Abstract
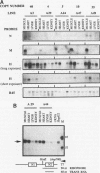
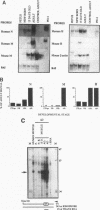
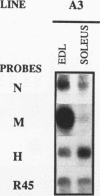
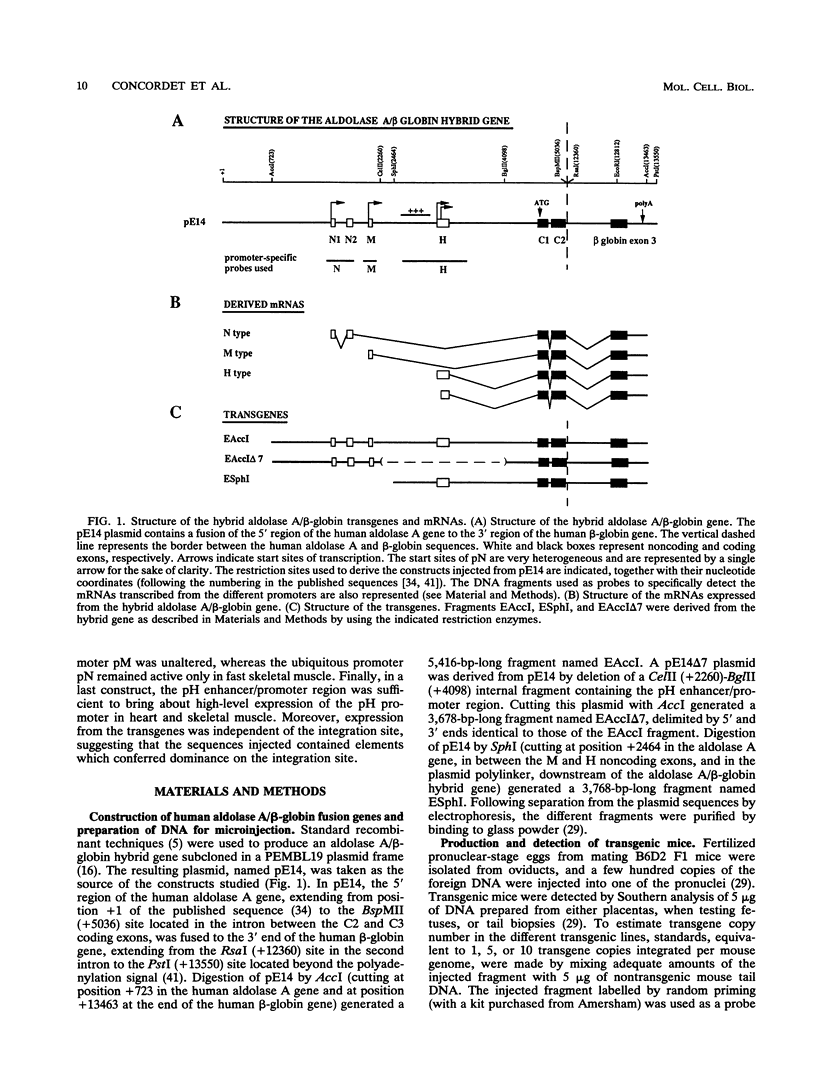
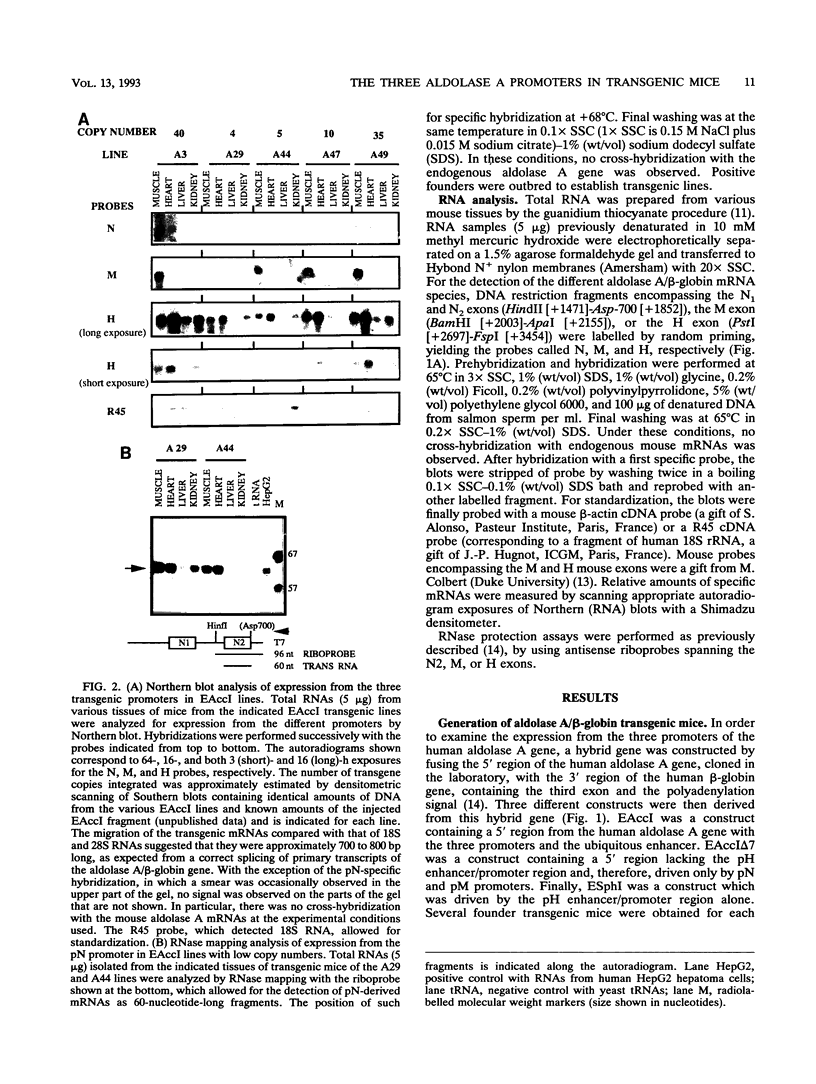
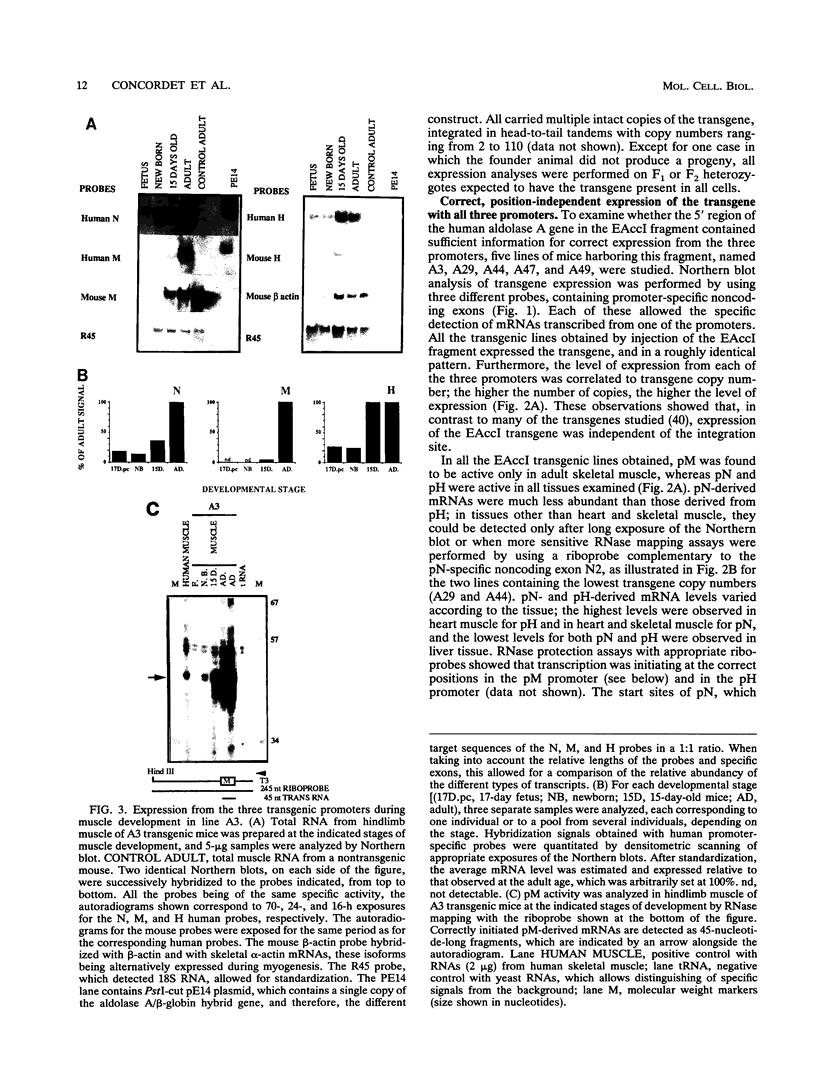
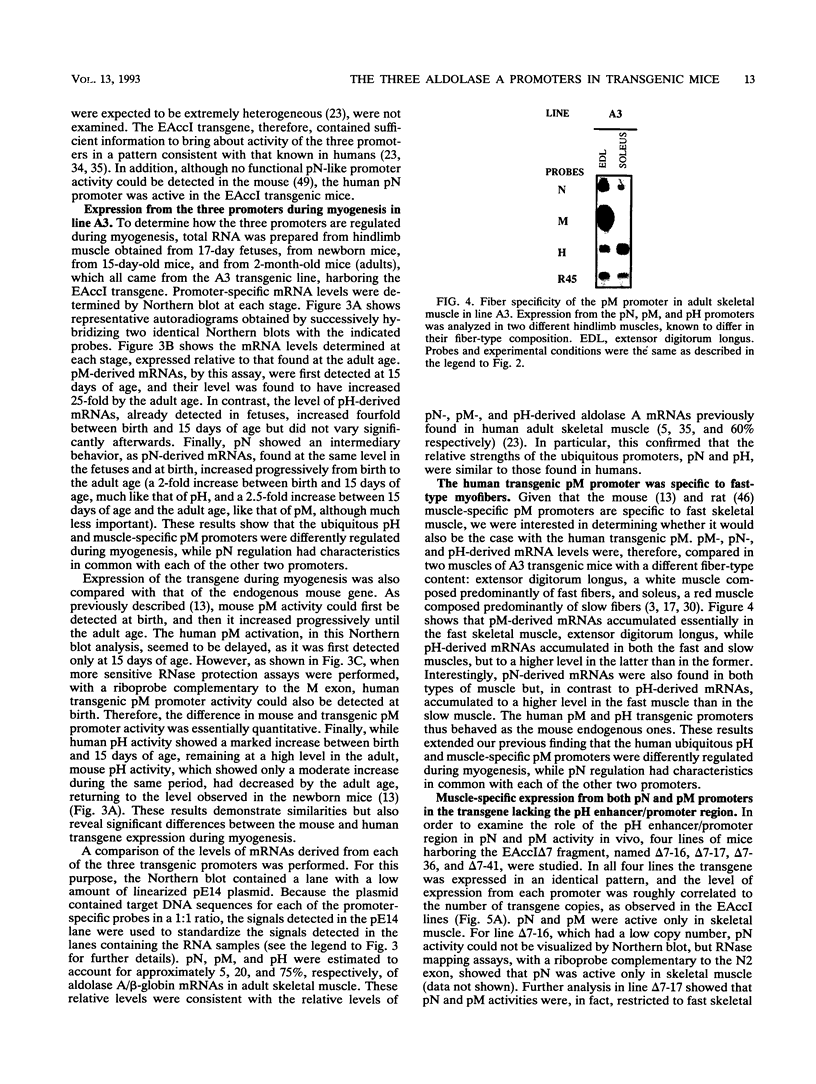
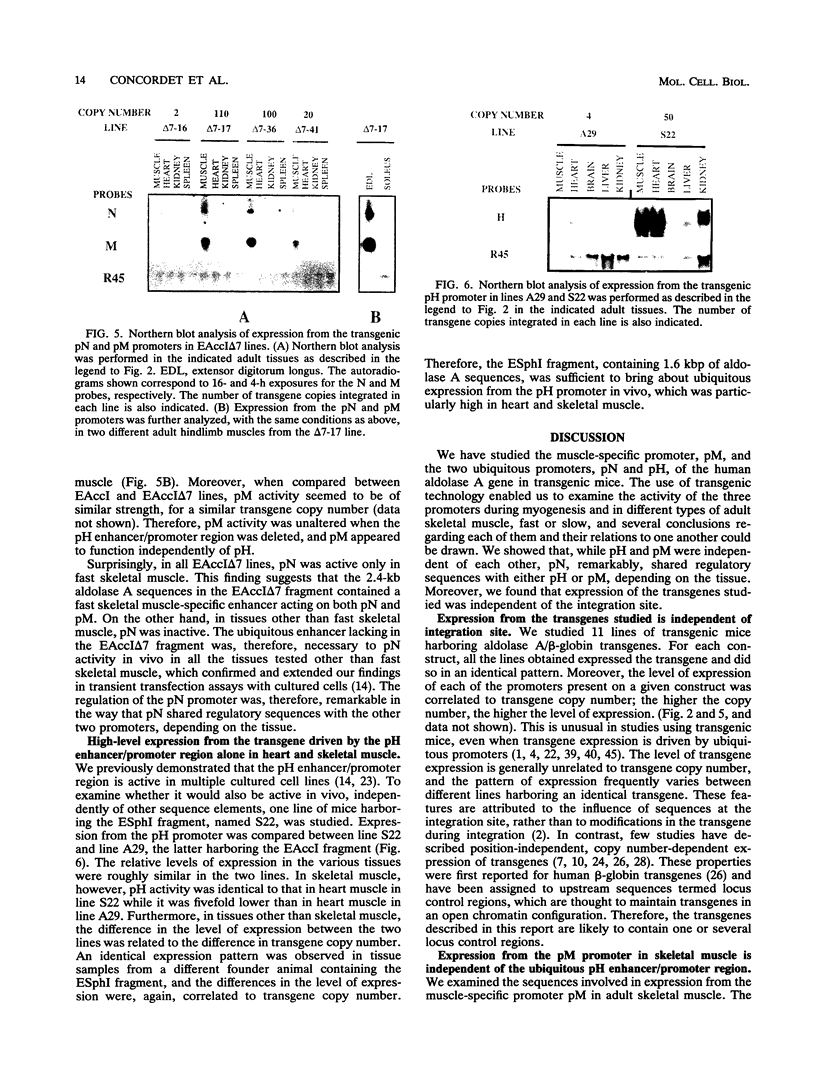
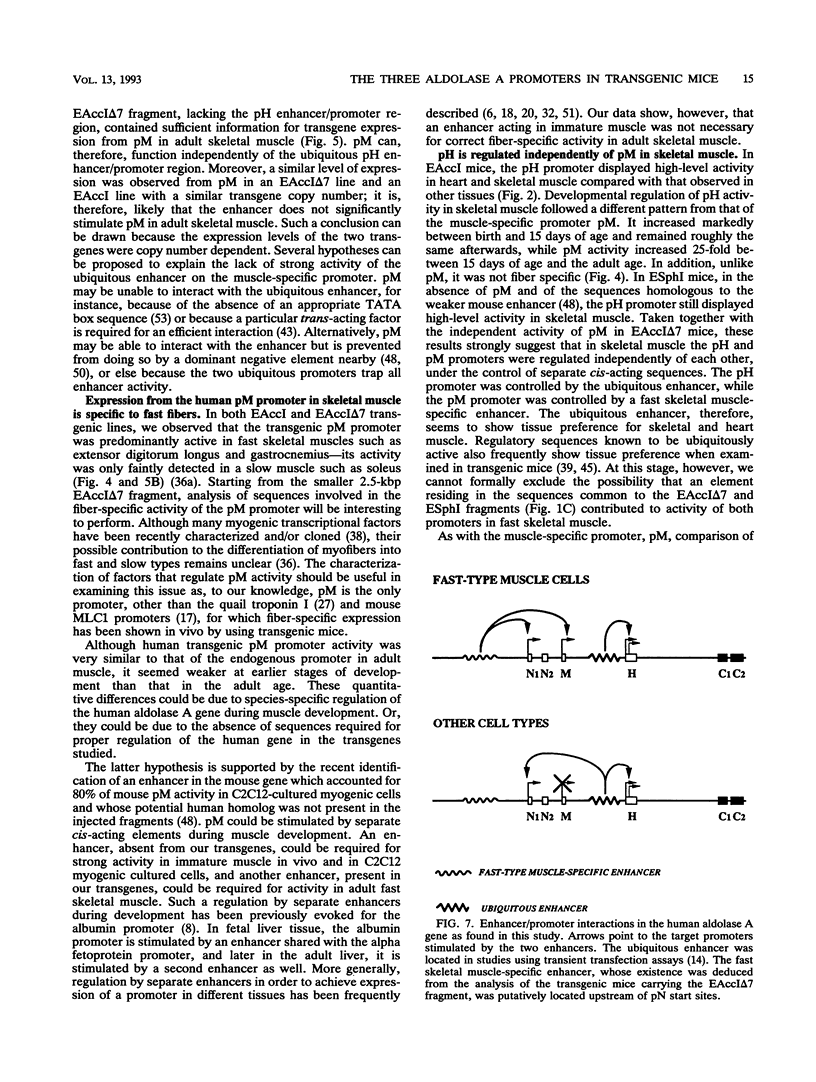
The human aldolase A gene is transcribed from three different promoters, pN, pM, and pH, all of which are clustered within a small 1.6-kbp DNA domain. pM, which is highly specific to adult skeletal muscle, lies in between pN and pH, which are ubiquitous but particularly active in heart and skeletal muscle. A ubiquitous enhancer, located just upstream of pH start sites, is necessary for the activity of both pH and pN in transient transfection assays. Using transgenic mice, we studied the sequence controlling the muscle-specific promoter pM and the relations between the three promoters and the ubiquitous enhancer. A 4.3-kbp fragment containing the three promoters and the ubiquitous enhancer showed an expression pattern consistent with that known in humans. In addition, while pH was active in both fast and slow skeletal muscles, pM was active only in fast muscle. pM activity was unaltered by the deletion of a 1.8-kbp region containing the ubiquitous enhancer and the pH promoter, whereas pN remained active only in fast skeletal muscle. These findings suggest that in fast skeletal muscle, a tissue-specific enhancer was acting on both pN and pM, whereas in other tissues, the ubiquitous enhancer was necessary for pN activity. Finally, a 2.6-kbp region containing the ubiquitous enhancer and only the pH promoter was sufficient to bring about high-level expression of pH in cardiac and