Abstract
Of somatosensory modalities, cold is one of the more ambiguous percepts, evoking the pleasant sensation of cooling, the stinging bite of cold pain, and welcome relief from chronic pain. Moreover, unlike the precipitous thermal thresholds for heat activation of thermosensitive afferent neurons, thresholds for cold fibers are across a range of cool to cold temperatures that spans over 30 °C. Until recently, how cold produces this myriad of biological effects has been poorly studied, yet new advances in our understanding of cold mechanisms may portend a better understanding of sensory perception as well as provide novel therapeutic approaches. Chief among these was the identification of a number of ion channels that either serve as the initial detectors of cold as a stimulus in the peripheral nervous system, or are part of rather sophisticated differential expression patterns of channels that conduct electrical signals, thereby endowing select neurons with properties that are amenable to electrical signaling in the cold. This review highlights the current understanding of the channels involved in cold transduction as well as presents a hypothetical model to account for the broad range of cold thermal thresholds and distinct functions of cold fibers in perception, pain, and analgesia.
Keywords: cold, TRPM8, pain, thermosensation, menthol, TRP channel
Temperature acuity is a finely tuned part of our somatosensory system, allowing us to both avoid thermal conditions in nature that may be potentially harmful, as well as attract organisms to the thermal clime that is the most amenable to survival.1 Our perception of temperature falls into four loosely segregated categories: innocuously cool or warm and painfully hot or cold. Each is a uniquely distinct percept, yet innocuous temperatures, warm or cool, are those that animals will seek out depending on their environment, whereas noxious hot or cold can be tremendously aversive and evoke robust and instinctive withdrawal responses.
Generally, the perception of innocuously cool temperatures occurs when the skin is cooled as little as 1 °C from a basal temperature of 32 °C, and studies of thermal acuity in humans finds the thermal thresholds for warm and cold detection near ∼34 and 31 °C, respectively, showing little deviation between subjects with response threshold ranging by only a few degrees.2 Cold and heat pain thresholds were reported at ∼12 and 45 °C, respectively; however, cold pain was felt over a much broader temperature range, from 0 to 28 °C, compared to heat pain, which ranged from 39 to 50 °C, demonstrating cold to be a more indefinite percept in comparison to heat. Similarly, there are prominent differences in the manner in which cold pain is perceived compared to the other thermal percepts.3 For instance, when subjects were asked to provide verbal descriptors for the sensation of a noxious cold stimulus versus that of noxious heat, a wider range of words were used for cold than for heat (largely burning), with cold pain felt as sharp, stinging, aching, and pricking. In a further distinction of noxious cold, when subjects were exposed to cool, warm, or hot temperatures, the latency to cessation of these distinct percepts after the stimulus was removed was only a few seconds, whereas the pain felt by noxious cold lasted approximately four times longer.3 These results highlight the murkiness with which we perceive cold temperatures, psychophysical data that is only now being correlated to cellular responses.
When considering temperature perception at the cellular level, afferent sensory neurons of either the dorsal root (DRG) or trigeminal ganglia (TG) are the site of detection with select afferent populations, primarily small diameter C-fibers and medium diameter Aδ-fibers, activated at distinct temperature thresholds.1 For example, the thermal activation threshold of a large population of presumptive noxious heat-sensing sensory afferents occurs near 45 °C in vitro, coinciding with the temperature at which noxious heat is perceived psychophysically.2,4 Similarly there are afferents that respond at thermal thresholds in the warm (30–40 °C), innocuous cool (<30–15 °C), and noxious cold (<15 °C) range with their coordinated signals providing the overall sense of any given temperature.4−7 This review focuses on the low end of thermometer and those temperatures that are perceived as cold, and describes our current understanding of the cellular mechanisms leading to the activation of cold afferents.
Cold Responsiveness at the Cellular Level
Seminal studies starting in the mid-20th century and extending into today identified single nerve fibers that respond to cold with differing temperature thresholds, response properties that allowed these afferents to be categorized as cold thermoreceptors responsible for detecting innocuous cool sensations and cold nociceptors that evoke the perception of painful cold.8−12 Many cold thermoreceptors in both humans and rodents show spontaneous activity at normal skin temperature, with cold stimuli inducing an increase in their rate of firing, while warm temperatures reduce this activity,13−15 results suggesting a level of tonic excitatory drive from cold thermoreceptors to the spinal cord dorsal horn where these cells synapse. These properties are distinct from cold nociceptors that likely mediate cold pain, which are silent at normal skin temperature and only become active when the skin is cooled to those in the noxious cold range,10,11 properties consistent with the classical definition of nociceptors.16 However, the exact proportion of thermoreceptors or nociceptors that respond to cold temperatures is not clear, with reported percentages of neurons deemed cold sensitive in electrophysiological recordings ranging near 10–15% for cool to cold temperatures, and up to 100% if the stimulus intensity drops below freezing.9,10,13 Moreover, the mechanism whereby an enzymatically unfavorable decrease in temperature could lead to increased neural activity was a grand mystery for many decades.
What then is the transduction mechanism for cold? Intuitively, one that incorporated cold-mediated inhibition of proteins involved in maintaining ionic gradients in excitable neurons was an attractive model. Data showing that inhibition of the Na+/K+ pump increased the static discharge frequency of cold fibers was one of the first hypothetical models.17 Indeed several reports found that oubain-mediated inhibition of the Na+/K+-ATPase affected cold-fiber responses,17−20 but later data demonstrating that the magnitude of the depolarization was largely subthreshold discounted this as a transduction mechanism.21 Following this same reasoning, several groups suggested that inhibition of potassium conductances normally serving as excitability brakes to depolarization could provoke cold-based nerve firing.21−23 Indeed, normally cold-insensitive neurons could be made cold-sensitive via inhibition of a slow transient potassium current likely composed of Kv channels,22 however, to this day the molecular identities of putative cold relevant K+-channels have not yet been determined. Similarly, the loss of the two-pore K+ channels TREK-1 and TRAAK lowers the threshold for neural activation by cold.24 These channels act as leak currents and regulate membrane potential, likely playing an important role in membrane excitability in sensory afferents, and their absence makes cold receptors more readily depolarized. However, these and other proposed mediators of cold are largely ubiquitously expressed regulators of membrane polarization, suggesting that these proteins may have modulatory roles in cold neuron activity, in addition to other cellular functions, and are not determinants of cold sensitivity.21,24,25
If inhibition is not involved, could cold actually stimulate activity of a cold receptor protein? Suto and Gotoh presented some of the first evidence suggesting cold-dependent changes in cell function when they observed a cold-induced increase in intracellular Ca2+ concentration in cultured rat DRG neurons when the bath temperature was lowered to 20 °C.26 These responses were due to Ca2+ influx as they required the presence of external Ca2+ and were also independent of external sodium, suggesting that Na+-dependent membrane depolarization was not involved. This and subsequent studies observed that approximately 10–15% of sensory neurons isolated from TG or DRG respond to cold temperatures at thresholds for activation ranging from ∼35 to near 15 °C.25−30 These responses were remarkably similar to those evoked by noxious heat,31 yet the scarcity of cold sensitive neurons made more direct measures of neuronal activity, such as electrophysiology, challenging.22,25,29,30 When examined in more detail, several studies find the presence of two distinct groups of cold neurons, distinguished by response properties indicative of cool thermoreceptors (low-threshold (LT) activation temperature near 30 °C) and a second class of neurons suggested to be the cold nociceptor subset (high-threshold (HT) activation temperature below 20 °C).25,28,32 These different activation thresholds suggest the former cells to be an in vitro model for innocuously cool signaling afferents, while the latter may be analogous to those mediating noxious cold. Indeed the HT population was largely capsaicin sensitive, further implicating these cells as nociceptors.28 However, it appears that scarcity of cells within a ganglion that are cold-sensitive may have been one of the most significant hindrances in our understanding of cold transduction mechanisms.
