Abstract
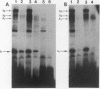
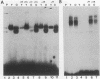
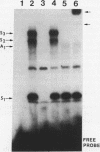
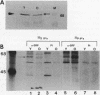
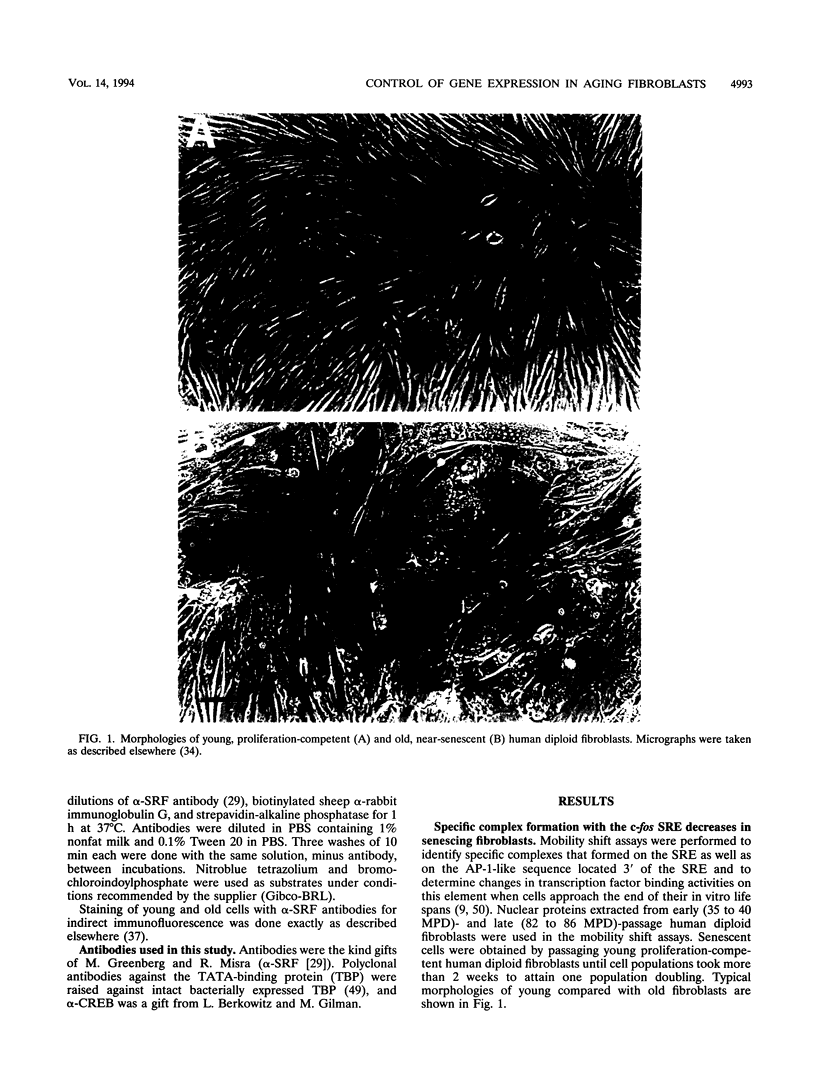
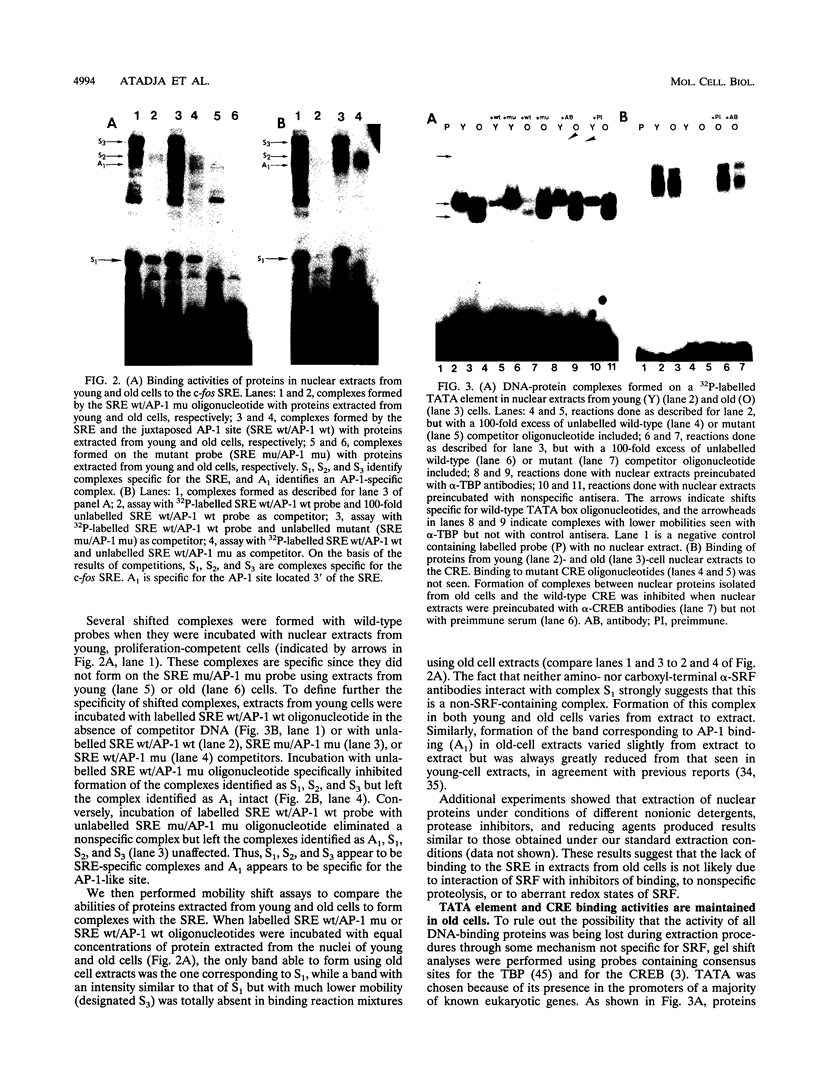
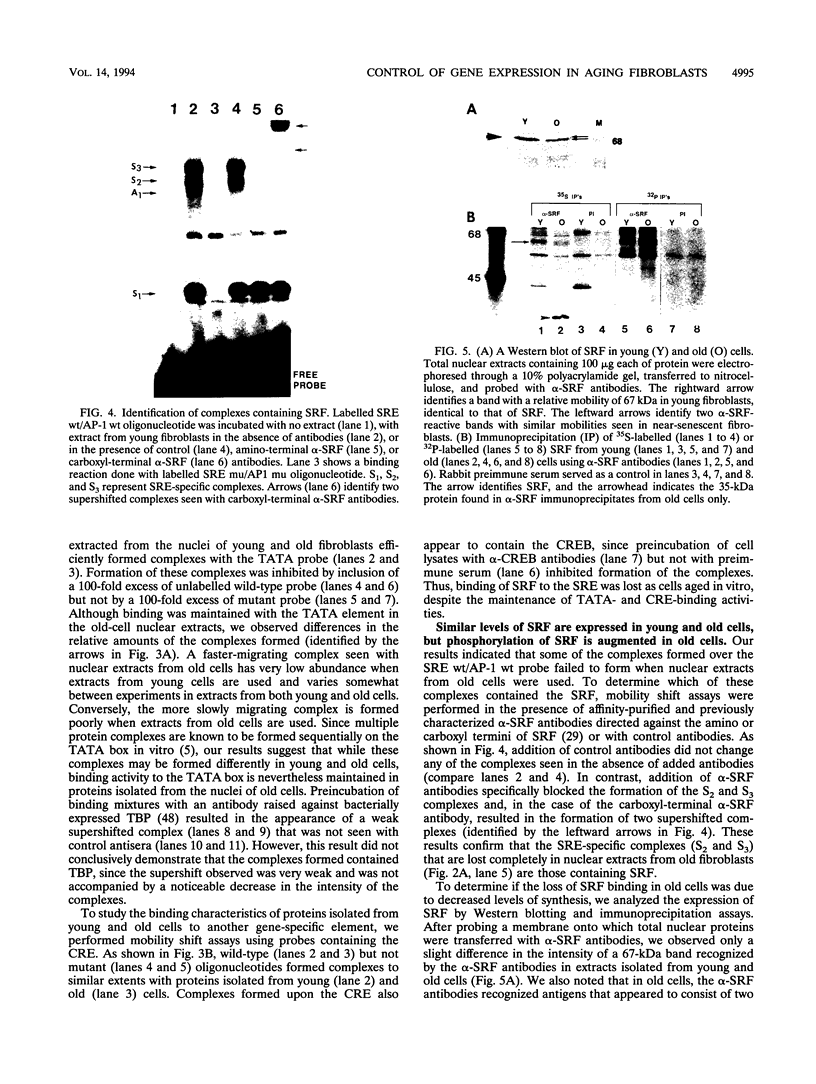
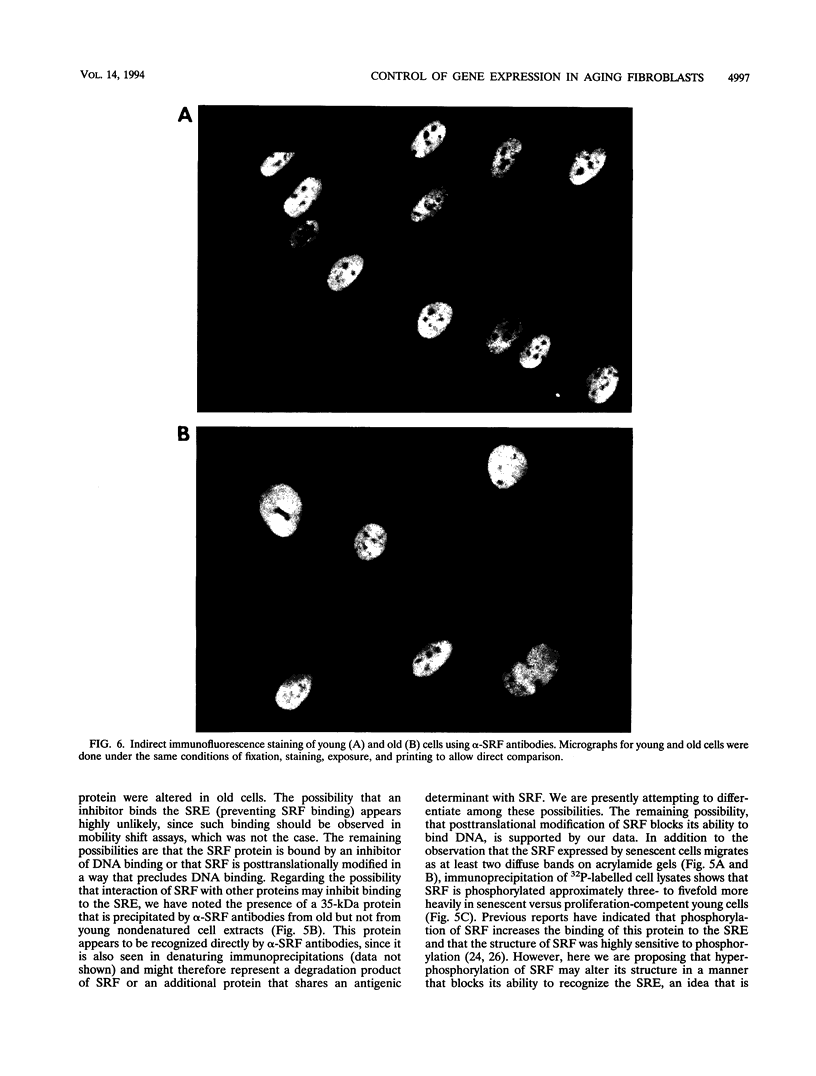
Human diploid fibroblasts undergo a limited number of population doublings in vitro and are used widely as a model of cellular aging. Despite growing evidence that cellular aging occurs as a consequence of altered gene expression, little is known about the activity of transcription factors in aging cells. Here, we report a dramatic reduction in the ability of proteins extracted from the nuclei of near-senescent fibroblasts to bind the serum response element which is necessary for serum-induced transcription of the c-fos gene. In contrast, the activities of proteins binding to the RNA polymerase core element, TATA, as well as to the cyclic AMP response element were maintained during cellular aging. While no major differences in the expression of the serum response factor (SRF) that binds the serum response element were seen between early-passage and late-passage cells, hyperphosphorylation of SRF was observed in near-senescent cells. Furthermore, removal of phosphatase inhibitors during the isolation of endogenous nuclear proteins restored the ability of SRF isolated from old cells to bind the SRE. These data, therefore, indicate that hyperphosphorylation of SRF plays a role in altering the ability of this protein to bind to DNA and regulate gene expression in senescent cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almendral J. M., Sommer D., Macdonald-Bravo H., Burckhardt J., Perera J., Bravo R. Complexity of the early genetic response to growth factors in mouse fibroblasts. Mol Cell Biol. 1988 May;8(5):2140–2148. doi: 10.1128/mcb.8.5.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz L. A., Riabowol K. T., Gilman M. Z. Multiple sequence elements of a single functional class are required for cyclic AMP responsiveness of the mouse c-fos promoter. Mol Cell Biol. 1989 Oct;9(10):4272–4281. doi: 10.1128/mcb.9.10.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. T. Genetic diseases of premature aging as models of senescence. Annu Rev Gerontol Geriatr. 1990;10:23–42. doi: 10.1007/978-3-662-38445-9_2. [DOI] [PubMed] [Google Scholar]
