Abstract
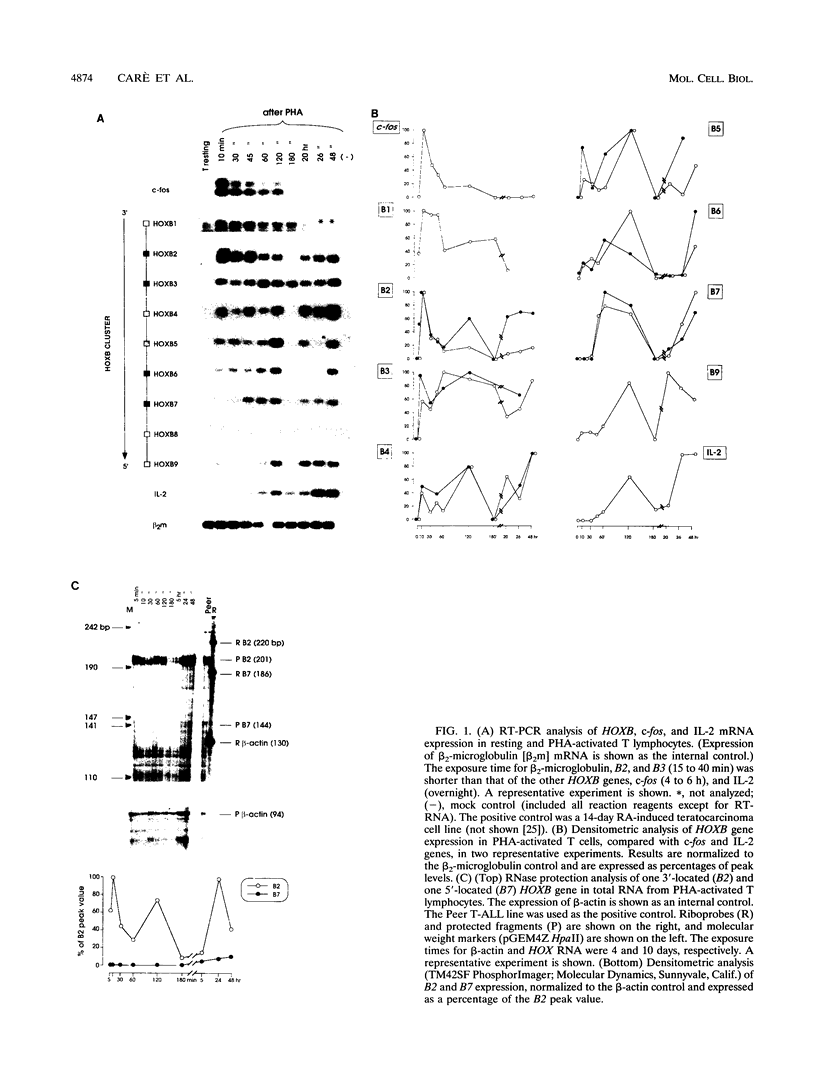
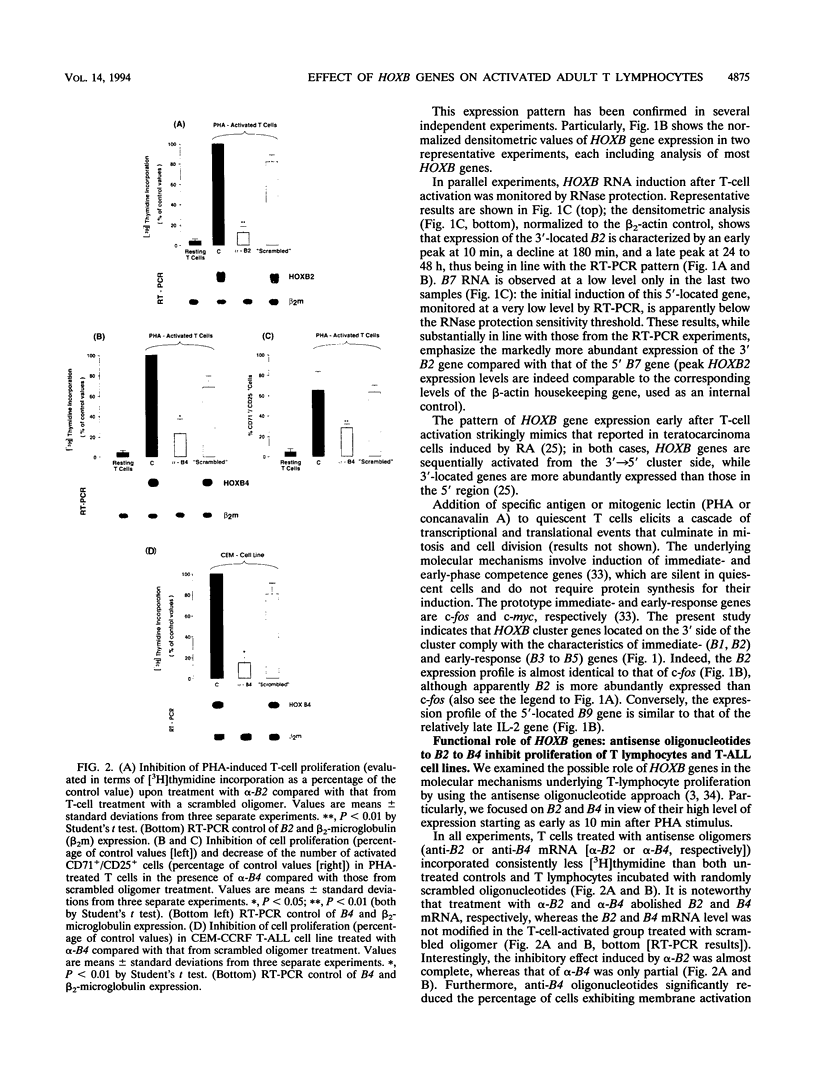
We investigated the expression of HOXB cluster genes in purified phytohemagglutinin (PHA)-activated T lymphocytes from normal adult peripheral blood by reverse transcription PCR and RNase protection. These genes are not expressed in quiescent T cells, except for barely detectable B1 RNA. After the PHA stimulus, HOXB gene activation initiates coordinately as a rapid induction wave in the 3'-->5' cluster direction (i.e., from HOXB1 through B9 genes). Thus, (i) expression of the foremost 3'-located B1 and B2 genes peaks 10 min after PHA addition and then rapidly declines, (ii) activation of B3, B4, and B5 begins 10 min after PHA addition and peaks at later times (i.e., at 120 min for B5), (iii) B6, B7, and B9 are expressed at a low level starting at later times (45 to 60 min), and (iv) B8 remains silent. Treatment of PHA-activated T lymphocytes with antisense oligonucleotides to B2 or B4 mRNA causes a drastic inhibition of T-cell proliferation and a decreased expression of T-cell activation markers (i.e., interleukin 2 and transferrin receptors). Similarly, treatment of CEM-CCRF, Peer, and SEZ627 T acute lymphocytic leukemia cell lines with anti-B4 oligomer markedly inhibits cell proliferation. Finally, T cells stimulated by a low dosage of PHA in the presence of 1 microM retinoic acid show a marked increase of both HOXB expression, particularly B2, and cell proliferation. These studies provide novel evidence on the role of HOX genes in adult cell proliferation. (i) Coordinate, early activation of HOXB genes from the 3'-->5' cluster side apparently underlies T-cell activation. (ii) The expression pattern in adult PHA-activated T cells is strikingly similar to that observed in retinoic acid-induced teratocarcinoma cells (A. Simeone, D. Acampora, L. Arcioni, P. W. Andres, E. Boncinelli, and F. Mavilio, Nature (London) 346:763-766, 1990), thus suggesting that molecular mechanisms underlying HOX gene expression in the earliest stages of development may also operate in activated adult T lymphocytes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acampora D., D'Esposito M., Faiella A., Pannese M., Migliaccio E., Morelli F., Stornaiuolo A., Nigro V., Simeone A., Boncinelli E. The human HOX gene family. Nucleic Acids Res. 1989 Dec 25;17(24):10385–10402. doi: 10.1093/nar/17.24.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck J., Grün F., Derguini F., Chen Y., Kimura S., Noy N., Hämmerling U. Anhydroretinol: a naturally occurring inhibitor of lymphocyte physiology. J Exp Med. 1993 Aug 1;178(2):675–680. doi: 10.1084/jem.178.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caracciolo D., Valtieri M., Venturelli D., Peschle C., Gewirtz A. M., Calabretta B. Lineage-specific requirement of c-abl function in normal hematopoiesis. Science. 1989 Sep 8;245(4922):1107–1110. doi: 10.1126/science.2672339. [DOI] [PubMed] [Google Scholar]