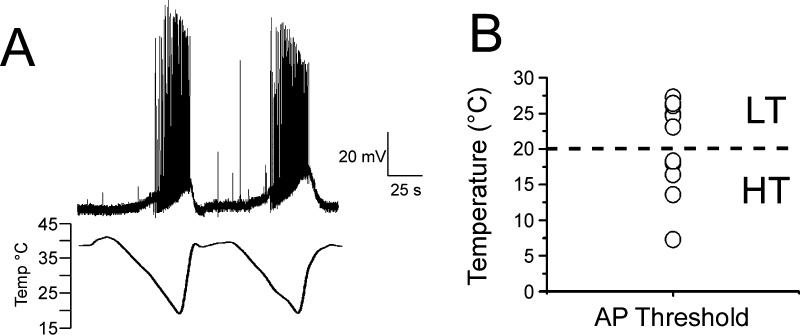
Natural products produced by plants have been fundamental in our understanding of nervous system function. This is also true for cold in that the vast majority of cold sensitive neurons respond to the cold-mimetic menthol, a cyclic terpene alcohol found in leaves of the genus Mentha and commonly included in many commercially available products.33 The sensation of pleasantly cool evoked by menthol is well-known, but at higher concentrations it can cause burning, irritation, and pain,34−36 yet it was unclear how menthol related to cold activation of sensory afferents. Seminal studies by Hensel and Zotterman examining cold sensitivity in cat lingual nerve recordings found that menthol raised the cold activation temperature of cold fibers, results suggesting that these effects were due to modulation of an as yet unidentified cold detection mechanism.37 Indeed they proposed that menthol would “...exert its action upon an enzyme, which is concerned in the thermally conditioned regulation of the discharge of the cold receptors.”37 It took over 5 decades, but validation for this hypothesis came from study of cultured sensory neurons where ∼15% of excitable cells were menthol-sensitive (assayed largely with Ca2+-microfluorimetry), and in most reports there was a strong correlation with menthol and cold sensitivity at the cellular level.22,29,30,38 Moreover, rigorous experiments in which Ca2+-imaging based screens for cold- and menthol-sensitive neurons coupled with electrophysiology were the first to show that both cold and menthol activated a nonselective cation conductance in sensory afferents, with biophysical properties reminiscent of heat-gated, and presumably TRPV1 mediated responses.29,30 Menthol-evoked currents are observed in cold-sensitive neurons of both the LT and HT classes, but are more robust in the former compared to the latter.25,28 More recently, the use of transgenic reporter mice labeling cold-sensitive afferents both in vivo and in vitro39−41 has allowed a direct analysis of putative cold-responsive neurons (Figure 1A). In current-clamp mode, we find that presumptive cold neurons in culture also respond over a wide-range of cold thermal thresholds (Figure 1B), suggesting that Ca2+ imaging approaches can be a reasonable first approximation in identifying different cold-sensitive cell types. Thus, these cultured sensory afferents have been a useful experimental model and provided insights into the mechanisms of cold signaling.
Figure 1.
Broad range of cold temperature thresholds in TRPM8-expressing sensory neurons. (A) Current-clamp recordings of cold-evoked action potentials in genetically labeled cold-sensitive trigeminal neurons. Figure adapted from Daniels et al. 2009.39 (B) Scatter plot of cold activation thresholds in neurons recorded as in (A) displays the range of temperature thresholds in cold-sensitive sensory afferent neurons.
Cold Responses Mediated by TRPM8
Cold- and menthol-evoked activity of a biophysically identifiably cation conductance suggested the presence of a specific protein (as Hensel and Zotterman proposed) mediating cold neural activity. Moreover, the similarity in the properties of this current to those of TRPV1 channels suggested a similar transduction mechanism. These two hypotheses were used as the basis for two independent screens that identified TRPM8 (also called Trp-p8 or CMR1) as a cold- and menthol-gated ion channel.30,42 TRPM8 was originally discovered in a subtractive screen for molecules highly expressed in transformed prostate epithelial cells, and is considered a cancer cell marker in this context.43−46 However, it had yet to be identified in sensory ganglia. One approach was founded on a genomic screen for TRP ion channels with robust expression in sensory ganglia.42 This intuitive strategy was based on the previous identification of the heat-sensitive channel TRPV1 in sensory neurons47 and, in addition to TRPM8, was instrumental in the identification of other somatosensory relevant TRP channels such as TRPA1.48−51 The second strategy used an expression cloning paradigm similar to that used to identify TRPV1, and followed Hensel and Zotterman’s original posit that menthol’s action is “upon an enzyme” involved in cold transduction.37 Thus, if the molecular mediator of menthol’s action were to be identified, like that previously for capsaicin,47 this would provide insights into cold transduction.30 A screen for trigeminal neuron transcripts that could confer menthol-sensitivity to heterologous cells also identified TRPM8.30 Once cloned, the functional properties of recombinant TRPM8 channels were characterized and found to be remarkably similar to that observed for menthol- and cold-gated currents in neurons including temperature sensitivity, menthol sensitivity, cation selectivity, channel rectification, and Ca2+-dependent adaptation.30,38,42 Moreover, this protein fit with Hensel and Zotterman’s model in that menthol shifts the thermal sensitivity of TRPM8 toward warmer temperatures (Figure 2), thereby providing a clear mechanism for why menthol feels cold.30
Figure 2.
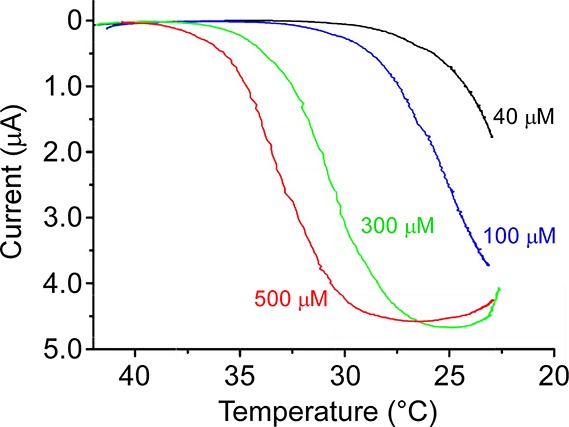
Menthol shifts the temperature dependence of TRPM8 channels toward warmer temperatures. Representative whole-cell voltage-clamp current recordings (−60 mV) versus bath temperature (ramp from ∼43 to 22 °C) of Xenopus laevis oocytes expressing recombinant rTRPM8 channels bathed in increasing concentrations of menthol (see ref (39)).