- Buratowski S., Hahn S., Guarente L., Sharp P. A. Five intermediate complexes in transcription initiation by RNA polymerase II. Cell. 1989 Feb 24;56(4):549–561. doi: 10.1016/0092-8674(89)90578-3. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisch T. M., Prywes R., Roeder R. G. c-fos sequence necessary for basal expression and induction by epidermal growth factor, 12-O-tetradecanoyl phorbol-13-acetate and the calcium ionophore. Mol Cell Biol. 1987 Oct;7(10):3490–3502. doi: 10.1128/mcb.7.10.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman M. Z. The c-fos serum response element responds to protein kinase C-dependent and -independent signals but not to cyclic AMP. Genes Dev. 1988 Apr;2(4):394–402. doi: 10.1101/gad.2.4.394. [DOI] [PubMed] [Google Scholar]
- Gilman M. Z., Wilson R. N., Weinberg R. A. Multiple protein-binding sites in the 5'-flanking region regulate c-fos expression. Mol Cell Biol. 1986 Dec;6(12):4305–4316. doi: 10.1128/mcb.6.12.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano T., Foster D. N. Identification of a highly abundant cDNA isolated from senescent WI-38 cells. Exp Cell Res. 1989 Dec;185(2):399–406. doi: 10.1016/0014-4827(89)90310-8. [DOI] [PubMed] [Google Scholar]
- Goldstein S. Replicative senescence: the human fibroblast comes of age. Science. 1990 Sep 7;249(4973):1129–1133. doi: 10.1126/science.2204114. [DOI] [PubMed] [Google Scholar]
- Graham R., Gilman M. Distinct protein targets for signals acting at the c-fos serum response element. Science. 1991 Jan 11;251(4990):189–192. doi: 10.1126/science.1898992. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Siegfried Z., Ziff E. B. Mutation of the c-fos gene dyad symmetry element inhibits serum inducibility of transcription in vivo and the nuclear regulatory factor binding in vitro. Mol Cell Biol. 1987 Mar;7(3):1217–1225. doi: 10.1128/mcb.7.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- HAYFLICK L., MOORHEAD P. S. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961 Dec;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- HAYFLICK L. THE LIMITED IN VITRO LIFETIME OF HUMAN DIPLOID CELL STRAINS. Exp Cell Res. 1965 Mar;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Hai T. W., Liu F., Allegretto E. A., Karin M., Green M. R. A family of immunologically related transcription factors that includes multiple forms of ATF and AP-1. Genes Dev. 1988 Oct;2(10):1216–1226. doi: 10.1101/gad.2.10.1216. [DOI] [PubMed] [Google Scholar]