skeletal muscle. Thus, while pH and pM function independently of each other, pN, remarkably, shares regulatory elements with each of them, depending on the tissue. Importantly, expression of the transgenes was independent of the integration site, as originally described for transgenes containing the beta-globin locus control region.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen N. D., Cran D. G., Barton S. C., Hettle S., Reik W., Surani M. A. Transgenes as probes for active chromosomal domains in mouse development. Nature. 1988 Jun 30;333(6176):852–855. doi: 10.1038/333852a0. [DOI] [PubMed] [Google Scholar]
- Ariano M. A., Armstrong R. B., Edgerton V. R. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973 Jan;21(1):51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- Aronow B., Lattier D., Silbiger R., Dusing M., Hutton J., Jones G., Stock J., McNeish J., Potter S., Witte D. Evidence for a complex regulatory array in the first intron of the human adenosine deaminase gene. Genes Dev. 1989 Sep;3(9):1384–1400. doi: 10.1101/gad.3.9.1384. [DOI] [PubMed] [Google Scholar]
- Benfey P. N., Chua N. H. The Cauliflower Mosaic Virus 35S Promoter: Combinatorial Regulation of Transcription in Plants. Science. 1990 Nov 16;250(4983):959–966. doi: 10.1126/science.250.4983.959. [DOI] [PubMed] [Google Scholar]
- Bonifer C., Vidal M., Grosveld F., Sippel A. E. Tissue specific and position independent expression of the complete gene domain for chicken lysozyme in transgenic mice. EMBO J. 1990 Sep;9(9):2843–2848. doi: 10.1002/j.1460-2075.1990.tb07473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camper S. A., Tilghman S. M. Postnatal repression of the alpha-fetoprotein gene is enhancer independent. Genes Dev. 1989 Apr;3(4):537–546. doi: 10.1101/gad.3.4.537. [DOI] [PubMed] [Google Scholar]