What endows TRPM8, and other thermosensitive TRP ion channels, with the ability to respond to temperature is still not clear, and has been extensively reviewed elsewhere.52−54 However, analysis of specific channel functions has begun to elucidate ways in which cold may activate TRPM8. For example, like many sensory systems, we adapt to cold stimuli5,13 as do recombinant TRPM8 channels.30 Several laboratories studying adaptation have found that TRPM8 activity is obligate for the presence of the membrane lipid phosphatidylinositol-4,5-bisphosphate (PIP2), the substrate for phospholipase C (PLC).55,56 Adaptation results from an influx of calcium ions through TRPM8 activating Ca2+-sensitive PLCδ isozymes, likely PLCδ3 or 4,39 which cleave PIP2, thereby reducing its levels in the inner leaflet of the plasma membrane. This reduces, or in some cases abolishes, channel activity, which can be restored by addition of exogenous PIP2.55,56 In addition to PIP2, binding of inorganic phosphates also modulates TRPM8 channel function,57,58 and TRPM8 channels have been shown to localize to lipid rafts where channel activity is inhibited, as evidenced by an increase in TRPM8 currents after raft disruption.59 Lastly, post-translational modification of TRPM8 channels by glycosylation can alter channel function.60
As PLC is a common downstream effector protein of many cell surface signaling receptors,61 this form of regulation likely strongly influences cold signaling via TRPM8.39 For example, cold and menthol evoked responses are diminished in DRG neurons treated with the inflammatory mediators such as bradykinin, prostaglandin E2, or histamine which activate their cognate G-protein-coupled receptors (GPCR), proposed to lower cold sensitivity under conditions of inflammation.62 While PLC activation downstream of these receptors likely plays an important role in decreased cold responses, recent evidence has shown that TRPM8 is regulated in a novel manner by direct binding of the G-proteins Gαq, inhibiting channel activity.63 Moreover, kinase activity is also reported to regulate channel function, although it is not clear if this is through a direct action on the channel.64 Taken together, these data suggest that regulation of channel activity is an amalgamation of multiple processes that may have distinct roles in regulating cold sensation in different cell types and under certain physiological conditions.54
The Role of the Irritant Receptor TRPA1 in Cold Sensation
Heat-gated TRPV channels are segregated by activation thresholds in the noxious heat range for TRPV1 and TRPV2, compared to TRPV3 and TRPV4 which are activated in the warm range,47,51,65,66 thereby providing an intuitive separation in the detection of pleasant from painful heat. Is a similar paradigm involved in cold sensation? The observation that the cold activation threshold of TRPM8 is approximately 23–26 °C suggested to many that the channel mediates innocuous cool, and that other transduction mechanisms are needed to account for noxious cold responses.67 In screens for TRP channels with robust expression in sensor neurons, TRPA1 was identified and shown to be activated by the TRPM8 agonist and supercooling compound icilin (AG-3–5)30,68 when expressed in heterologous expression systems.48 This original description of TRPA1 also reported that the channel is activated by cold. Calcium-imaging and voltage-clamp recordings of recombinant TRPA1 channels found that cold temperatures ranging from between 8 and 28 °C (average threshold of 17 °C) evoked an increase in intracellular Ca2+ and nonselective cation currents, respectively, that were blocked by ruthenium red, a blocker of several Ca2+-permeable channels.48 TRPA1 was first identified as a transformation-sensitive RNA transcript in human fibroblasts,69 whereas in sensory neurons transcripts were almost exclusively in nociceptive afferents that also express TRPV1, Substance P, and CGRP, but distinct from those expressing TRPM8.48 Thus, the expression pattern and activation threshold for TRPA1 made it attractive as the mediator of cold pain and, when coupled with the activation threshold of TRPM8, suggested a similar dual-sensor model for cold transduction as was known for heat.67,70
However, this hypothesis has proven controversial.67,71−75 Quickly after the identification of TRPA1 as a putative noxious cold sensor, it was found to be the receptor for several pungent irritants such as allyl isothiocyanate (the pungent ingredient in mustard oil and wasabi), allicin (the irritant in raw garlic), and cinnamaldehyde (the spicy component of cinnamon),72,76−78 all substances which do not evoke the psychophysical sensation of cold. In addition, several groups were unable to reproduce TRPA1 activation by cold in both heterologous and native cells innervating the periphery.72,74 However, cellular context seems critical in that one intriguing report found that TRPA1 channels expressed in dissociated neurons from DRG were not cold sensitive, whereas cold responses were recorded in isolated vagal afferents, and these were TRPA1-dependent.79 Such discrepancies regarding TRPA1 activation by cold at the cellular have been reviewed elsewhere67,71 and not repeated herein, but the channel clearly has a roles in cold hypersensitivity as discussed below.
The Role of Na+ and K+ Conductances in Cold Signaling
As described, sodium and potassium currents are fundamental in the cell’s ability to fire action potentials, and both types of conductances have been associated in cold responses.24,25,80−82 Nav1.8, a tetrodotoxin (TTX)-resistant voltage-gated sodium channel, is selectively expressed in approximately 75% of mouse peripheral sensory neurons, largely including those considered nociceptors.83 The channels involvement in cold signaling was first reported when it was shown to be resistant to inhibition by cold, a property not observed in other sodium channels.81 Normally, cold temperatures gradually increase voltage-dependent slow inactivation of TTX-sensitive Nav channels, thereby limiting excitability. However, inactivation of Nav1.8 channels is unaffected by cold. Moreover, cold reduces the voltage-activation threshold for Nav1.8, properties, suggesting that this channel is the primary impulse generator in the cold.81 Consistent with these in vitro data, mice lacking the Nav1.8 gene, or in which Nav1.8-expressing neurons are depleted, are reportedly unresponsive to noxious cold, yet show normal responses to measures of innocuous cool sensitivity.80,81 Thus, Nav1.8 clearly plays a role in cold transduction, presumably specifically involved in noxious cold signaling.
Similar to the necessity of voltage-gated sodium conductances in neuronal signaling, potassium conductances are also critical in regulating excitability and several mechanisms have been proposed for K+-channel mediated cold signaling.53 For example, cold was originally suggested to be mediated by cooling-induced closing of a background K+ current,21,22 causing depolarization and firing via an ever present cationic inward current, termed Ih, which was poorly inhibited by cold. In cold-insensitive neurons, cold-evoked firing is prevented by a slow, transient, 4-AP-sensitive K+ current (IKD) that acts as an excitability brake, and its absence, or minimal expression, in cold-sensitive neurons allows for depolarization in the cold.22 Pharmacological blockade of IKD induced thermosensitivity in cold-insensitive neurons, suggesting that cold activation was not the product of cold directly acting on a cold thermosensor, but that these cells were electrogenically tuned due to the differential expression of select K+-conductances.21,22 However, it is important to note that these studies were performed before the cloning of TRPM8,30,42 and subsequent analyses of cold and menthol-sensitive neurons finds a strong correlation between temperature threshold and the level of expression of IKD.25 Specifically, HT cells express high levels of this K+ brake current (and low levels of TRPM8), whereas neurons which fall into the LT subtype and likely mediate responses to innocuously cool temperatures express low levels of IKD (and high levels of TRPM8).25 However, the molecular identity of IKD has yet to be elucidated, but is suggested to be a member of the Kv channel family. Lastly, the two-pore domain K+ channels TREK-1 and TRAAK are activated by membrane stretch and free fatty acids, but have also been shown to be heat sensitive when expressed in heterologous expression systems.23,82 In regard to their role in cooling, it is proposed that heat-evoked activity of these channels polarizes cells, whereas cooling leads to channel closing, depolarization, and then subsequent action potential firing. Thus, as described for IKD currents, the absence of this K+-mediated brake current in the cold may contribute to cold neuronal activity.23,24,82
Measurement of Cold Sensation in Vivo
The above focuses largely on transduction pathways implicated in cold signaling at the molecular or channel level. However, in order to understand the necessity of these candidate cold transducers, their role in cold-evoked animal behaviors needed to be assessed. This has proven to be a considerable obstacle in our understanding of the cellular and molecular basis of cold due to the ambiguity in cold-evoked behaviors in rodents. Classical thermal behavioral assays typically include a heated or cooled plate from which the animals' thermal sensitivity is gauged by the latency or speed in which they lift or withdraw their hindpaw from the surface. The shorter the latency, the more sensitive the animal. While this test is relatively robust for heat, rodent behavior on a cold plate is spurious at best. Indeed, as compared to the hot plate test, the latencies to response in the cold plate test tend to be highly variable between studies, even those conducted in the same laboratory.84 For example, in analyses of different mouse lines in which the genes of putative cold transducers or those involved in conduction were disrupted (knockout or gene-nulls), latencies for hindpaw lifts at near freezing temperatures (0 to −1 °C) ranged from 5 to 200 s in wild-type or control mice.85 At these temperatures, mice generally assume a posture that largely limits contact with the cold surface by rearing up on their hindlimbs and not exposing their forepaws, rumps, and tails to the cold.86 These significant differences in animal behavior have motivated the field to identify and develop novel experimental approaches to assess cold.