- Hipskind R. A., Rao V. N., Mueller C. G., Reddy E. S., Nordheim A. Ets-related protein Elk-1 is homologous to the c-fos regulatory factor p62TCF. Nature. 1991 Dec 19;354(6354):531–534. doi: 10.1038/354531a0. [DOI] [PubMed] [Google Scholar]
- Holt J. T., Gopal T. V., Moulton A. D., Nienhuis A. W. Inducible production of c-fos antisense RNA inhibits 3T3 cell proliferation. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4794–4798. doi: 10.1073/pnas.83.13.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Karin M. The regulation of transcription by phosphorylation. Cell. 1992 Aug 7;70(3):375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- Kovary K., Bravo R. The jun and fos protein families are both required for cell cycle progression in fibroblasts. Mol Cell Biol. 1991 Sep;11(9):4466–4472. doi: 10.1128/mcb.11.9.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazaki T., Robetorye R. S., Robetorye S. C., Smith J. R. Fibronectin expression increases during in vitro cellular senescence: correlation with increased cell area. Exp Cell Res. 1991 Jul;195(1):13–19. doi: 10.1016/0014-4827(91)90494-f. [DOI] [PubMed] [Google Scholar]
- König H., Ponta H., Rahmsdorf U., Büscher M., Schönthal A., Rahmsdorf H. J., Herrlich P. Autoregulation of fos: the dyad symmetry element as the major target of repression. EMBO J. 1989 Sep;8(9):2559–2566. doi: 10.1002/j.1460-2075.1989.tb08394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manak J. R., Prywes R. Mutation of serum response factor phosphorylation sites and the mechanism by which its DNA-binding activity is increased by casein kinase II. Mol Cell Biol. 1991 Jul;11(7):3652–3659. doi: 10.1128/mcb.11.7.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais R. M., Hsuan J. J., McGuigan C., Wynne J., Treisman R. Casein kinase II phosphorylation increases the rate of serum response factor-binding site exchange. EMBO J. 1992 Jan;11(1):97–105. doi: 10.1002/j.1460-2075.1992.tb05032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais R., Wynne J., Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993 Apr 23;73(2):381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- Martin G. M., Sprague C. A., Epstein C. J. Replicative life-span of cultivated human cells. Effects of donor's age, tissue, and genotype. Lab Invest. 1970 Jul;23(1):86–92. [PubMed] [Google Scholar]