- Cavener D. R. Transgenic animal studies on the evolution of genetic regulatory circuitries. Bioessays. 1992 Apr;14(4):237–244. doi: 10.1002/bies.950140407. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. W., Vasavada H. A., Ganguly S., Weissman S. M. Identification of cis sequences controlling efficient position-independent tissue-specific expression of human major histocompatibility complex class I genes in transgenic mice. Mol Cell Biol. 1991 Jul;11(7):3564–3572. doi: 10.1128/mcb.11.7.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Choi O. R., Engel J. D. Developmental regulation of beta-globin gene switching. Cell. 1988 Oct 7;55(1):17–26. doi: 10.1016/0092-8674(88)90005-0. [DOI] [PubMed] [Google Scholar]
- Colbert M. C., Ciejek-Baez E. The proximal promoter of the aldolase A gene remains active during myogenesis in vitro and muscle development in vivo. Dev Biol. 1992 Jan;149(1):66–79. doi: 10.1016/0012-1606(92)90264-h. [DOI] [PubMed] [Google Scholar]
- Concordet J. P., Maire P., Kahn A., Daegelen D. A ubiquitous enhancer shared by two promoters in the human aldolase A gene. Nucleic Acids Res. 1991 Aug 11;19(15):4173–4180. doi: 10.1093/nar/19.15.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin V., Maniatis T. The role of specific enhancer-promoter interactions in the Drosophila Adh promoter switch. Genes Dev. 1989 Dec;3(12B):2191–2120. doi: 10.1101/gad.3.12b.2191. [DOI] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue M. J., Alvarez J. D., Merlie J. P., Sanes J. R. Fiber type- and position-dependent expression of a myosin light chain-CAT transgene detected with a novel histochemical stain for CAT. J Cell Biol. 1991 Oct;115(2):423–434. doi: 10.1083/jcb.115.