Abstract
Recent years have seen a dramatic change in the approach towards diagnosing and treating Multiple Myeloma. Newer and more target specific approach to treatment has prolonged the survival for patients with multiple myeloma. The proteasome inhibitors make an important class of anti-myeloma drugs that disrupts the proteolytic machinery of the tumor cells preferentially, enhancing their susceptibility to apoptosis. Bortezomib, in particular has shown significant clinical efficacy in myeloma treatment. It is the most commonly used proteasome inhibitor and has been tested to be effective in prolonging the overall survival in several trials. Its combinations with cyclophosphamide and dexamethasone are the treatment of choice for standard risk patients following the mSMART guidelines. The success with its lower dosage in elderly and its proven efficacious subcutaneous usage makes Bortezomib a useful agent for maximizing patient compliance and minimizing therapy related toxicity and costs. This review discusses several trials where Bortezomib has been used as a single/combination agent for front-line treatment of multiple myeloma.
Keywords: multiple myeloma, Bortezomib, first-line, efficacy, clinical outcomes
Introduction
Multiple Myeloma (MM) accounts for 10% of all hematological malignancies, with an incidence of 5 cases per 100,000/year and a median age at onset of 65–70 years; there is a slight male predominance. It is diagnosed by the presence of monoclonal plasma cell proliferation with more than 10% plasma cells in the bone marrow, presence of monoclonal proteins in serum, and/or in urine with one or more of end organ effects such as hypercalcemia, renal failure, anemia, or bone destruction (CRAB).1,2 Treatment regimens have undergone immense changes resulting in significant improvements in treatment tolerability. Additionally, improvements in overall survival have been achieved with newer therapies such as proteasome inhibitors and immunomodulatory drugs.3,4 The survival advantages have been more evident for patients less than 65 years of age, of whom 68% and 53% go on living beyond 5 year and 10 years respectively.5 Overall, decreasing trends in mortality for both newly diagnosed and relapsed myeloma patients suggest promising benefits due to the new drugs.6 Here, we have reviewed current evidence supporting the use of bortezomib in front-line treatment for previously untreated or newly diagnosed MM.
Diagnosis and treatment of newly diagnosed patients
MM requiring therapy is diagnosed according to the International Myeloma Working Group criteria. The presence of serum and/or urine M spike (except in true non-secretory MM cases) along with ≥10% clonalplasma cells in the bone marrow and one or more of the CRAB features indicates MM.7 Being part of a spectrum of closely associated plasma cell disorders, one must differentiate active myeloma disease from Smoldering Multiple Myeloma (SMM) or the more frequent and benign Monoclonal Gammopathy of Undetermined Significance (MGUS) syndrome, especially given the watch and wait approach for SMM and MGUS.8–11 Following the diagnosis, classification