- McCormick A., Campisi J. Cellular aging and senescence. Curr Opin Cell Biol. 1991 Apr;3(2):230–234. doi: 10.1016/0955-0674(91)90144-n. [DOI] [PubMed] [Google Scholar]
- Misra R. P., Rivera V. M., Wang J. M., Fan P. D., Greenberg M. E. The serum response factor is extensively modified by phosphorylation following its synthesis in serum-stimulated fibroblasts. Mol Cell Biol. 1991 Sep;11(9):4545–4554. doi: 10.1128/mcb.11.9.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y., Satake M., Yamaguchi-Iwai Y., Sakai M., Muramatsu M., Ito Y. The nuclear protooncogenes c-jun and c-fos as regulators of DNA replication. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3947–3951. doi: 10.1073/pnas.88.9.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murano S., Thweatt R., Shmookler Reis R. J., Jones R. A., Moerman E. J., Goldstein S. Diverse gene sequences are overexpressed in werner syndrome fibroblasts undergoing premature replicative senescence. Mol Cell Biol. 1991 Aug;11(8):3905–3914. doi: 10.1128/mcb.11.8.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikura K., Murray J. M. Antisense RNA of proto-oncogene c-fos blocks renewed growth of quiescent 3T3 cells. Mol Cell Biol. 1987 Feb;7(2):639–649. doi: 10.1128/mcb.7.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips P. D., Pignolo R. J., Nishikura K., Cristofalo V. J. Renewed DNA synthesis in senescent WI-38 cells by expression of an inducible chimeric c-fos construct. J Cell Physiol. 1992 Apr;151(1):206–212. doi: 10.1002/jcp.1041510126. [DOI] [PubMed] [Google Scholar]
- Riabowol K. T., Fink J. S., Gilman M. Z., Walsh D. A., Goodman R. H., Feramisco J. R. The catalytic subunit of cAMP-dependent protein kinase induces expression of genes containing cAMP-responsive enhancer elements. Nature. 1988 Nov 3;336(6194):83–86. doi: 10.1038/336083a0. [DOI] [PubMed] [Google Scholar]
- Riabowol K. T. Transcription factor activity during cellular aging of human diploid fibroblasts. Biochem Cell Biol. 1992 Oct-Nov;70(10-11):1064–1072. doi: 10.1139/o92-151. [DOI] [PubMed] [Google Scholar]