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin R. A., Gopal-Srivastava R., Wawrousek E. F., Piatigorsky J. Expression of the murine alpha B-crystallin gene in lens and skeletal muscle: identification of a muscle-preferred enhancer. Mol Cell Biol. 1991 Sep;11(9):4340–4349. doi: 10.1128/mcb.11.9.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enver T., Raich N., Ebens A. J., Papayannopoulou T., Costantini F., Stamatoyannopoulos G. Developmental regulation of human fetal-to-adult globin gene switching in transgenic mice. Nature. 1990 Mar 22;344(6264):309–313. doi: 10.1038/344309a0. [DOI] [PubMed] [Google Scholar]
- Fischer J. A., Maniatis T. Regulatory elements involved in Drosophila Adh gene expression are conserved in divergent species and separate elements mediate expression in different tissues. EMBO J. 1986 Jun;5(6):1275–1289. doi: 10.1002/j.1460-2075.1986.tb04357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley K. P., Engel J. D. Individual stage selector element mutations lead to reciprocal changes in beta- vs. epsilon-globin gene transcription: genetic confirmation of promoter competition during globin gene switching. Genes Dev. 1992 May;6(5):730–744. doi: 10.1101/gad.6.5.730. [DOI] [PubMed] [Google Scholar]
- Furth P. A., Hennighausen L., Baker C., Beatty B., Woychick R. The variability in activity of the universally expressed human cytomegalovirus immediate early gene 1 enhancer/promoter in transgenic mice. Nucleic Acids Res. 1991 Nov 25;19(22):6205–6208. doi: 10.1093/nar/19.22.6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautron S., Maire P., Hakim V., Kahn A. Regulation of the multiple promoters of the human aldolase A gene: response of its two ubiquitous promoters to agents promoting cell proliferation. Nucleic Acids Res. 1991 Feb 25;19(4):767–774. doi: 10.1093/nar/19.