- Deguchi Y., Moroney J. F., Kehrl J. H. Expression of the HOX-2.3 homeobox gene in human lymphocytes and lymphoid tissues. Blood. 1991 Jul 15;78(2):445–450. [PubMed] [Google Scholar]
- Deschamps J., Meijlink F. Mammalian homeobox genes in normal development and neoplasia. Crit Rev Oncog. 1992;3(1-2):117–173. [PubMed] [Google Scholar]
- Eppinger T. M., Buck J., Hämmerling U. Growth control or terminal differentiation: endogenous production and differential activities of vitamin A metabolites in HL-60 cells. J Exp Med. 1993 Dec 1;178(6):1995–2005. doi: 10.1084/jem.178.6.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbe A., Buck J., Hämmerling U. Retinoids are important cofactors in T cell activation. J Exp Med. 1992 Jul 1;176(1):109–117. doi: 10.1084/jem.176.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giampaolo A., Acampora D., Zappavigna V., Pannese M., D'Esposito M., Carè A., Faiella A., Stornaiuolo A., Russo G., Simeone A. Differential expression of human HOX-2 genes along the anterior-posterior axis in embryonic central nervous system. Differentiation. 1989 Jun;40(3):191–197. doi: 10.1111/j.1432-0436.1989.tb00598.x. [DOI] [PubMed] [Google Scholar]
- Graham A., Papalopulu N., Krumlauf R. The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell. 1989 May 5;57(3):367–378. doi: 10.1016/0092-8674(89)90912-4. [DOI] [PubMed] [Google Scholar]
- Hatano M., Roberts C. W., Minden M., Crist W. M., Korsmeyer S. J. Deregulation of a homeobox gene, HOX11, by the t(10;14) in T cell leukemia. Science. 1991 Jul 5;253(5015):79–82. doi: 10.1126/science.1676542. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Look A. T., Baltimore D. The human t(1;19) translocation in pre-B ALL produces multiple nuclear E2A-Pbx1 fusion proteins with differing transforming potentials. Genes Dev. 1991 Mar;5(3):358–368. doi: 10.1101/gad.5.3.358. [DOI] [PubMed] [Google Scholar]
- Lowney P., Corral J., Detmer K., LeBeau M. M., Deaven L., Lawrence H. J., Largman C. A human Hox 1 homeobox gene exhibits myeloid-specific expression of alternative transcripts in human hematopoietic cells. Nucleic Acids Res. 1991 Jun 25;19(12):3443–3449. doi: 10.1093/nar/19.12.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magli M. C., Barba P., Celetti A., De Vita G., Cillo C., Boncinelli E. Coordinate regulation of HOX genes in human hematopoietic cells. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6348–6352. doi: 10.1073/pnas.88.14.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall H., Nonchev S., Sham M. H., Muchamore I., Lumsden A., Krumlauf R. Retinoic acid alters hindbrain Hox code and induces transformation of rhombomeres 2/3 into a 4/5 identity. Nature. 1992 Dec 24;360(6406):737–741. doi: 10.1038/360737a0. [DOI] [PubMed] [Google Scholar]
