doi: 10.1038/oby.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Uranga AP, Levine J, Jensen MD. Isotope tracer measures of meal fatty acid metabolism: reproducibility and effects of the menstrual cycle. Am. J. Physiol. Endocrinol. Metab. 2005;288:E547–E555. doi: 10.1152/ajpendo.00340.2004. [DOI] [PubMed] [Google Scholar]
- 63.Evans K, et al. Regulation of dietary fatty acid entrapment in subcutaneous adipose tissue and skeletal muscle. Diabetes. 2002;51(9):2684–2690. doi: 10.2337/diabetes.51.9.2684. [DOI] [PubMed] [Google Scholar]
- 64.Hodson L, Frayn KN. Hepatic fatty acid partitioning. Curr. Opin. Lipidol. 2011;22(3):216–224. doi: 10.1097/MOL.0b013e3283462e16. [DOI] [PubMed] [Google Scholar]
- 65.Kien CL, et al. Short-term effects of dietary fatty acids on muscle lipid composition and serum acylcarnitine profile in human subjects. Obesity. 2011;19(2):305–311. doi: 10.1038/oby.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Hees AM, et al. Effects of dietary fat modification on skeletal muscle fatty acid handling in the metabolic syndrome. Int. J. Obes. 2010;34(5):859–870. doi: 10.1038/ijo.2010.6. [DOI] [PubMed] [Google Scholar]
- 67.Schrauwen-Hinderling VB, et al. Intramyocellular lipid content andmolecular adaptations in response to a 1-week high-fat diet. Obes. Res. 2005;13(12):2088–2094. doi: 10.1038/oby.2005.259. [DOI] [PubMed] [Google Scholar]
- 68.van Hees AM, et al. Skeletal muscle fatty acid handling in insulin resistant men. Obesity. 2011;19(7):1350–1359. doi: 10.1038/oby.2011.10. [DOI] [PubMed] [Google Scholar]
- 69.Labbé SM, et al. Organ-specific dietary fatty acid uptake in humans using positron emission tomography coupled to computed tomography. Am. J. Physiol. Endocrinol. Metab. 2011;300(3):E445–E453. doi: 10.1152/ajpendo.00579.2010. [DOI] [PubMed] [Google Scholar]
- 70.Labbé SM, et al. Normal postprandial nonesterified fatty acid uptake in muscles despite increased circulating fatty acids in type 2 diabetes. Diabetes. 2011;60(2):408–415. doi: 10.2337/db10-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Szczepaniak LS, et al. Forgotten but not gone: the rediscovery of fatty heart, the most common unrecognized disease in America. Circ. Res. 2007;101(8):759–767. doi: 10.1161/CIRCRESAHA.107.160457. [DOI] [PubMed] [Google Scholar]
- 72.Rolls BJ, Shide DJ. The influence of dietary fat on food intake and body weight. Nutr. Rev. 1993;50(10):283–290. doi: 10.1111/j.1753-4887.1992.tb02466.x. [DOI] [PubMed] [Google Scholar]
- 73.Lichtenstein AH, et al. Dietary fat consumption and health. Nutr. Rev. 1998;56(5 Pt 2):S3–S19. doi: 10.1111/j.1753-4887.1998.tb01728.x. discussion S19-28. [DOI] [PubMed] [Google Scholar]
- 74.Grundy SM. Dietary fat: at the heart of the matter. Science. 2001;293(5531):801–804. doi: 10.1126/science.293.5531.801d. [DOI] [PubMed] [Google Scholar]
- 75.Melanson EL, Astrup A, Donahoo WT. The relationship between dietary fat and fatty acid intake and body weight, diabetes, and the metabolic syndrome. Ann. Nutr. Metab. 2009;55(1–3):229–243. doi: 10.1159/000229004. [DOI] [PubMed] [Google Scholar]
- 76.Ravikumar B, et al. Real-time assessment of postprandial fat storage in liver and skeletal muscle in health and type 2 diabetes. Am. J. Physiol. 2005;288(4):E789–E797. doi: 10.1152/ajpendo.00557.2004. [DOI] [PubMed] [Google Scholar]
- 77.Barrows BR, Parks EJ. Contributions of different fatty acid sources to VLDLtriacylglycerol in the fasted and fed-states. J. Clin. Endocrinol. Metab. 2006;91(4):1446–1452. doi: 10.1210/jc.2005-1709. [DOI] [PubMed] [Google Scholar]
- 78.Timlin MT, Barrows BR, Parks EJ. Increased dietary substrate delivery alters hepatic fatty acid recycling in healthy men. Diabetes. 2005;54:2694–2701. doi: 10.2337/diabetes.54.9.2694. [DOI] [PubMed] [Google Scholar]
- 79.Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011;60(10):2441–2449. doi: 10.2337/db11-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Greco D, et al. Gene expression in human NAFLD. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;294(5):G1281–G1287. doi: 10.1152/ajpgi.00074.2008. [DOI] [PubMed] [Google Scholar]
- 81.Burdge GC, et al. Effect of meal sequence on postprandial lipid, glucose and insulin responses in young men. Eur. J. Clin. Nutr. 2003;57(12):1536–1544. doi: 10.1038/sj.ejcn.1601722. [DOI] [PubMed] [Google Scholar]