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves D. R., Wilson F. D., Lang G., Kioussis D. Human CD2 3'-flanking sequences confer high-level, T cell-specific, position-independent gene expression in transgenic mice. Cell. 1989 Mar 24;56(6):979–986. doi: 10.1016/0092-8674(89)90631-4. [DOI] [PubMed] [Google Scholar]
- Grossniklaus U., Pearson R. K., Gehring W. J. The Drosophila sloppy paired locus encodes two proteins involved in segmentation that show homology to mammalian transcription factors. Genes Dev. 1992 Jun;6(6):1030–1051. doi: 10.1101/gad.6.6.1030. [DOI] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Hallauer P. L., Hastings K. E., Peterson A. C. Fast skeletal muscle-specific expression of a quail troponin I gene in transgenic mice. Mol Cell Biol. 1988 Dec;8(12):5072–5079. doi: 10.1128/mcb.8.12.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs D. R., Wood W. G., Jarman A. P., Sharpe J., Lida J., Pretorius I. M., Ayyub H. A major positive regulatory region located far upstream of the human alpha-globin gene locus. Genes Dev. 1990 Sep;4(9):1588–1601. doi: 10.1101/gad.4.9.1588. [DOI] [PubMed] [Google Scholar]
- Hughes S. M., Blau H. M. Muscle fiber pattern is independent of cell lineage in postnatal rodent development. Cell. 1992 Feb 21;68(4):659–671. doi: 10.1016/0092-8674(92)90142-y. [DOI] [PubMed] [Google Scholar]
- Izzo P., Costanzo P., Lupo A., Rippa E., Paolella G., Salvatore F. Human aldolase A gene. Structural organization and tissue-specific expression by multiple promoters and alternate mRNA processing. Eur J Biochem. 1988 Jul 1;174(4):569–578. doi: 10.1111/j.1432-1033.1988.tb14136.x. [DOI] [PubMed] [Google Scholar]
