Abstract
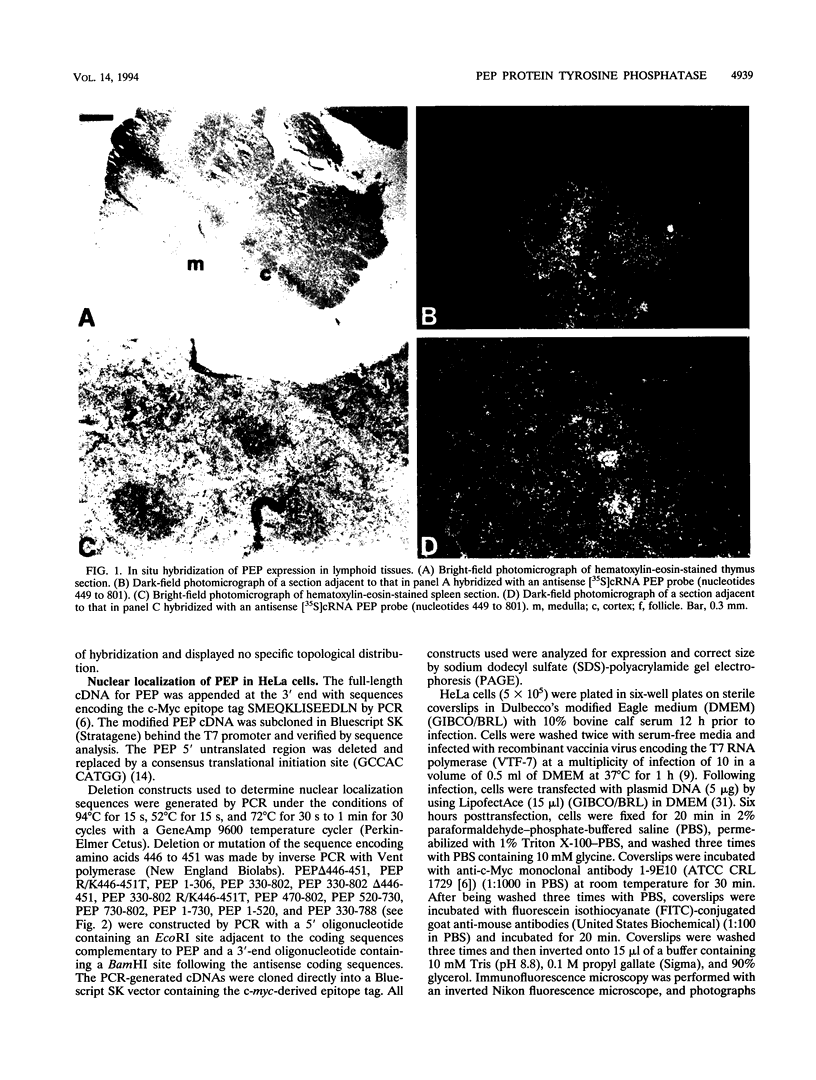
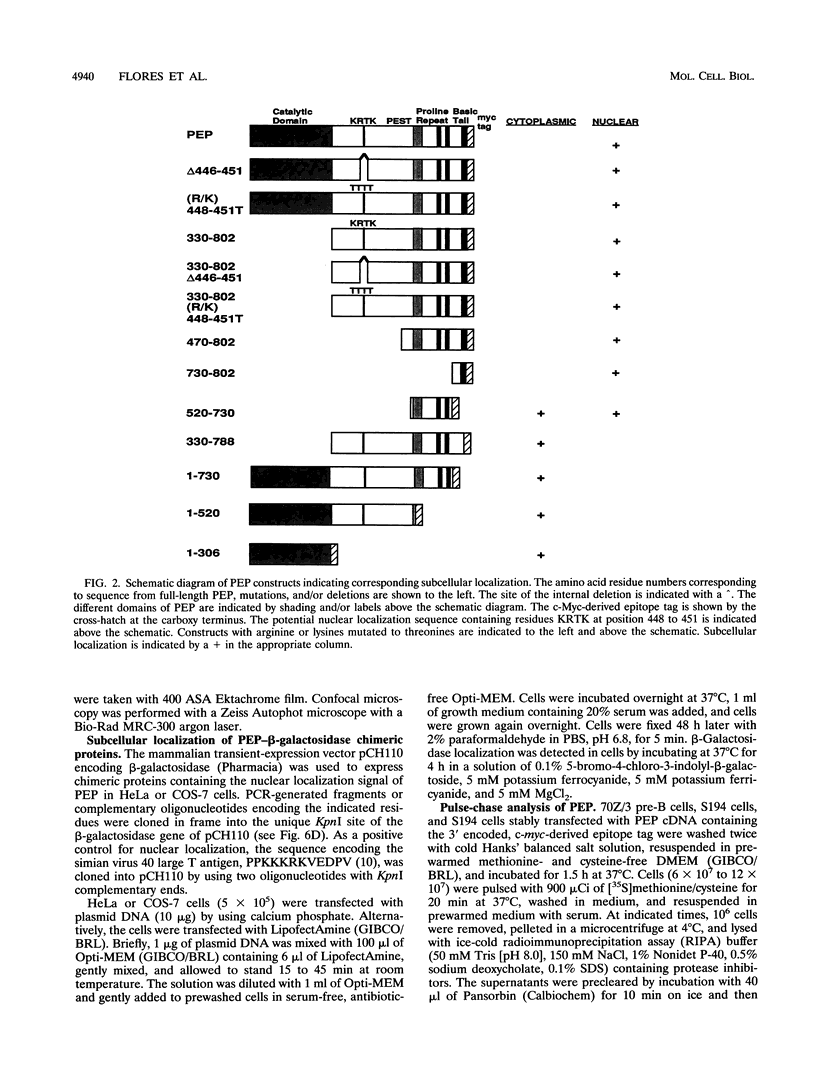
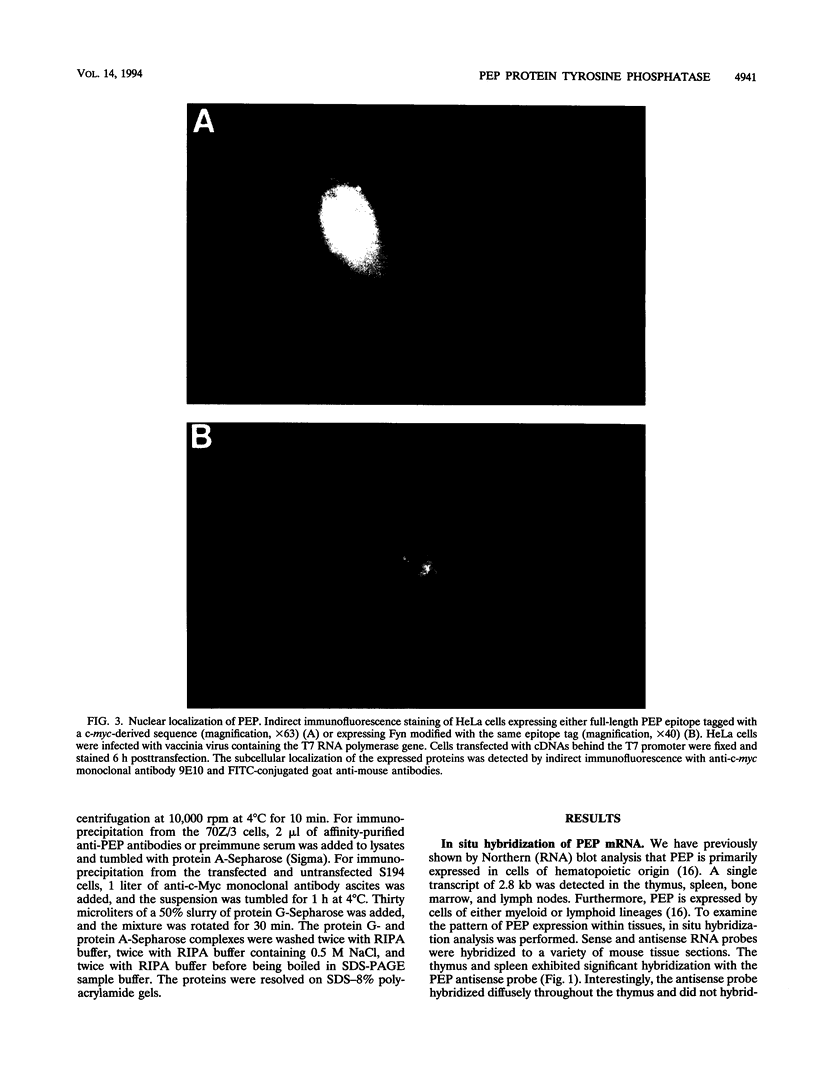
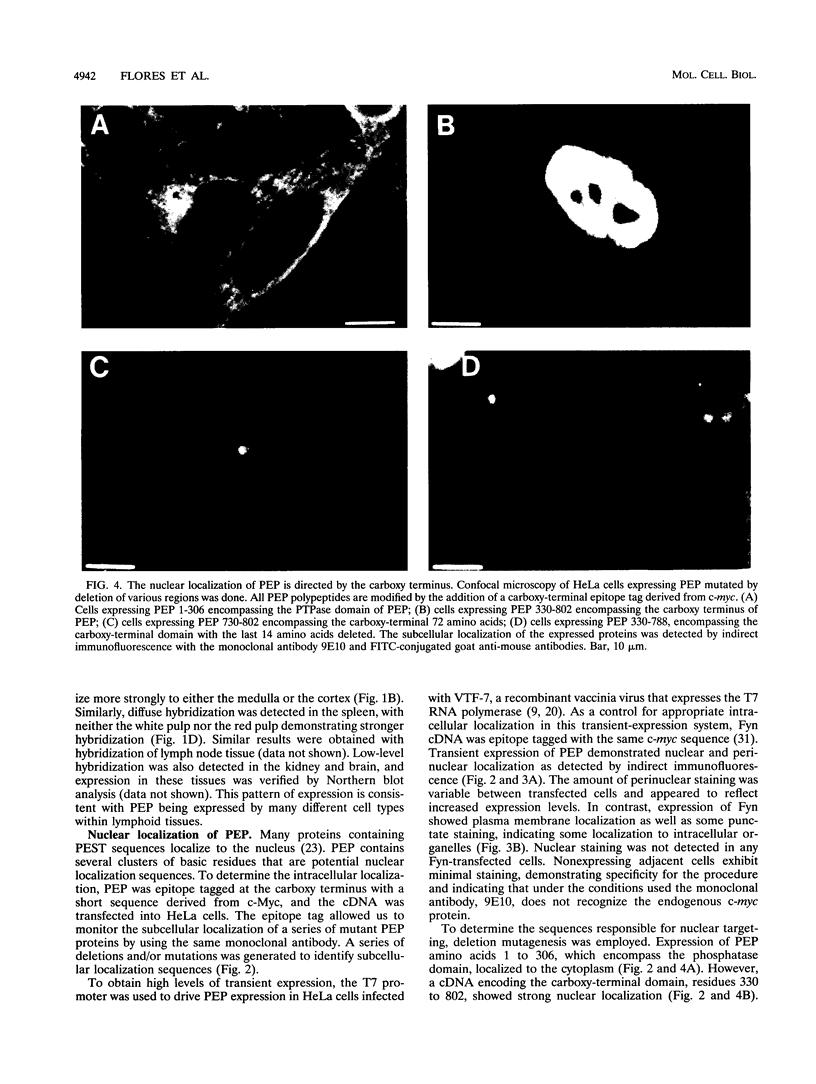
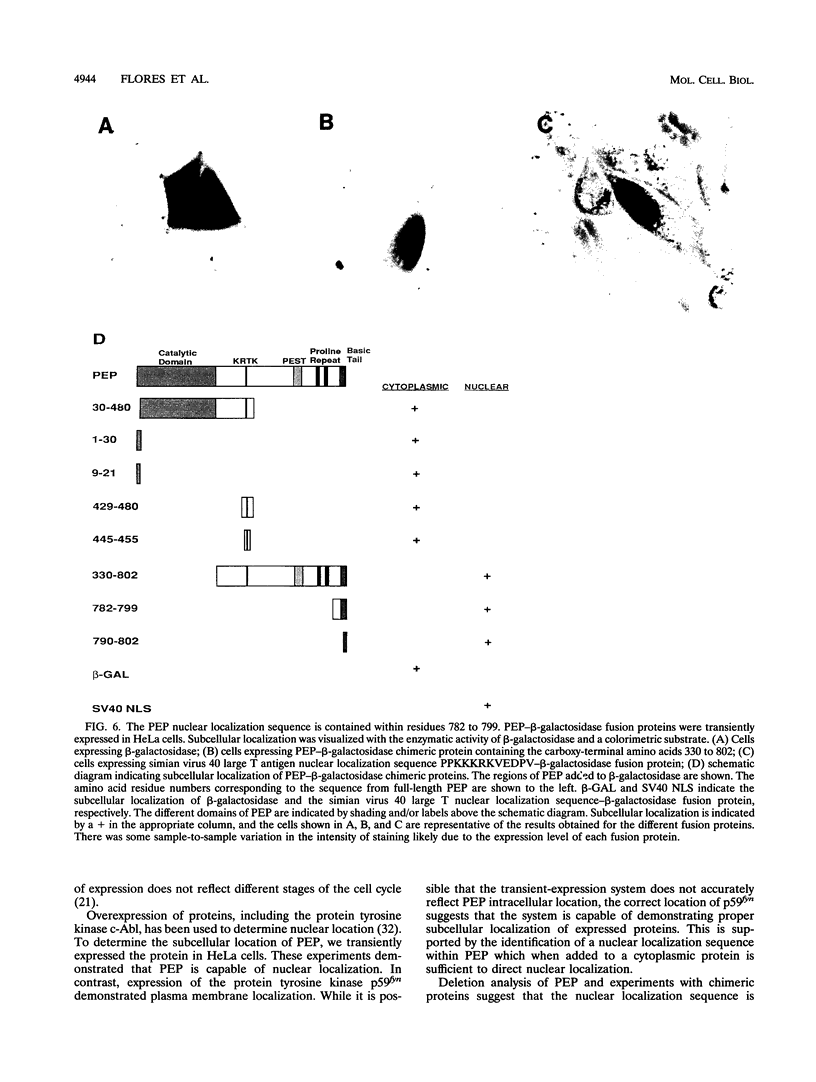
PEP is an intracellular protein tyrosine phosphatase expressed primarily by cells of hematopoietic origin that can be divided structurally into a catalytic domain and a large carboxy-terminal domain. The carboxy-terminal domain is enriched in proline, glutamic acid, serine, and threonine residues (PEST sequences) and contains a nonperfect tandem repeat sequence enriched in proline residues and a carboxy terminus enriched in basic amino acids. Here we show that PEP is diffusely expressed in lymphoid tissues, consistent with expression by many different cell types. Analysis of the PEP protein identifies a nuclear localization sequence within the extreme carboxy terminus. Transfer of 18 amino acids from the carboxy terminus of PEP to beta-galactosidase conferred nuclear localization, indicating that this sequence was sufficient for nuclear localization. Proteins enriched in PEST sequences are often rapidly degraded. However, pulse-chase analysis indicates that PEP has a half-life of greater than 5 h.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Charbonneau H., Tonks N. K. 1002 protein phosphatases? Annu Rev Cell Biol. 1992;8:463–493. doi: 10.1146/annurev.cb.08.110192.002335. [DOI] [PubMed] [Google Scholar]