For example, a variation of the cold plate that uses lightly restrained mice was recently reported.87 This method allows for easier measurements of both paws independently as only one is placed on a cold plate at a time. Additionally, this assay eliminates any confusion caused by whole body exposure to cold and subsequent reduction in mobility as seen in the cold plate assay. However, the acts of habituating the animals to being restrained can be problematic. Nonetheless, latencies to lifts at 0 °C were less than 10 s, a duration consistent with responses to noxious heat.87 Similarly, a dynamic thermal plate approach has been reported where the animal is placed on the surface warmed to near 32 °C and then the temperature is slowly reduced until the animal performs an escape behavior, which was near 5 °C in control mice.88 Another attractive technique that is reminiscent of the Hargreaves assay which uses radiant heat to measure paw withdrawal in freely moving animals,89 employs a compressed pellet of dry ice that is pressed to the underneath surface of a glass plate in which the animal is standing.90 Temperature at the surface-hindpaw interface varies over time with the thickness of the glass, but remarkably, the glass temperature that induced a lift was near 24 °C, a temperature not typically considered nociceptive. Lastly, many laboratories have employed the evaporative cooling assay to measure cold responses, a methodology that uses a droplet of acetone placed on the animal’s hindpaw.91 As the liquid evaporates the surface skin temperature drops to near 17 °C, inducing flinching, licking, and guarding behaviors depending on the animal’s sensitivity. While this is generally considered a measurement of innocuous cold, the behaviors are reminiscent of nocifensive responses, suggesting that temperatures in this range may indeed be bothersome to the animal.
The two-temperature choice assay has been very informative in characterizing cold sensation.92−94 Animals are placed in a chamber on two thermally controlled plates and then allowed to freely move across both surfaces. When both are maintained at the same temperature, animals will explore the entire chamber and spend an equal amount of time on each surface. To test thermal sensitivity, one plate is held constant (typically 30 °C) with the other varied to either warmer or colder temperatures and the animals preference is determined by which surface it spends the majority of the recording period, whereas the animal’s avoidance of a temperature can be inferred by how often it transitions between temperatures.54 These approaches have been used in various combinations to test the necessity of putative cold-relevant molecules in animal behavior, which we summarize below.
The Role of Candidate Molecules in Cold Sensation
To date the majority of analyses of cold-induced behaviors have focused on TRPM8 and TRPA1, with the prevailing hypothesis that the channels exclusively mediate innocuous cool and noxious cold, respectively.67 However, for many years, this hypothesis has been strongly put to the test. For the former, TRPM8 is clearly involved in innocuous cool sensation, but a role for the channel in cold pain has been controversial. Three independent groups created Trpm8–/– mouse lines, reporting these animals to have severe deficits in cold sensation, yet there were striking differences between their findings.84,92−94 One consistent measure of cold deficits in Trpm8–/– mice has been the evaporative cooling assay which has clearly shown a lack of cold sensitivity in these mice.86,92,94 However, results using the cold plate have proved to be less clear. Two groups reported no difference between wild-type and Trpm8–/– mice using the cold plate tests at 10, 0, −1, −5 or, −10 °C,92,94 while a third found a significant difference in withdrawal latency at 0 °C, results suggesting the animals were deficient in noxious cold sensation.93 As above, the time to paw withdrawal at near freezing temperatures for wild-type mice ranged from 5 to 50 s (5, 20, and 50 s) between the three studies, highlighting the inconsistency with the cold plate. Subsequently, it was reported that lightly restrained Trpm8–/– mice have significantly longer withdrawal latencies than wild-type when their hindpaws were placed on a 10 °C plate.87 Moreover, Trpm8–/– mice were completely unresponsive in the dynamic cold plate assay, suggesting they do not perceive cold temperatures at all.88 Lastly, while mice rarely show representative nocifensive behaviors with their hindpaws under these conditions, two robust and reproducible behaviors involving the forepaw are readily visible.86 The first presents as a flinching of both forepaws which occurs shortly after wild-type animals are placed on a 0 °C surface, and a second manifests as a wringing or licking of the forepaw, suggestive of intense irritation. Control animals respond with latencies of less than 10 s, whereas Trpm8–/– mice rarely show these behaviors up to the 60 s cutoff time for this noxious stimulus.86 Thus, depending on the experimental approach, cold plate behavioral data suggests these animals are at least partially deficient in cold nociception.
Interpretation of cold deficits in Trpm8–/– mice using the two-plate choice assay has also been problematic. These animals show a robust deficit in thermal preference and are unable to discern the difference between the 30 °C surface and when the test plate is held at temperatures down to 15 °C,75,92−94 demonstrating that they cannot discriminate between warm and putatively innocuously cool temperatures. However, once temperatures drop below 15 °C, Trpm8–/– mice do prefer the warm side, albeit less than what is observed for wild-type mice,75,92 results implying that other transduction mechanisms predominate below 15 °C. Further complicating the interpretation of these data, Colburn et al. reported a clear phenotype when the temperatures were set to room temperature (∼25 °C) versus 5 °C with wild-types showing a strong preference for the warmer temperature whereas the knockouts showed no preference between the two.93 What then underlies this preference for warmth at extreme cold temperatures? The answer may lie in how we interpret the motivation behind this behavior in this experimental paradigm. It is not clear if this preference for warmth is due to a drive to avoid an unpleasant stimulus, or if it is prompted by a drive to seek out a comfortably warm environment, something that is critical for the maintenance of core body temperatures. Therefore, Trpm8–/– mice may not be able to detect the noxious cold signal when given the choice of two surfaces held at 30 °C and <15 °C, but are able to discern that the warm surface is preferable due to input from warm fibers.54 In support of this hypothesis, when the number of times an animal crosses from the 30 °C surface to the test surface and back again is counted, wild-type mice show a significant drop in the number of crossing events as one plate is cooled.75,86 Indeed, when the test plate is held at 5 °C, wild-type mice on average cross over and back from the cold surface once and largely never return, whereas Trpm8–/– mice freely transition between plates showing no signs of aversion.75
An alternative explanation for the residual preference for warmth in the Trpm8–/– mice is the presence of another cold transducer that functions in the noxious cold range, a hypothesis consistent with the model that TRPA1 is a noxious cold receptor.95 However, in mice in which both TRPM8 and TRPA1 genes were disrupted, there was no difference in preference or avoidance behaviors beyond those already present in Trpm8–/– mice.75 Certainly, as perplexing as the role for TRPM8 in cold nociception has been, establishing the necessity of TRPA1 in acute cold sensation has been confounding.71 As with menthol and cold responses,37 the prediction would be that TRPA1 agonists such as cinnamaldehyde (CA) or mustard oil (MO) should sensitize cold responses, yet studies found no differences in cold withdrawal threshold when these are topically applied to anesthetized rats.96,97 Similarly, when MO was topically applied, no increases in cold-evoked spikes were seen in spinal wide dynamic range (WDR) neurons. However, when CA was injected into the rat paw, a significant drop in paw lick/jump latency was seen in response to both 0 and 5 °C plate tests in one report, continuing the ambiguity.98 Similarly, two independently generated Trpa1–/– lines were analyzed for deficits in cold transduction with mixed results. In one, no deficits in cold-induced behaviors were observed, including responses to evaporative cooling, cold plate withdrawal behaviors, shivering, and thermal preference.92,99 However, the other Trpa1–/– line was reported to have deficits in evaporative cooling and cold withdrawal behaviors, although curiously these differences were only significant in female mice and not males.100 Moreover, this line of Trpa1–/– was were reported to have reduced escape behaviors (jumping when placed on a 0 °C cold plate) as well as longer latencies to tail flicks when the tail was immersed in −10 °C liquid.101 Indeed, the story has not become clearer with time, nor can the discrepancies between studies be correlated to the two lines as del Camino et al. recently reported no differences in cold evoked behaviors with the latter line.102
However, this study may be the most informative and offers insight into TRPA1’s role in cold in that they observed that TRPA1 is important for cold hypersensitivity after injury. Previous reports had suggested that TRPA1 served an important role in inflammatory cold hypersensitivity.103−105 Inflammation-induced increase in cold sensitivity was blocked using a TRPA1 antagonist, as well as reduced by a reduction in TRPA1 transcript expression.102,103 Moreover, cold hypersensitivity was induced by injection of an endogenous TRPA1 agonist in wild-type but not Trpa1–/– mice.102 What may have been a more important result was the observation that TRPA1 channels, both native and heterologously expressed, were not sensitive to cold temperatures, but cold did amplify agonist evoked TRPA1 currents.102 Thus, under the context of inflammation or nerve injury when TRPA1 channels are likely stimulated by endogenous mediators, cold may further potentiate these responses and lead to cold evoked pain. Indeed TRPA1 likely serves in this context to all modalities and is now considered a “gatekeeper” for chronic pain (see ref (106) for review). As cold hypersensitivity is also reduced in Trpm8–/– mice,93,107 what remains to be determined is how TRPA1 activation leads to cold hypersensitivity and if this process works through TRPM8 or other transduction channels.