- Mathews C. H., Detmer K., Boncinelli E., Lawrence H. J., Largman C. Erythroid-restricted expression of homeobox genes of the human HOX 2 locus. Blood. 1991 Nov 1;78(9):2248–2252. [PubMed] [Google Scholar]
- Mavilio F. Regulation of vertebrate homeobox-containing genes by morphogens. Eur J Biochem. 1993 Mar 1;212(2):273–288. doi: 10.1111/j.1432-1033.1993.tb17660.x. [DOI] [PubMed] [Google Scholar]
- Mavilio F., Simeone A., Giampaolo A., Faiella A., Zappavigna V., Acampora D., Poiana G., Russo G., Peschle C., Boncinelli E. Differential and stage-related expression in embryonic tissues of a new human homoeobox gene. Nature. 1986 Dec 18;324(6098):664–668. doi: 10.1038/324664a0. [DOI] [PubMed] [Google Scholar]
- Nourse J., Mellentin J. D., Galili N., Wilkinson J., Stanbridge E., Smith S. D., Cleary M. L. Chromosomal translocation t(1;19) results in synthesis of a homeobox fusion mRNA that codes for a potential chimeric transcription factor. Cell. 1990 Feb 23;60(4):535–545. doi: 10.1016/0092-8674(90)90657-z. [DOI] [PubMed] [Google Scholar]
- Perkins A. C., Cory S. Conditional immortalization of mouse myelomonocytic, megakaryocytic and mast cell progenitors by the Hox-2.4 homeobox gene. EMBO J. 1993 Oct;12(10):3835–3846. doi: 10.1002/j.1460-2075.1993.tb06062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins A., Kongsuwan K., Visvader J., Adams J. M., Cory S. Homeobox gene expression plus autocrine growth factor production elicits myeloid leukemia. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8398–8402. doi: 10.1073/pnas.87.21.8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini M., Quaranta M. T., Testa U., Samoggia P., Tritarelli E., Carè A., Cianetti L., Valtieri M., Barletta C., Peschle C. Expression of selected human HOX-2 genes in B/T acute lymphoid leukemia and interleukin-2/interleukin-1 beta-stimulated natural killer lymphocytes. Blood. 1992 Jul 1;80(1):185–193. [PubMed] [Google Scholar]
- Privitera E., Kamps M. P., Hayashi Y., Inaba T., Shapiro L. H., Raimondi S. C., Behm F., Hendershot L., Carroll A. J., Baltimore D. Different molecular consequences of the 1;19 chromosomal translocation in childhood B-cell precursor acute lymphoblastic leukemia. Blood. 1992 Apr 1;79(7):1781–1788. [PubMed] [Google Scholar]
- Simeone A., Acampora D., Arcioni L., Andrews P. W., Boncinelli E., Mavilio F. Sequential activation of HOX2 homeobox genes by retinoic acid in human embryonal carcinoma cells. Nature. 1990 Aug 23;346(6286):763–766. doi: 10.1038/346763a0. [DOI] [PubMed] [Google Scholar]
- Simeone A., Acampora D., Nigro V., Faiella A., D'Esposito M., Stornaiuolo A., Mavilio F., Boncinelli E. Differential regulation by retinoic acid of the homeobox genes of the four HOX loci in human embryonal carcinoma cells. Mech Dev. 1991 Mar;33(3):215–227. doi: 10.1016/0925-4773(91)90029-6. [DOI] [PubMed] [Google Scholar]