- Cicchetti P., Mayer B. J., Thiel G., Baltimore D. Identification of a protein that binds to the SH3 region of Abl and is similar to Bcr and GAP-rho. Science. 1992 Aug 7;257(5071):803–806. doi: 10.1126/science.1379745. [DOI] [PubMed] [Google Scholar]
- David M., Grimley P. M., Finbloom D. S., Larner A. C. A nuclear tyrosine phosphatase downregulates interferon-induced gene expression. Mol Cell Biol. 1993 Dec;13(12):7515–7521. doi: 10.1128/mcb.13.12.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan G. I., Lewis G. K., Ramsay G., Bishop J. M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985 Dec;5(12):3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E. H., Charbonneau H., Tonks N. K. Protein tyrosine phosphatases: a diverse family of intracellular and transmembrane enzymes. Science. 1991 Jul 26;253(5018):401–406. doi: 10.1126/science.1650499. [DOI] [PubMed] [Google Scholar]
- Fu X. Y. A transcription factor with SH2 and SH3 domains is directly activated by an interferon alpha-induced cytoplasmic protein tyrosine kinase(s). Cell. 1992 Jul 24;70(2):323–335. doi: 10.1016/0092-8674(92)90106-m. [DOI] [PubMed] [Google Scholar]
- Fuerst T. R., Earl P. L., Moss B. Use of a hybrid vaccinia virus-T7 RNA polymerase system for expression of target genes. Mol Cell Biol. 1987 Jul;7(7):2538–2544. doi: 10.1128/mcb.7.7.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bustos J., Heitman J., Hall M. N. Nuclear protein localization. Biochim Biophys Acta. 1991 Mar 7;1071(1):83–101. doi: 10.1016/0304-4157(91)90013-m. [DOI] [PubMed] [Google Scholar]