systems such as the Durie Salmon Staging system and the International staging system can be utilized for clinical staging and can be helpful in deciding on timing of initiation of therapy and determining prognosis.12,13 Once diagnosed, MM requires a multi-parametric risk stratification approach for determining the appropriate therapy. Additionally, the decision to pursue further consolidation or maintenance therapy following initial disease control requires scrutiny of disease severity and the response achieved after induction regimens.7,14
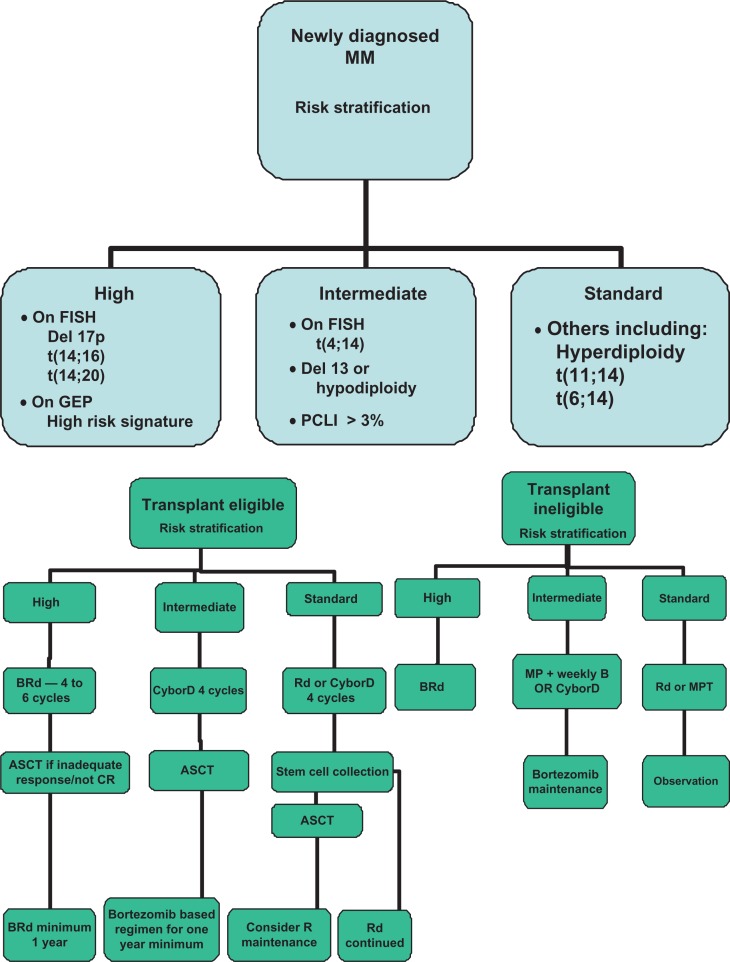
Given the significant heterogeneity in the outcomes as seen in this disease despite similar clinical stage classifications, it is now crucial to consider genetic risk factors for optimum therapeutic recommendation.15–18 Ideally, the risk stratification system should incorporate the known independent prognostic factors and allow us to determine prognosis, as well as allow us to select specific therapies for individual groups of patients. Over the years, we have developed such a system that has been since widely adopted (http://www.mSMART.org). The approach is detailed in Figure 1A and B.
Figure 1.
(A and B) A brief summary of the mSMART Consensus Guidelines.
Abbreviations: MM, Multiple Myeloma; ASCT, Autologous stem cell transplantation; GEP, Gene expression profiling; B, Bortezomib/Velcade; M, Melphalan; R, Revlimid/Lenalidomide; d/D, Dexamethasone; P, Prednisone; Cy, Cyclophosphamide; T, Thalidomide.102
Proteasome inhibition as therapy in multiple myeloma
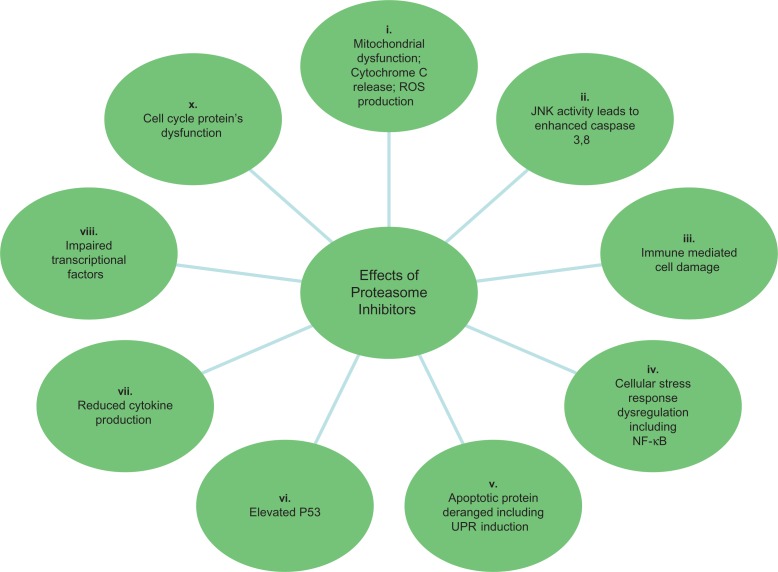
The discovery of the Ubiquitin Proteasome system and its role in protein degradation affecting the cell division has been a major breakthrough in our understanding of the cellular systems degrading unwanted molecules.19,20 We now understand that for sequential degradation, proteins undergo a highly regulated and specific ATP driven tagging of their lysine moieties by a small protein molecule capable of forming chains called Ubiquitin; this involves the ubiquitin associated activating, conjugating, and the ligase enzymes.21–23 These ubiquinated molecules are recognized by large multienzyme ATP-dependent complexes called proteasome which are found in cytoplasm and the nucleus.24 Proteasomes are comprised of complex inner (α1 to α7 subunits) and outer (β1 to β7 subunits) cylindrical structures with three compartments performing catalytic (20S) and regulatory (19S) processes.25 The 20S subunit core eliminates the ubiquinated proteins via its Chymotrypsin-like (CT-L), Trypsin-like (T-L), and post-glutamyl peptide hydrolyzing (PGPH) proteases activity.25,26 Several important cellular regulatory proteins (eg, I-κB, Cyclins, p53, Bax) undergo ubiquinated degradation. Thus, proteasome inhibition deranges their metabolism, causing cellular dysfunction that culminates with apoptosis (Fig. 2).22,25,27,28 Given that cancer cells display increased susceptibility to proteasome inhibition due to faulty/decreased checkpoint mechanisms, development has begun on proteasome inhibitors for use in cancer treatment.29,30
Figure 2.