In addition to TRPM8 and TRPA1, behavioral analyses have found that Nav1.8 and the potassium channels TREK-1 and TRAAK contribute to cold responsiveness in mice. For the former, Nav1.8–/– mice showed reduced lifts and jumping behavior on a 0 °C cold plate compared to wild-type animals, as well as there was a reduction in menthol-sensitized cold responses in the skin-nerve preparation.81 Similarly, mice in which Nav1.8 neurons were genetically ablated showed less sensitivity on the cold plate (0 °C), but interestingly were identical to wild-types in the evaporative cooling assay, suggesting they retained normal innocuous cold perception.80 These data are consistent with Nav1.8’s functional properties in the cold and suggest it is required for conduction in extreme cold, but is not needed for innocuous cool transmission. Conduction of cold signals also appears to rely in part on both TREK-1 and TRAAK channels. Double knockout mice for both channels are reported to be more sensitive to cold on the cold plate at temperatures ranging from 10 to 20 °C, as well as show deficits in thermal preference in the cool range (from 21 to 15 °C).24 Lastly, a recent report found evidence that homomeric TRPC5 channels are cold-sensitive in the innocuous range; however, no deficits in cold behaviors were observed in Trpc5–/– mice, suggesting an as yet undetermined modulatory role for this channel in cold sensation.108
A Model for the Cellular Basis of Cold Sensation
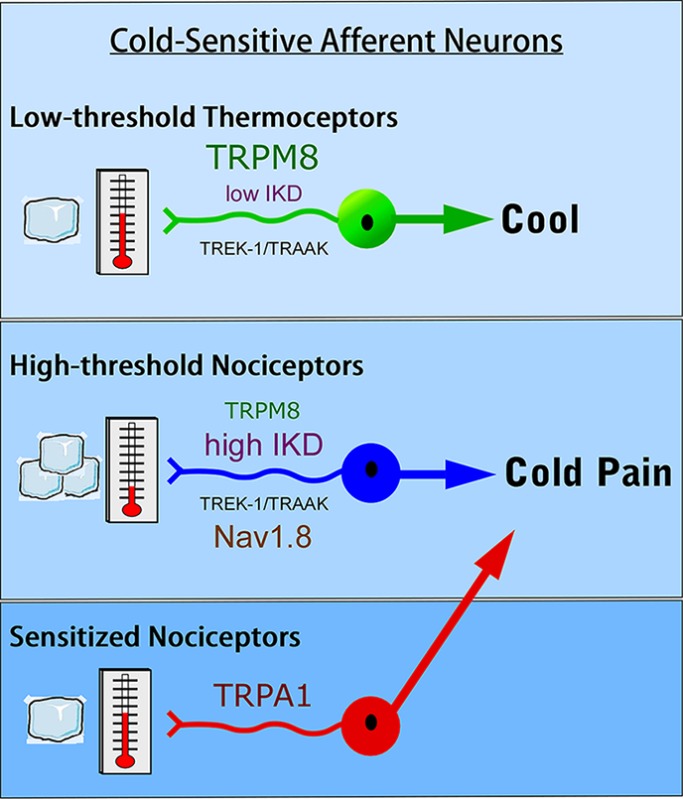
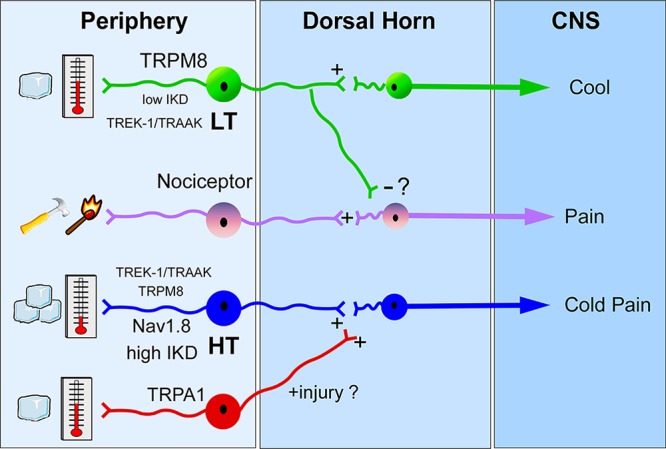
The past decade has been a boom period in our understanding of cold transduction and now enables serious consideration of potential cellular models that account for mammalian cold sensation. Specifically, a model (Figure 3) has been proposed for how the peripheral nervous system is able to detect ambient cold temperature and discriminate between those that are innocuous from those that are noxious,25,53,54 a fundamental part for survival. At the core of the model is TRPM8 which, unlike the other molecular candidates for cold transduction, appears to be exclusively involved in cold signaling and not mediating other modalities of somatosensation. For simplicity, this model also separates cold nerve fibers into those mediating innocuous cool, the LT subtype, from those mediating noxious cold, the HT subtype. This is by far an oversimplification as recordings at all levels of analysis show a continuum of cold activation thresholds. Nonetheless, the model provides a framework for the testing of the key roles of each molecular player; insights should also be applicable to those cells with more intermediate thermal thresholds.
Figure 3.
Model for the differential perception of innocuous cool and noxious cold temperatures in the low-threshold (LT) and high-threshold (HT) populations, respectively.
How can TRPM8 channels generate responses in high-threshold cold-sensitive nerves if it is activated initially at temperatures in the innocuous range? Mechanistically, thermoceptors such as TRPM8 provide the receptor potential to initiate firing, much in the way ionotropic glutamate receptors mediate postsynaptic potentials. Thus, other electrogenic properties of the nerve, such as channel density, current magnitude, and neuronal excitability, are fundamental in impulse generation. For the LT subtype, TRPM8 clearly plays a dominant role in that channel expression is higher than in the HT population.25,28 These cells are also readily excitable as expression of K+ brake conductances are low, thereby limiting the contribution of hyperpolarizing conductances.22,24,25 For HT cells, expression of TRPM8 is reduced, whereas high levels of IKD currents, attributed to Kv1 channels, are expressed, thereby necessitating a more robust thermal stimulus to activate sufficient TRPM8 currents in order to overcome the excitability brake established by the K+ conductances. Moreover, Nav1.8 is likely expressed predominantly in the HT population in order to enable axonal conduction under these cold conditions.80,81 Lastly, both TRPM8 and TRPA1 are required for cold pain after injury, and signal independently due to their reported expression in distinct cell types, via an as yet undefined nociceptor cell type expressing both channels or through an indirect mechanism whereby activation of TRPA1 afferents sensitizes TRPM8-expressing HT nerves such that they fire at a lower (warmer) temperature threshold.
Acknowledgments
This work was supported by U.S. National Institutes of Health grants (D.D.M.; NS071364 and NS078530).