- Johnson J. E., Wold B. J., Hauschka S. D. Muscle creatine kinase sequence elements regulating skeletal and cardiac muscle expression in transgenic mice. Mol Cell Biol. 1989 Aug;9(8):3393–3399. doi: 10.1128/mcb.9.8.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsey G. D., Povey S., Bygrave A. E., Lovell-Badge R. H. Species- and tissue-specific expression of human alpha 1-antitrypsin in transgenic mice. Genes Dev. 1987 Apr;1(2):161–171. doi: 10.1101/gad.1.2.161. [DOI] [PubMed] [Google Scholar]
- Maire P., Gautron S., Hakim V., Gregori C., Mennecier F., Kahn A. Characterization of three optional promoters in the 5' region of the human aldolase A gene. J Mol Biol. 1987 Oct 5;197(3):425–438. doi: 10.1016/0022-2836(87)90556-0. [DOI] [PubMed] [Google Scholar]
- Mennecier F., Daegelen D., Schweighoffer F., Levin M., Kahn A. Expression of aldolase A messenger RNAs in human adult and foetal tissues and in hepatoma. Biochem Biophys Res Commun. 1986 Feb 13;134(3):1093–1100. doi: 10.1016/0006-291x(86)90363-3. [DOI] [PubMed] [Google Scholar]
- Miller J. B. Myoblast diversity in skeletal myogenesis: how much and to what end? Cell. 1992 Apr 3;69(1):1–3. doi: 10.1016/0092-8674(92)90111-o. [DOI] [PubMed] [Google Scholar]
- Mukai T., Arai Y., Yatsuki H., Joh K., Hori K. An additional promoter functions in the human aldolase A gene, but not in rat. Eur J Biochem. 1991 Feb 14;195(3):781–787. doi: 10.1111/j.1432-1033.1991.tb15766.x. [DOI] [PubMed] [Google Scholar]
- Olson E. N. MyoD family: a paradigm for development? Genes Dev. 1990 Sep;4(9):1454–1461. doi: 10.1101/gad.4.9.1454. [DOI] [PubMed] [Google Scholar]