An overview of some major effects of proteasome inhibition.
Notes: i. 103–105, 106 ii. 107 iii. 108,109 iv. 110–112 v. 113–119 vi. 120 vii. 121–123 viii. 124 ix. 111,112,125 x. 40
Abbreviations: JNK, c-Jun-N-terminalkinase; ROS, Reactive Oxygen Species.
Bortezomib (PS341, Velcade)
Unlike its non-specific and less potent predecessors, bortezomib’s development was conceived as a potent and specific proteasome inhibitor suitable for clinicaluse.28,31–33 Its very specific 26S proteasome inhibitory function has been attributed to the reversible but strong covalent bond formed between its dipeptidyl boronic acid moiety and the threonine proteases of the 20S subunit.34–36 Following the promising activity seen in early phase clinicaltrials,37–40 a series of clinical trials followed, eventually leading to its FDA approval in 2003 for treating patients with relapsed MM.
Mechanism of action
Bortezomib’s mechanisms of action are plethoric and hence a detailed discussion is beyond the scope of this review. We will review the major mechanisms implicated in its efficacy against MM cells.
Bortezomib stabilizes Iκ-B, a small regulatory unit of NF-κB. NF-κB is a heterodimeric cytoplasmic transcription factor and promotes transcription of several anti-apoptotic growth factors, proteins, and cytokines, in response to cellular stress and after its nuclear translocation.41 NF-κB is also constitutively upregulated in several MM cell lines. Stabilized Iκ-B binds NF-κB, hindering its nuclear translocation and thus preventing the transcription of the following: (1) anti-apoptotic proteins such as Bcl-2, A1, cIAP-2, and XIAP; (2) cell cycle regulators cyclin-D1 and c-Myc; (3) growth and anti-apoptotic factors such as IL-6 and VEGF, which is essential for myeloma cells and bone marrow stromal-cell signalling; and (4) adhesion molecules such as vascular adhesion molecule (VCAM-1) and intracellular adhesion molecule (ICAM-1) (Fig. 2).41,42 As NF-κB is highly expressed in myeloma cells,43,44 its inhibition by bortezomib promotes their apoptosis. Other mechanisms include: (a) stimulation of classical stress response proteins; (b) mitochondrial calcium transport and membrane potential disruption generating ROS. These mechanisms are also associated with bortezomib mediated apoptosis; (c) activation of JNK releasing mitochondrial second mitochondria-derived activator of caspases (Smac) and cytochrome-c causing caspase activation and (d) induction of intrinsic and extrinsic apoptotic pathways. These preceding mechanisms are the other significant NF-κB independent pathways causing apoptosis in bortezomib treated Myeloma cells (Fig. 2).
Pharmacology
Bortezomib is recommended intravenously or subcutaneously at a dose of 1.3 mg/m2 twice weekly for two weeks and with a treatment free week in a 21 day cycle.45,46 However, doses of 1.0 to 1.6 mg/m2 have been used in various trials, either in combination with dexamethasone or as part of multidrug combinations.47 On intravenous administration, bortezomib is 80% plasma protein bound and shows rapid tissue redistribution, with a first dose elimination half-life varying between 9–15 hours and repeated dosage reducing its plasma clearance substantially.48 While subcutaneous administration takes longer to reach peak serum concentration compared with intravenous route (30 minutes versus 5 minutes), the mean systemic exposure (56.8 nghr/mL versus 42.9 nghr/mL) and the 20S inhibitory activity (63.7% versus 69.7%) of bortezomib displays close similarity between subcutaneous and intravenous routes, suggesting similarities in their elimination half-lives.46,49
In the body, bortezomib displays tissue selectivity with predominant accumulation in the gastrointestinal system, which contrasts its weak distribution in the subcutaneous fat and skin and complete inaccessibility of the centralnervous system, testis, and eye.28 Hepatic CYP-450 isoenzymes metabolizes it via oxidative deboronation forming M1 and M2, two minor metabolites which undergo biliary clearance, making it a safer alternative for patients with the concurrent kidney disorders which can be rather commonly associated with Myeloma.28,50–52
Peripheral neuropathy is a major non-hematologic toxicity induced by bortezomib and can require mid therapy dose reductions or even discontinuation.53–55 Other significant toxicities include cytopenias, gastrointestinal distress, fatigue, cardiac side effects, infections, re-activation of shingles, and, rarely treatment-related mortality.56 Once-weekly and reduced intensity regimens have been effective at maintaining therapeutic advantages, with reduced toxicity and treatment associated weekly patient visits.57,58 Recent trials using bortezomib’s subcutaneous administration have reported a reduction in the neurotoxicity along with the possibility of self administration. Such changes can significantly alter patient’s quality of life and hence need further investigation.46