- Hao Q. L., Ferris D. K., White G., Heisterkamp N., Groffen J. Nuclear and cytoplasmic location of the FER tyrosine kinase. Mol Cell Biol. 1991 Feb;11(2):1180–1183. doi: 10.1128/mcb.11.2.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart G. W., Haltiwanger R. S., Holt G. D., Kelly W. G. Glycosylation in the nucleus and cytoplasm. Annu Rev Biochem. 1989;58:841–874. doi: 10.1146/annurev.bi.58.070189.004205. [DOI] [PubMed] [Google Scholar]
- Khan K. D., Shuai K., Lindwall G., Maher S. E., Darnell J. E., Jr, Bothwell A. L. Induction of the Ly-6A/E gene by interferon alpha/beta and gamma requires a DNA element to which a tyrosine-phosphorylated 91-kDa protein binds. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6806–6810. doi: 10.1073/pnas.90.14.6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of vertebrate mRNA sequences: intimations of translational control. J Cell Biol. 1991 Nov;115(4):887–903. doi: 10.1083/jcb.115.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larner A. C., David M., Feldman G. M., Igarashi K., Hackett R. H., Webb D. S., Sweitzer S. M., Petricoin E. F., 3rd, Finbloom D. S. Tyrosine phosphorylation of DNA binding proteins by multiple cytokines. Science. 1993 Sep 24;261(5129):1730–1733. doi: 10.1126/science.8378773. [DOI] [PubMed] [Google Scholar]
- Matthews R. J., Bowne D. B., Flores E., Thomas M. L. Characterization of hematopoietic intracellular protein tyrosine phosphatases: description of a phosphatase containing an SH2 domain and another enriched in proline-, glutamic acid-, serine-, and threonine-rich sequences. Mol Cell Biol. 1992 May;12(5):2396–2405. doi: 10.1128/mcb.12.