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- McKemy D. D. (2007) Temperature sensing across species. Pflugers Arch. 454, 777–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erpelding N.; Moayedi M.; Davis K. D. (2012) Cortical thickness correlates of pain and temperature sensitivity. Pain 153, 1602–1609. [DOI] [PubMed] [Google Scholar]
- Morin C.; Bushnell M. C. (1998) Temporal and qualitative properties of cold pain and heat pain: a psychophysical study. Pain 74, 67–73. [DOI] [PubMed] [Google Scholar]
- Bessou P.; Perl E. R. (1969) Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J. Neurophysiol. 32, 1025–1043. [DOI] [PubMed] [Google Scholar]
- Darian-Smith I.; Johnson K. O.; Dykes R. (1973) “Cold” fiber population innervating palmar and digital skin of the monkey: responses to cooling pulses. J. Neurophysiol. 36, 325–346. [DOI] [PubMed] [Google Scholar]
- Kenshalo D. R.; Duclaux R. (1977) Response characteristics of cutaneous cold receptors in the monkey. J. Neurophysiol. 40, 319–332. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M.; Stucky C. L.; Lewin G. R. (1997) Receptive properties of mouse sensory neurons innervating hairy skin. J. Neurophysiol. 78, 1841–1850. [DOI] [PubMed] [Google Scholar]
- Hensel H. (1973) Neural processes in thermoregulation. Physiol. Rev. 53, 948–1017. [DOI] [PubMed] [Google Scholar]
- Simone D. A.; Kajander K. C. (1996) Excitation of rat cutaneous nociceptors by noxious cold. Neurosci. Lett. 213, 53–56. [DOI] [PubMed] [Google Scholar]
- Simone D. A.; Kajander K. C. (1997) Responses of cutaneous A-fiber nociceptors to noxious cold. J. Neurophysiol. 77, 2049–2060. [DOI] [PubMed] [Google Scholar]
- LaMotte R. H.; Thalhammer J. G. (1982) Response properties of high-threshold cutaneous cold receptors in the primate. Brain Res. 244, 279–287. [DOI] [PubMed] [Google Scholar]
- Carr R. W.; Pianova S.; McKemy D. D.; Brock J. A. (2009) Action potential initiation in the peripheral terminals of cold-sensitive neurones innervating the guinea-pig cornea. J. Physiol. 587, 1249–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campero M.; Serra J.; Bostock H.; Ochoa J. L. (2001) Slowly conducting afferents activated by innocuous low temperature in human skin. J. Physiol. 535, 855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock J. A.; Pianova S.; Belmonte C. (2001) Differences between nerve terminal impulses of polymodal nociceptors and cold sensory receptors of the guinea-pig cornea. J. Physiol. 533, 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid R.; Donovan-Rodriguez T.; Meseguer V.; Acosta M. C.; Belmonte C.; Viana F. (2006) Contribution of TRPM8 channels to cold transduction in primary sensory neurons and peripheral nerve terminals. J. Neurosci. 26, 12512–12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington C. (1906) The integrative action of the nervous system, Scribner, New York. [Google Scholar]
- Pierau F. K.; Torrey P.; Carpenter D. O. (1974) Mammalian cold receptor afferents: role of an electrogenic sodium pump in sensory transduction. Brain Res. 73, 156–160. [DOI] [PubMed] [Google Scholar]
- Pierau F. K.; Torrey P.; Carpenter D. (1975) Effect of ouabain and potassium-free solution on mammalian thermosensitive afferents in vitro. Pflugers Arch. 359, 349–356. [DOI] [PubMed] [Google Scholar]
- Spray D. C. (1974) Characteristics, specificity, and efferent control of frog cutaneous cold receptors. J. Physiol. 237, 15–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer K.; Braun H. A. (1990) Modulation of periodic cold receptor activity by ouabain. Pflugers Arch. 417, 91–99. [DOI] [PubMed] [Google Scholar]
- Reid G.; Flonta M. (2001) Cold transduction by inhibition of a background potassium conductance in rat primary sensory neurones. Neurosci. Lett. 297, 171–174. [DOI] [PubMed] [Google Scholar]
- Viana F.; de la Pena E.; Belmonte C. (2002) Specificity of cold thermotransduction is determined by differential ionic channel expression. Nat. Neurosci. 5, 254–260. [DOI] [PubMed] [Google Scholar]
- Maingret F.; Lauritzen I.; Patel A. J.; Heurteaux C.; Reyes R.; Lesage F.; Lazdunski M.; Honore E. (2000) TREK-1 is a heat-activated background K(+) channel. EMBO J. 19, 2483–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel J.; Zimmermann K.; Busserolles J.; Deval E.; Alloui A.; Diochot S.; Guy N.; Borsotto M.; Reeh P.; Eschalier A.; Lazdunski M. (2009) The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J. 28, 1308–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid R.; de la Pena E.; Donovan-Rodriguez T.; Belmonte C.; Viana F. (2009) Variable threshold of trigeminal cold-thermosensitive neurons is determined by a balance between TRPM8 and Kv1 potassium channels. J. Neurosci. 29, 3120–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto K.; Gotoh H. (1999) Calcium signaling in cold cells studied in cultured dorsal root ganglion neurons. Neuroscience 92, 1131–1135. [DOI] [PubMed] [Google Scholar]
- Babes A.; Zorzon D.; Reid G. (2004) Two populations of cold-sensitive neurons in rat dorsal root ganglia and their modulation by nerve growth factor. Eur. J. Neurosci. 20, 2276–2282. [DOI] [PubMed] [Google Scholar]
- Xing H.; Ling J.; Chen M.; Gu J. G. (2006) Chemical and cold sensitivity of two distinct populations of TRPM8-expressing somatosensory neurons. J. Neurophysiol. 95, 1221–1230. [DOI] [PubMed] [Google Scholar]
- Reid G.; Flonta M. L. (2001) Physiology. Cold current in thermoreceptive neurons. Nature 413, 480. [DOI] [PubMed] [Google Scholar]
- McKemy D. D.; Neuhausser W. M.; Julius D. (2002) Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416, 52–58. [DOI] [PubMed] [Google Scholar]
- Cesare P.; McNaughton P. (1996) A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proc. Natl. Acad. Sci. U.S.A. 93, 15435–15439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut P. D.; Wrigley D.; Gold M. S. (2003) Cold transduction in rat trigeminal ganglia neurons in vitro. Neuroscience 119, 1071–1083. [DOI] [PubMed] [Google Scholar]
- Patel T.; Ishiuji Y.; Yosipovitch G. (2007) Menthol: A refreshing look at this ancient compound. J. Am. Acad. Dermatol. 57, 873–878. [DOI] [PubMed] [Google Scholar]
- Cliff M. A.; Green B. G. (1994) Sensory irritation and coolness produced by menthol: evidence for selective desensitization of irritation. Physiol. Behav. 56, 1021–1029. [DOI] [PubMed] [Google Scholar]
- Green B. G. (1992) The sensory effects of l-menthol on human skin. Somatosens. Motor Res. 9, 235–244. [DOI] [PubMed] [Google Scholar]
- Green B. G.; Schoen K. L. (2007) Thermal and nociceptive sensations from menthol and their suppression by dynamic contact. Behav. Brain Res. 176, 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel H.; Zotterman Y. (1951) The effect of menthol on the thermoreceptors. Acta Physiol. Scand. 24, 27–34. [DOI] [PubMed] [Google Scholar]
- Reid G.; Flonta M. L. (2002) Ion channels activated by cold and menthol in cultured rat dorsal root ganglion neurones. Neurosci. Lett. 324, 164–168. [DOI] [PubMed] [Google Scholar]
- Daniels R. L.; Takashima Y.; McKemy D. D. (2009) Activity of the Neuronal Cold Sensor TRPM8 Is Regulated by Phospholipase C via the Phospholipid Phosphoinositol 4,5-Bisphosphate. J. Biol. Chem. 284, 1570–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima Y.; Daniels R. L.; Knowlton W.; Teng J.; Liman E. R.; McKemy D. D. (2007) Diversity in the neural circuitry of cold sensing revealed by genetic axonal labeling of transient receptor potential melastatin 8 neurons. J. Neurosci. 27, 14147–14157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra A.; Madrid R.; Echevarria D.; del Olmo S.; Morenilla-Palao C.; Acosta M. C.; Gallar J.; Dhaka A.; Viana F.; Belmonte C. (2012) Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat. Med. 16, 1396–1399. [DOI] [PubMed] [Google Scholar]
- Peier A. M.; Moqrich A.; Hergarden A. C.; Reeve A. J.; Andersson D. A.; Story G. M.; Earley T. J.; Dragoni I.; McIntyre P.; Bevan S.; Patapoutian A. (2002) A TRP channel that senses cold stimuli and menthol. Cell 108, 705–715. [DOI] [PubMed] [Google Scholar]
- Bai V. U.; Murthy S.; Chinnakannu K.; Muhletaler F.; Tejwani S.; Barrack E. R.; Kim S. H.; Menon M.; Veer Reddy G. P. (2010) Androgen regulated TRPM8 expression: a potential mRNA marker for metastatic prostate cancer detection in body fluids. Int. J. Oncol. 36, 443–450. [PubMed] [Google Scholar]
- Zhang L.; Barritt G. J. (2004) Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells. Cancer Res. 64, 8365–8373. [DOI] [PubMed] [Google Scholar]