- Overbeek P. A., Lai S. P., Van Quill K. R., Westphal H. Tissue-specific expression in transgenic mice of a fused gene containing RSV terminal sequences. Science. 1986 Mar 28;231(4745):1574–1577. doi: 10.1126/science.3006249. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncz M., Schwartz E., Ballantine M., Surrey S. Nucleotide sequence analysis of the delta beta-globin gene region in humans. J Biol Chem. 1983 Oct 10;258(19):11599–11609. [PubMed] [Google Scholar]
- Proudfoot N. J. Transcriptional interference and termination between duplicated alpha-globin gene constructs suggests a novel mechanism for gene regulation. Nature. 1986 Aug 7;322(6079):562–565. doi: 10.1038/322562a0. [DOI] [PubMed] [Google Scholar]
- Schatt M. D., Rusconi S., Schaffner W. A single DNA-binding transcription factor is sufficient for activation from a distant enhancer and/or from a promoter position. EMBO J. 1990 Feb;9(2):481–487. doi: 10.1002/j.1460-2075.1990.tb08134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler U., Sierra F. Alternative promoters in developmental gene expression. Annu Rev Genet. 1987;21:237–257. doi: 10.1146/annurev.ge.21.120187.001321. [DOI] [PubMed] [Google Scholar]
- Schmidt E. V., Christoph G., Zeller R., Leder P. The cytomegalovirus enhancer: a pan-active control element in transgenic mice. Mol Cell Biol. 1990 Aug;10(8):4406–4411. doi: 10.1128/mcb.10.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighoffer F., Maire P., Tuil D., Gautron S., Daegelen D., Bachner L., Kahn A. In vivo developmental modifications of the expression of genes encoding muscle-specific enzymes in rat. J Biol Chem. 1986 Aug 5;261(22):10271–10276. [PubMed] [Google Scholar]
- Sham M. H., Hunt P., Nonchev S., Papalopulu N., Graham A., Boncinelli E., Krumlauf R. Analysis of the murine Hox-2.7 gene: conserved alternative transcripts with differential distributions in the nervous system and the potential for shared regulatory regions. EMBO J. 1992 May;11(5):1825–1836. doi: 