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S., Dixon J. E. Alternative splicing gives rise to a nuclear protein tyrosine phosphatase in Drosophila. J Biol Chem. 1993 Apr 5;268(10):6839–6842. [PubMed] [Google Scholar]
- Meikrantz W., Smith D. M., Sladicka M. M., Schlegel R. A. Nuclear localization of an O-glycosylated protein phosphotyrosine phosphatase from human cells. J Cell Sci. 1991 Mar;98(Pt 3):303–307. doi: 10.1242/jcs.98.3.303. [DOI] [PubMed] [Google Scholar]
- Millar J. B., Blevitt J., Gerace L., Sadhu K., Featherstone C., Russell P. p55CDC25 is a nuclear protein required for the initiation of mitosis in human cells. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10500–10504. doi: 10.1073/pnas.88.23.10500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. Vaccinia virus: a tool for research and vaccine development. Science. 1991 Jun 21;252(5013):1662–1667. doi: 10.1126/science.2047875. [DOI] [PubMed] [Google Scholar]
- Nargi J. L., Woodford-Thomas T. A. Cloning and characterization of a cdc25 phosphatase from mouse lymphocytes. Immunogenetics. 1994;39(2):99–108. doi: 10.1007/BF00188612. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Rogers S., Wells R., Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986 Oct 17;234(4774):364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Rohan P. J., Davis P., Moskaluk C. A., Kearns M., Krutzsch H., Siebenlist U., Kelly K. PAC-1: a mitogen-induced nuclear protein tyrosine phosphatase. Science. 1993 Mar 19;259(5102):1763–1766. doi: 10.1126/science.7681221. [DOI] [PubMed] [Google Scholar]