- Simeone A., Mavilio F., Acampora D., Giampaolo A., Faiella A., Zappavigna V., D'Esposito M., Pannese M., Russo G., Boncinelli E. Two human homeobox genes, c1 and c8: structure analysis and expression in embryonic development. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4914–4918. doi: 10.1073/pnas.84.14.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone A., Mavilio F., Bottero L., Giampaolo A., Russo G., Faiella A., Boncinelli E., Peschle C. A human homoeo box gene specifically expressed in spinal cord during embryonic development. Nature. 1986 Apr 24;320(6064):763–765. doi: 10.1038/320763a0. [DOI] [PubMed] [Google Scholar]
- Sposi N. M., Zon L. I., Carè A., Valtieri M., Testa U., Gabbianelli M., Mariani G., Bottero L., Mather C., Orkin S. H. Cell cycle-dependent initiation and lineage-dependent abrogation of GATA-1 expression in pure differentiating hematopoietic progenitors. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6353–6357. doi: 10.1073/pnas.89.14.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita K., Bollekens J. A., Hijiya N., Ratajczak M., Ruddle F. H., Gewirtz A. M. A homeobox gene of the Antennapedia class is required for human adult erythropoiesis. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3535–3538. doi: 10.1073/pnas.90.8.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulmé J. J., Hélène C. Antimessenger oligodeoxyribonucleotides: an alternative to antisense RNA for artificial regulation of gene expression--a review. Gene. 1988 Dec 10;72(1-2):51–58. doi: 10.1016/0378-1119(88)90127-8. [DOI] [PubMed] [Google Scholar]
- Tuggle C. K., Zakany J., Cianetti L., Peschle C., Nguyen-Huu M. C. Region-specific enhancers near two mammalian homeo box genes define adjacent rostrocaudal domains in the central nervous system. Genes Dev. 1990 Feb;4(2):180–189. doi: 10.1101/gad.4.2.180. [DOI] [PubMed] [Google Scholar]
- Ullman K. S., Northrop J. P., Verweij C. L., Crabtree G. R. Transmission of signals from the T lymphocyte antigen receptor to the genes responsible for cell proliferation and immune function: the missing link. Annu Rev Immunol. 1990;8:421–452. doi: 10.1146/annurev.iy.08.040190.002225. [DOI] [PubMed] [Google Scholar]
- Valtieri M., Venturelli D., Caré A., Fossati C., Pelosi E., Labbaye C., Mattia G., Gewirtz A. M., Calabretta B., Peschle C. Antisense myb inhibition of purified erythroid progenitors in development and differentiation is linked to cycling activity and expression of DNA polymerase alpha. Blood. 1991 Mar 15;77(6):1181–1190. [PubMed] [Google Scholar]
- Zappavigna V., Renucci A., Izpisúa-Belmonte J. C., Urier G., Peschle C., Duboule D. HOX4 genes encode transcription factors with potential auto- and cross-regulatory capacities. EMBO J. 1991 Dec;10(13):4177–4187. doi: 10.1002/j.1460-2075.1991.tb04996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]