10.1002/j.1460-2075.1992.tb05234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer J. K., Ciejek-Baez E. Autonomous activity of the alternate aldolase A muscle promoter is maintained by a sequestering mechanism. Nucleic Acids Res. 1992 Jan 25;20(2):327–336. doi: 10.1093/nar/20.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer J. K., Colbert M. C., Ciejek-Baez E. Nonconservative utilization of aldolase A alternative promoters. J Biol Chem. 1990 Jul 15;265(20):11773–11782. [PubMed] [Google Scholar]
- Vacher J., Tilghman S. M. Dominant negative regulation of the mouse alpha-fetoprotein gene in adult liver. Science. 1990 Dec 21;250(4988):1732–1735. doi: 10.1126/science.1702902. [DOI] [PubMed] [Google Scholar]
- Vidal M., Morris R., Grosveld F., Spanopoulou E. Tissue-specific control elements of the Thy-1 gene. EMBO J. 1990 Mar;9(3):833–840. doi: 10.1002/j.1460-2075.1990.tb08180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas P., Vickers M. A., Simmons D. L., Ayyub H., Craddock C. F., Higgs D. R. Cis-acting sequences regulating expression of the human alpha-globin cluster lie within constitutively open chromatin. Cell. 1992 May 29;69(5):781–793. doi: 10.1016/0092-8674(92)90290-s. [DOI] [PubMed] [Google Scholar]
- Wefald F. C., Devlin B. H., Williams R. S. Functional heterogeneity of mammalian TATA-box sequences revealed by interaction with a cell-specific enhancer. Nature. 1990 Mar 15;344(6263):260–262. doi: 10.1038/344260a0. [DOI] [PubMed] [Google Scholar]
- al-Shawi R., Kinnaird J., Burke J., Bishop J. O. Expression of a foreign gene in a line of transgenic mice is modulated by a chromosomal position effect. Mol Cell Biol. 1990 Mar;10(3):1192–1198. doi: 10.1128/mcb.10.3.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]