- Ruff-Jamison S., Chen K., Cohen S. Induction by EGF and interferon-gamma of tyrosine phosphorylated DNA binding proteins in mouse liver nuclei. Science. 1993 Sep 24;261(5129):1733–1736. doi: 10.1126/science.8378774. [DOI] [PubMed] [Google Scholar]
- Schindler C., Shuai K., Prezioso V. R., Darnell J. E., Jr Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor. Science. 1992 Aug 7;257(5071):809–813. doi: 10.1126/science.1496401. [DOI] [PubMed] [Google Scholar]
- Shuai K., Schindler C., Prezioso V. R., Darnell J. E., Jr Activation of transcription by IFN-gamma: tyrosine phosphorylation of a 91-kD DNA binding protein. Science. 1992 Dec 11;258(5089):1808–1812. doi: 10.1126/science.1281555. [DOI] [PubMed] [Google Scholar]
- Shuai K., Stark G. R., Kerr I. M., Darnell J. E., Jr A single phosphotyrosine residue of Stat91 required for gene activation by interferon-gamma. Science. 1993 Sep 24;261(5129):1744–1746. doi: 10.1126/science.7690989. [DOI] [PubMed] [Google Scholar]
- Silvennoinen O., Schindler C., Schlessinger J., Levy D. E. Ras-independent growth factor signaling by transcription factor tyrosine phosphorylation. Science. 1993 Sep 24;261(5129):1736–1739. doi: 10.1126/science.8378775. [DOI] [PubMed] [Google Scholar]
- Takekawa M., Itoh F., Hinoda Y., Arimura Y., Toyota M., Sekiya M., Adachi M., Imai K., Yachi A. Cloning and characterization of a human cDNA encoding a novel putative cytoplasmic protein-tyrosine-phosphatase. Biochem Biophys Res Commun. 1992 Dec 15;189(2):1223–1230. doi: 10.1016/0006-291x(92)92335-u. [DOI] [PubMed] [Google Scholar]