Clinical Results
Bortezomib ± dexamethasone
Bortezomib was first used as a frontline single agent for treatment naive myeloma patients by Jagannath et al.55 This open label Phase II study enrolled patients with newly diagnosed myeloma with no prior therapy or minimal exposure to steroids. Bortezomib was administered at a dose of 1.3 mg/m2 and the responses achieved were measured using EBMT criteria. Of 32 patients, 13 responded by the second cycle, resulting in 1 complete response (CR), 3 near complete responses (nCR), and 9 partial responses (PR). With the addition of dexamethasone in 22 patients, additional responses were observed in 15 of these patients. While bortezomib alone achieved a 40% response rate (RR), the RR further improved to 88% in combination with dexamethasone. An estimated 87% survival at 12 months was noted. Ten patients experienced grade 2 or 3 neuropathy while 8 patients reported painful neuropathy. Neuropathic pain disappeared within a median of 3 months of stopping bortezomib. Other noted side effects were myalgia, fatigue, and constipation of varying severity. In their extended follow-up,59 the overall response among patients receiving the BD combination was 90%, with 42 in VGPR or better (19% in CR/nCR). The estimated survival at 4 years was 67%.60–62
Richardson et al63 reported a time to progression (TTP) of 17.3 months post-bortezomib treatment in 64 patients, with 41% ≥ PR. In that study, bortezomib was administered up to 8 cycles or 2 more cycles post CR achievement while on the regimen. No significant correlation between adverse cytogenetics features and the responses achieved were noted. Median Overall Survival (OS) was not reached and a 30-month OS was estimated as 79%, with a trend towards better OS for patients receiving SCT of 82% compared with 78% OS for bortezomib-only treated patients.
PETHEMA phase II trial utilized alternating bortezomib and dexamethasone administered to 44 younger patients as induction regimens. The trial achieved a rapid M spike drop (>80%) within the first couple of treatment cycles. Post-induction, 65% patients had PR or better, showing 88% response rates after the ASCT with 33% immunofixation negative. Common side effects included neutropenia (72%), peripheral neuropathy (25%), thrombocytopenia (27%), and gastrointestinal distress, with other less frequent side effects including skin rash, fatigue, and hepatotoxicity. This trial highlighted the potential of using bortezomib and dexamethasone as potential induction regimens with quick responses, no stem cell collection issues, high post-ASCT response rates, and manageable toxicity.64
These trials showed promising results from using bortezomib for newly diagnosed patients and increased the possible spectrum of future drug combinations.
Bortezomib Combinations
Bortezomib-Melphalan-Prednisone (VMP)
In their phase 1/2 trial with 60 elderly untreated MM patients, Mateos et al highlighted the benefits of using bortezomib in combination with melphalan and prednisone (VMP) when compared to the standard MP regimen. Seventy percent of patients showed an objective response within the first cycle. Overall response rate was 89% (32% CR) with VMP compared with 42% overall response rate for MP. In addition, the 16-month event free survival rate was significantly higher with VMP than MP (83% as compared to 51%, P < 0.001).65 With a longer follow-up of 38 months, the results remained significant, showing improved survival for patients treated with VMP when compared to MP (85% compared to 38%, P < 0.0001). Adverse prognostic markers such as t(4;16), and t(14;16) had no impact on outcome, suggesting the beneficial effect of bortezomib for these high-risk patients.66
The VISTA trial56 was the first prospective, randomized open labelled phase III trial which tested the efficacy of VMP as front line treatment in previously untreated MM patients. Importantly, the median age of treated patients was 71 years, who would traditionally not classify for high dose therapy and SCT. Overall, 682 patients of whom 340 received VMP and 337 received MP. The median time to progression was 24 months in the Bortezomib arm as compared with 16.6 months in the MP arm.