- Bidaux G.; Roudbaraki M.; Merle C.; Crepin A.; Delcourt P.; Slomianny C.; Thebault S.; Bonnal J. L.; Benahmed M.; Cabon F.; Mauroy B.; Prevarskaya N. (2005) Evidence for specific TRPM8 expression in human prostate secretory epithelial cells: functional androgen receptor requirement. Endocr.-Relat. Cancer 12, 367–382. [DOI] [PubMed] [Google Scholar]
- Tsavaler L.; Shapero M. H.; Morkowski S.; Laus R. (2001) Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res. 61, 3760–3769. [PubMed] [Google Scholar]
- Caterina M. J.; Schumacher M. A.; Tominaga M.; Rosen T. A.; Levine J. D.; Julius D. (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824. [DOI] [PubMed] [Google Scholar]
- Story G. M.; Peier A. M.; Reeve A. J.; Eid S. R.; Mosbacher J.; Hricik T. R.; Earley T. J.; Hergarden A. C.; Andersson D. A.; Hwang S. W.; McIntyre P.; Jegla T.; Bevan S.; Patapoutian A. (2003) ANKTM1, a TRP-like Channel Expressed in Nociceptive Neurons, Is Activated by Cold Temperatures. Cell 112, 819–829. [DOI] [PubMed] [Google Scholar]
- Peier A. M.; Reeve A. J.; Andersson D. A.; Moqrich A.; Earley T. J.; Hergarden A. C.; Story G. M.; Colley S.; Hogenesch J. B.; McIntyre P.; Bevan S.; Patapoutian A. (2002) A heat-sensitive TRP channel expressed in keratinocytes. Science 296, 2046–2049. [DOI] [PubMed] [Google Scholar]
- Smith G. D.; Gunthorpe M. J.; Kelsell R. E.; Hayes P. D.; Reilly P.; Facer P.; Wright J. E.; Jerman J. C.; Walhin J. P.; Ooi L.; Egerton J.; Charles K. J.; Smart D.; Randall A. D.; Anand P.; Davis J. B. (2002) TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 418, 186–190. [DOI] [PubMed] [Google Scholar]
- Xu H.; Ramsey I. S.; Kotecha S. A.; Moran M. M.; Chong J. A.; Lawson D.; Ge P.; Lilly J.; Silos-Santiago I.; Xie Y.; DiStefano P. S.; Curtis R.; Clapham D. E. (2002) TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 418, 181–186. [DOI] [PubMed] [Google Scholar]
- Latorre R.; Brauchi S.; Madrid R.; Orio P. (2011) A cool channel in cold transduction. Physiology 26, 273–285. [DOI] [PubMed] [Google Scholar]
- Belmonte C.; Brock J. A.; Viana F. (2009) Converting cold into pain. Exp. Brain Res. 196, 13–30. [DOI] [PubMed] [Google Scholar]
- McCoy D. D.; Knowlton W. M.; McKemy D. D. (2011) Scraping through the ice: uncovering the role of TRPM8 in cold transduction. Am. J. Physiol.: Regul., Integr. Comp. Physiol. 300, R1278–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohacs T.; Lopes C. M.; Michailidis I.; Logothetis D. E. (2005) PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat. Neurosci. 8, 626–634. [DOI] [PubMed] [Google Scholar]
- Liu B.; Qin F. (2005) Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4,5-bisphosphate. J. Neurosci. 25, 1674–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharian E.; Cao C.; Rohacs T. (2010) Gating of transient receptor potential melastatin 8 (TRPM8) channels activated by cold and chemical agonists in planar lipid bilayers. J. Neurosci. 30, 12526–12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharian E.; Thyagarajan B.; French R. J.; Pavlov E.; Rohacs T. (2009) Inorganic polyphosphate modulates TRPM8 channels. PloS One 4, e5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morenilla-Palao C.; Pertusa M.; Meseguer V.; Cabedo H.; Viana F. (2009) Lipid raft segregation modulates TRPM8 channel activity. J. Biol. Chem. 284, 9215–9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertusa M.; Madrid R.; Morenilla-Palao C.; Belmonte C.; Viana F. (2012) N-glycosylation of TRPM8 ion channels modulates temperature sensitivity of cold thermoreceptor neurons. J. Biol. Chem. 287, 18218–18229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum A. I.; Bautista D. M.; Scherrer G.; Julius D. (2009) Cellular and molecular mechanisms of pain. Cell 139, 267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linte R. M.; Ciobanu C.; Reid G.; Babes A. (2007) Desensitization of cold- and menthol-sensitive rat dorsal root ganglion neurones by inflammatory mediators. Exp. Brain Res. 178, 89–98. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Mak S.; Li L.; Parra A.; Denlinger B.; Belmonte C.; McNaughton P. A. (2012) Direct inhibition of the cold-activated TRPM8 ion channel by Galphaq. Na. Cell Biol. 14, 851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar L. S.; Raisinghani M.; Pingle S. C.; Long C.; Pimentel F. (2005) Downregulation of transient receptor potential melastatin 8 by protein kinase C-mediated dephosphorylation. J. Neurosci. 25, 11322–11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina M. J.; Rosen T. A.; Tominaga M.; Brake A. J.; Julius D. (1999) A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 398, 436–441. [DOI] [PubMed] [Google Scholar]
- Guler A. D.; Lee H.; Iida T.; Shimizu I.; Tominaga M.; Caterina M. (2002) Heat-evoked activation of the ion channel, TRPV4. J. Neurosci. 22, 6408–6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy D. D. (2005) How cold is it? TRPM8 and TRPA1 in the molecular logic of cold sensation. Mol. Pain 1, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei E. T.; Seid D. A. (1983) AG-3–5: a chemical producing sensations of cold. J. Pharm. Pharmacol. 35, 110–112. [DOI] [PubMed] [Google Scholar]
- Jaquemar D.; Schenker T.; Trueb B. (1999) An ankyrin-like protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. J. Biol. Chem. 274, 7325–7333. [DOI] [PubMed] [Google Scholar]
- Patapoutian A.; Peier A. M.; Story G. M.; Viswanath V. (2003) ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat. Rev. Neurosci. 4, 529–539. [DOI] [PubMed] [Google Scholar]
- Caspani O.; Heppenstall P. A. (2009) TRPA1 and cold transduction: an unresolved issue?. J. Gen. Physiol. 133, 245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt S. E.; Bautista D. M.; Chuang H. H.; McKemy D. D.; Zygmunt P. M.; Hogestatt E. D.; Meng I. D.; Julius D. (2004) Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427, 260–265. [DOI] [PubMed] [Google Scholar]
- Nagata K.; Duggan A.; Kumar G.; Garcia-Anoveros J. (2005) Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J. Neurosci. 25, 4052–4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurborg S.; Yurgionas B.; Jira J. A.; Caspani O.; Heppenstall P. A. (2007) Direct activation of the ion channel TRPA1 by Ca2. Nat. Neurosci. 10, 277–279. [DOI] [PubMed] [Google Scholar]
- Knowlton W. M.; Fisher A.; Bautista D. M.; McKemy D. D. (2010) TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain 150, 340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista D. M.; Movahed P.; Hinman A.; Axelsson H. E.; Sterner O.; Hogestatt E. D.; Julius D.; Jordt S. E.; Zygmunt P. M. (2005) Pungent products from garlic activate the sensory ion channel TRPA1. Proc. Natl. Acad. Sci. U.S.A. 102, 12248–12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson L. J.; Geierstanger B. H.; Viswanath V.; Bandell M.; Eid S. R.; Hwang S.; Patapoutian A. (2005) The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr. Biol. 15, 929–934. [DOI] [PubMed] [Google Scholar]
- Bandell M.; Story G. M.; Hwang S. W.; Viswanath V.; Eid S. R.; Petrus M. J.; Earley T. J.; Patapoutian A. (2004) Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41, 849–857. [DOI] [PubMed] [Google Scholar]
- Fajardo O.; Meseguer V.; Belmonte C.; Viana F. (2008) TRPA1 channels mediate cold temperature sensing in mammalian vagal sensory neurons: pharmacological and genetic evidence. J. Neurosci. 28, 7863–7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamsen B.; Zhao J.; Asante C. O.; Cendan C. M.; Marsh S.; Martinez-Barbera J. P.; Nassar M. A.; Dickenson A. H.; Wood J. N. (2008) The cell and molecular basis of mechanical, cold, and inflammatory pain. Science 321, 702–705. [DOI] [PubMed] [Google Scholar]
- Zimmermann K.; Leffler A.; Babes A.; Cendan C. M.; Carr R. W.; Kobayashi J.; Nau C.; Wood J. N.; Reeh P. W. (2007) Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature 447, 855–858. [DOI] [PubMed] [Google Scholar]
- Kang D.; Choe C.; Kim D. (2005) Thermosensitivity of the two-pore domain K+ channels TREK-2 and TRAAK. J. Physiol. 564, 103–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields S. D.; Ahn H. S.; Yang Y.; Han C.; Seal R. P.; Wood J. N.; Waxman S. G.; Dib-Hajj S. D. (2012) Na(v)1.8 expression is not restricted to nociceptors in mouse peripheral nervous system. Pain 153, 2017–2030. [DOI] [PubMed] [Google Scholar]
- Daniels R. L.; McKemy D. D. (2007) Mice left out in the cold: commentary on the phenotype of TRPM8-nulls. Molecular pain 3, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy D. D. (2010) Therapeutic potential of TRPM8 modulators. Open Drug Discovery J. 2, 80–87. [Google Scholar]
- Knowlton W. M., Palkar R., Lippoldt E. K., and McKemy D. D. Unpublished observations.