- Riabowol K. T., Vosatka R. J., Ziff E. B., Lamb N. J., Feramisco J. R. Microinjection of fos-specific antibodies blocks DNA synthesis in fibroblast cells. Mol Cell Biol. 1988 Apr;8(4):1670–1676. doi: 10.1128/mcb.8.4.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K. H., Afshari C. A., Annab L. A., Burkhart B. A., Owen R. D., Boyd J., Barrett J. C. Down-regulation of cdc2 in senescent human and hamster cells. Cancer Res. 1991 Nov 1;51(21):6010–6013. [PubMed] [Google Scholar]
- Rittling S. R., Brooks K. M., Cristofalo V. J., Baserga R. Expression of cell cycle-dependent genes in young and senescent WI-38 fibroblasts. Proc Natl Acad Sci U S A. 1986 May;83(10):3316–3320. doi: 10.1073/pnas.83.10.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera V. M., Greenberg M. E. Growth factor-induced gene expression: the ups and downs of c-fos regulation. New Biol. 1990 Sep;2(9):751–758. [PubMed] [Google Scholar]
- Ryan W. A., Jr, Franza B. R., Jr, Gilman M. Z. Two distinct cellular phosphoproteins bind to the c-fos serum response element. EMBO J. 1989 Jun;8(6):1785–1792. doi: 10.1002/j.1460-2075.1989.tb03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhme D. Evidence for a relationship between longevity of mammalian species and life spans of normal fibroblasts in vitro and erythrocytes in vivo. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5009–5013. doi: 10.1073/pnas.78.8.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski H. B., Gilman M. Z. Cell-free activation of a DNA-binding protein by epidermal growth factor. Nature. 1993 Mar 4;362(6415):79–83. doi: 10.1038/362079a0. [DOI] [PubMed] [Google Scholar]
- Schröter H., Mueller C. G., Meese K., Nordheim A. Synergism in ternary complex formation between the dimeric glycoprotein p67SRF, polypeptide p62TCF and the c-fos serum response element. EMBO J. 1990 Apr;9(4):1123–1130. doi: 10.1002/j.1460-2075.1990.tb08218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri T., Campisi J. Repression of c-fos transcription and an altered genetic program in senescent human fibroblasts. Science. 1990 Jan 12;247(4939):205–209. doi: 10.1126/science.2104680. [DOI] [PubMed] [Google Scholar]
- Sharp P. A. TATA-binding protein is a classless factor. Cell. 1992 Mar 6;68(5):819–821. doi: 10.1016/0092-8674(92)90023-6. [DOI] [PubMed] [Google Scholar]