CR rates were higher with VMP than MP (30% and 4% respectively, P < 0.001). More importantly, age and renal impairment did not affect the results for patients in the VMP arm as the CR rates achieved and the median time to progression (TTP) were identical. Patients with high risk cytogenetics subgroup [including t(4;14), t(14;16), and deletion 17p] had similar TTPs as compared to the subgroup with standard risk cytogenetics. A three year follow-up showed a 35% reduction in risk of mortality for VMP treated patients and an overall survival of 43.1 months in the MP arm and not reached for the patients on VMP arm. OS of patients below 75 years of age was better than the older subpopulation but no such significant trends were seen between groups based on creatinine clearance or cytogenetic abnormalities. More importantly, peripheral neuropathy resolved in the majority of patients within a median of 5.7 months.67 This landmark trial displayed the potential usage of bortezomib in achieving reduced toxicities and greater therapeutic advantage amongst elderly patients and diverse subgroups of newly diagnosed patients with MM, prompting further trials of bortezomib in various combinations.
Mateos et al then compared VMP to VTP as first randomization 1:1 induction therapy, followed by VT versus VP maintenance in second randomization elderly untreated patients, using reduced intensity bortezomib in each arm.57 Of 260 patients enrolled, 130 were treated in each arm and achieved comparable RR (≥PR) of 80% and 81% in VMP and VTP respectively. After VT or VP maintenance, complete remission rates of 44% and 39% respectively were noted; these were higher than the 30% response rate reported in the VISTA trial. Higher toxicities were noticed in the VMP arm, with 39% of patients experiencing thrombocytopenia compared with 12% in the VTP group. More serious adverse events were noted in the VTP (31%) arm when compared to VMP (15%) arm, which led to an increased discontinuation of therapy in the VTP arm. Patients from both arms were stratified based on cytogenetic abnormalities into high and standard risk groups and showed significant differences in the 3 year OS (55% vs. 77%—first randomization) and (60% vs. 85%—second randomization). Second randomization depicts patients who after successfully completing induction, and then went on to receive maintenance upto 3 years for high versus low risk respectively. The survival data didn’t vary in either arm irrespective of the maintenance therapy used and emphasized the use of such regimens for elderly patients.
In 44 transplant eligible patients, Gasperatto et al68 used a short induction of VMP with plans for ASCT after 2 to 6 cycles. High responses were achieved post-induction with 95% RR, including 18% ≥ CR, 27% VGPR, and 50% PR. More importantly they demonstrated rapid achievement of response to VMP as previously noted in the VISTA trial. Such short courses of therapy could reduce the side effects and potentially enhance adherence to the treatment regimen.
As a conditioning regimen, bortezomib combined with high dose melphalan has been tried effectively by the IFM group in their phase 2 trial, wherein 53 untreated patients were dosed with 1 mg/m2 of bortezomib on days −6, −3, −1, 1 and 4, along with melphalan 200 mg/m2 on day −2.69 The results were promising, with 70% and 32% VGPR and CR rates respectively, much higher than the matched control (11% CR). No engraftment failure was reported and all patients completed the conditioning with no exacerbation of any pre-existing neuropathy. While 5 serious adverse events were noted, no deaths occurred. The trial showed promising use of bortezomib as a combination option with HDM prior to ASCT.
Bortezomib-Doxorubicin-Dexamethasone (BAD/PAD/BDD)
Several trials have assessed the outcomes among patients induced with bortezomib, doxorubicin and dexamethasone (BDD) followed by stem cell transplantation.47,70,71One such trial compared combinations of 1.3 mg/m2 of bortezomib with doxorubicin and dexamethasone (PAD1) to 1.0 mg/m2 bortezomib with doxorubicin and dexamethasone (PAD2) in their phase I and II trials.47,70 Pre-transplant, PAD1/PAD2 induction had a 24%/11% CR, 33%/26% VGPR and high ORR’s (95%/89%). After transplantation, VGPR increased in both PAD1 and PAD2 arms by 81% and 53% respectively. The 2 year OS was 95% and 73% for PAD1 and PAD2 respectively, favoring PAD1 over PAD2.