- Gentry C.; Stoakley N.; Andersson D. A.; Bevan S. (2010) The roles of iPLA2, TRPM8 and TRPA1 in chemically induced cold hypersensitivity. Mol. Pain 6, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descoeur J.; Pereira V.; Pizzoccaro A.; Francois A.; Ling B.; Maffre V.; Couette B.; Busserolles J.; Courteix C.; Noel J.; Lazdunski M.; Eschalier A.; Authier N.; Bourinet E. (2011) Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors. EMBO Mol. Med. 3, 266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K.; Dubner R.; Brown F.; Flores C.; Joris J. (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32, 77–88. [DOI] [PubMed] [Google Scholar]
- Brenner D. S.; Golden J. P.; Gereau R. W. t. (2012) A novel behavioral assay for measuring cold sensation in mice. PloS One 7, e39765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y.; Yoon Y. W.; Na H. S.; Kim S. H.; Chung J. M. (1994) Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain 59, 369–376. [DOI] [PubMed] [Google Scholar]
- Bautista D. M.; Siemens J.; Glazer J. M.; Tsuruda P. R.; Basbaum A. I.; Stucky C. L.; Jordt S. E.; Julius D. (2007) The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448, 204–208. [DOI] [PubMed] [Google Scholar]
- Colburn R. W.; Lubin M. L.; Stone D. J. Jr.; Wang Y.; Lawrence D.; D’Andrea M. R.; Brandt M. R.; Liu Y.; Flores C. M.; Qin N. (2007) Attenuated cold sensitivity in TRPM8 null mice. Neuron 54, 379–386. [DOI] [PubMed] [Google Scholar]
- Dhaka A.; Murray A. N.; Mathur J.; Earley T. J.; Petrus M. J.; Patapoutian A. (2007) TRPM8 is required for cold sensation in mice. Neuron 54, 371–378. [DOI] [PubMed] [Google Scholar]
- Chung M. K.; Caterina M. J. (2007) TRP channel knockout mice lose their cool. Neuron 54, 345–347. [DOI] [PubMed] [Google Scholar]
- Dunham J. P.; Leith J. L.; Lumb B. M.; Donaldson L. F. (2010) Transient receptor potential channel A1 and noxious cold responses in rat cutaneous nociceptors. Neuroscience 165, 1412–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer C. M.; Carstens M. I.; Carstens E. (2009) Mustard oil enhances spinal neuronal responses to noxious heat but not cooling. Neurosci. Lett. 461, 271–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsagareli M. G.; Tsiklauri N.; Zanotto K. L.; Carstens M. I.; Klein A. H.; Sawyer C. M.; Gurtskaia G.; Abzianidze E.; Carstens E. (2010) Behavioral evidence of thermal hyperalgesia and mechanical allodynia induced by intradermal cinnamaldehyde in rats. Neurosci. Lett. 473, 233–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista D. M.; Jordt S. E.; Nikai T.; Tsuruda P. R.; Read A. J.; Poblete J.; Yamoah E. N.; Basbaum A. I.; Julius D. (2006) TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124, 1269–1282. [DOI] [PubMed] [Google Scholar]
- Kwan K. Y.; Allchorne A. J.; Vollrath M. A.; Christensen A. P.; Zhang D.-S.; Woolf C. J.; Corey D. P. (2006) TRPA1 Contributes to Cold, Mechanical, and Chemical Nociception but Is Not Essential for Hair-Cell Transduction. Neuron 50, 277–289. [DOI] [PubMed] [Google Scholar]
- Karashima Y.; Talavera K.; Everaerts W.; Janssens A.; Kwan K. Y.; Vennekens R.; Nilius B.; Voets T. (2009) TRPA1 acts as a cold sensor in vitro and in vivo. Proc. Natl. Acad. Sci. U.S.A. 106, 1273–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Camino D.; Murphy S.; Heiry M.; Barrett L. B.; Earley T. J.; Cook C. A.; Petrus M. J.; Zhao M.; D’Amours M.; Deering N.; Brenner G. J.; Costigan M.; Hayward N. J.; Chong J. A.; Fanger C. M.; Woolf C. J.; Patapoutian A.; Moran M. M. (2010) TRPA1 contributes to cold hypersensitivity. J. Neurosci 30, 15165–15174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa D. S.; Meotti F. C.; Andrade E. L.; Leal P. C.; Motta E. M.; Calixto J. B. (2010) The involvement of the transient receptor potential A1 (TRPA1) in the maintenance of mechanical and cold hyperalgesia in persistent inflammation. Pain 148, 431–437. [DOI] [PubMed] [Google Scholar]
- Dai Y.; Wang S.; Tominaga M.; Yamamoto S.; Fukuoka T.; Higashi T.; Kobayashi K.; Obata K.; Yamanaka H.; Noguchi K. (2007) Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J. Clin. Invest. 117, 1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K.; Katsura H.; Mizushima T.; Yamanaka H.; Kobayashi K.; Dai Y.; Fukuoka T.; Tokunaga A.; Tominaga M.; Noguchi K. (2005) TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest 115, 2393–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista D. M., Pellegrino M., and Tsunozaki M. (2012) TRPA1: A Gatekeeper for Inflammation. Annu. Rev. Physiol. DOI: 10.1146/annurev-physiol-030212-183811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton W. M.; Daniels R. L.; Palkar R.; McCoy D. D.; McKemy D. D. (2011) Pharmacological Blockade of TRPM8 Ion Channels Alters Cold and Cold Pain Responses in Mice. PloS One 6, e25894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann K.; Lennerz J. K.; Hein A.; Link A. S.; Kaczmarek J. S.; Delling M.; Uysal S.; Pfeifer J. D.; Riccio A.; Clapham D. E. (2011) Transient receptor potential cation channel, subfamily C, member 5 (TRPC5) is a cold-transducer in the peripheral nervous system. Proc. Natl. Acad. Sci. U.S.A. 108, 18114–18119. [DOI] [PMC free article] [PubMed] [Google Scholar]