- Timson Gauen L. K., Kong A. N., Samelson L. E., Shaw A. S. p59fyn tyrosine kinase associates with multiple T-cell receptor subunits through its unique amino-terminal domain. Mol Cell Biol. 1992 Dec;12(12):5438–5446. doi: 10.1128/mcb.12.12.5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten R. A., Jackson P., Baltimore D. The mouse type IV c-abl gene product is a nuclear protein, and activation of transforming ability is associated with cytoplasmic localization. Cell. 1989 Aug 25;58(4):669–678. doi: 10.1016/0092-8674(89)90102-5. [DOI] [PubMed] [Google Scholar]
- Velazquez L., Fellous M., Stark G. R., Pellegrini S. A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell. 1992 Jul 24;70(2):313–322. doi: 10.1016/0092-8674(92)90105-l. [DOI] [PubMed] [Google Scholar]
- Walton K. M., Dixon J. E. Protein tyrosine phosphatases. Annu Rev Biochem. 1993;62:101–120. doi: 10.1146/annurev.bi.62.070193.000533. [DOI] [PubMed] [Google Scholar]
- Yang Q., Co D., Sommercorn J., Tonks N. K. Cloning and expression of PTP-PEST. A novel, human, nontransmembrane protein tyrosine phosphatase. J Biol Chem. 1993 Mar 25;268(9):6622–6628. [PubMed] [Google Scholar]
- den Hertog J., Pals C. E., Jonk L. J., Kruijer W. Differential expression of a novel murine non-receptor protein tyrosine phosphatase during differentiation of P19 embryonal carcinoma cells. Biochem Biophys Res Commun. 1992 May 15;184(3):1241–1249. doi: 10.1016/s0006-291x(05)80015-4. [DOI] [PubMed] [Google Scholar]