- Smith J. R., Pereira-Smith O. M. Altered gene expression during cellular aging. Genome. 1989;31(1):386–389. doi: 10.1139/g89-058. [DOI] [PubMed] [Google Scholar]
- Stein G. H., Drullinger L. F., Robetorye R. S., Pereira-Smith O. M., Smith J. R. Senescent cells fail to express cdc2, cycA, and cycB in response to mitogen stimulation. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11012–11016. doi: 10.1073/pnas.88.24.11012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer K. F., Ingles C. J., Greenblatt J. Direct and selective binding of an acidic transcriptional activation domain to the TATA-box factor TFIID. Nature. 1990 Jun 28;345(6278):783–786. doi: 10.1038/345783a0. [DOI] [PubMed] [Google Scholar]
- Treisman R. Identification of a protein-binding site that mediates transcriptional response of the c-fos gene to serum factors. Cell. 1986 Aug 15;46(4):567–574. doi: 10.1016/0092-8674(86)90882-2. [DOI] [PubMed] [Google Scholar]
- Treisman R. The serum response element. Trends Biochem Sci. 1992 Oct;17(10):423–426. doi: 10.1016/0968-0004(92)90013-y. [DOI] [PubMed] [Google Scholar]
- Tsai L. H., Lees E., Faha B., Harlow E., Riabowol K. The cdk2 kinase is required for the G1-to-S transition in mammalian cells. Oncogene. 1993 Jun;8(6):1593–1602. [PubMed] [Google Scholar]
- Wagner B. J., Hayes T. E., Hoban C. J., Cochran B. H. The SIF binding element confers sis/PDGF inducibility onto the c-fos promoter. EMBO J. 1990 Dec;9(13):4477–4484. doi: 10.1002/j.1460-2075.1990.tb07898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E. A 57,000-mol-wt protein uniquely present in nonproliferating cells and senescent human fibroblasts. J Cell Biol. 1985 Feb;100(2):545–551. doi: 10.1083/jcb.100.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West M. D., Pereira-Smith O. M., Smith J. R. Replicative senescence of human skin fibroblasts correlates with a loss of regulation and overexpression of collagenase activity. Exp Cell Res. 1989 Sep;184(1):138–147. doi: 10.1016/0014-4827(89)90372-8. [DOI] [PubMed] [Google Scholar]
- Winkles J. A., O'Connor M. L., Friesel R. Altered regulation of platelet-derived growth factor A-chain and c-fos gene expression in senescent progeria fibroblasts. J Cell Physiol. 1990 Aug;144(2):313–325. doi: 10.1002/jcp.1041440218. [DOI] [PubMed] [Google Scholar]