Jakubowiak et al71 evaluated pegylated liposomal doxorubicin (30 mg/m2) in combination with bortezomib (1.3 mg/m2) and dexamethasone (20 to 40 mg/day). This phase II, single arm, 40 patient trial had 6 three-week cycles of therapy. After 6 cycles, over 37.5% nCR and 57.5% nCR and VGPR were achieved. Thirty patients who underwent SCT further improved their responses to 76.6% ≥ VGPR from a previous level of 57.5%. Those who achieved ≥VGPR showed significantly better PFS at 1 year (100%) than those who didn’t (82%). Additionally, the estimated PFS was significantly higher in VGPR patients than those who had PR or less (93% vs. 63%, P = 0.3). No differences were noted amongst patients when risk stratified according to chromosomal aberrations. With a manageable toxicity profile, the combination offers an induction option for patients going to SCT.
A phase II study specifically aimed at high risk patients (ISS stage II, III, and EMD) was conducted by Landau et al using BDD.72 Depending on the response post 3 cycles of BDD, patients either got 2 cycles of thalidomide (T) and dexamethasone (D) (if ≥ PR) or 2 cycles of BTD (SD, PD). Of the 42 patients, 34 (81%) responded to treatment, with 26% in CR/nCR and 40% ≥ PR. The 19 patients who had a suboptimal response (≤PR) post BDD therapy further received sequential BTD; 8 patients attained deeper response to the additional therapy. With a median TTP of 39 months and 2 year OS of 83%, the regimen showed promise not only for the high risk patients, but also for patients with renal failure, as a majority of patients with acute renal failure showed significant improvement.71
Two cycles each of VAD and BTD consecutively followed by weekly (4 out of every six weeks, up to 4 cycles) bortezomib maintenance, was examined by Kim et al in a phase II trial of 71 patients.73 They reported high response rates of 97% ≥ PR and 54% ≥VGPR pre-transplant, which further spiked over 98% ≥ PR, 82% ≥ VGPR, and 75% ≤ CR/nCR after SCT. These responses were much higher than those achieved in other comparable trials.53,54 Although small, the poor cytogenetics patient subgroup responded well, showing no increased risk of mortality or adverse effects on stem cell collection. Median PFS was 29.4 months, while the median OS was not reached after 52.7 month follow-up.
The HOVON-65/GMMG-HD4 randomized phase III trial recently compared the efficacy of vincristine doxorubicin dexamethasone (VAD) versus bortezomib doxorubicin dexamethasone (PAD) as induction agents along with thalidomide (T) or bortezomib (B) maintenance therapy, respectively. Post induction, patients received high dose melphalan followed by autologous transplant. A total of 833 patients were randomized with 414 in the VAD arm and 413 in the PAD arm. Throughout the treatment protocol, the VAD arm in comparison to PAD showed significantly (P < 0.001) lower CR/nCR (9%/21% vs. 12%/26% three months after HDM-1 and 12%/26% vs. 12%/38% on protocol) and VGPR (60% vs. 40% post three months of HDM-1 and 75% vs. 61% on protocol) rates. PFS at 3 years was 48% versus 42% and OS at 66 months was 78% versus 71% on comparing PAD with VAD respectively. These results also favored bortezomib maintenance over thalidomide, as OS was higher in the PAD group. Among patients with renal insufficiency, bortezomib showed significant improvement in PFS (30 vs. 13 months, P = 0.004) and OS (54 vs. 21 months, P < 0.001) when compared to the VAD/T arm. Similar significant results were noted in high risk cytogenetics (−13/13q- and −17p) when compared to standard risk patients. This trial showed the unprecedented advantage of using bortezomib as both induction and maintenance, especially in patients with high risk features.74
Bortezomib-Cyclophosphamide-Dexamethasone (Cybor-D)
CyborD was used as induction therapy for 33 newly diagnosed patients in a single arm phase II study at the Mayo Clinic,75 demonstrating high response rates after four 28 days cycles of therapy. Among the 28 patients who completed 4 induction cycles, the ORR was 96% with 46% in CR + nCR and 71% ≥ VGPR. Additionally, all treated patients had ORR of 88% (≥ PR) with 61% ≥ VGPR. Rapidity of response to therapy was most evident within the first two therapeutic cycles. Cytopenias and hyperglycemia along with grade I PN (46%) were the commonly experienced toxicities. However, this regimen used high dose dexamethasone, which is currently no in common usage.
Kropff et al used a standard dosage of dexamethasone and bortezomib together with 900 mg/m2 of cyclophosphamide (maximum tolerated dose) as the induction regimen. After four cycles of induction, high response rates with ≥77% PR and 10% CR were achieved; 28 of 30 patients had an adequate stem cell harvest, of which the majority had adequate stem cell collection in one apheresis. Patients with −17p and t[4;14] showed inferior ORR compared to standard risk patients (62.5% vs. 86.5% ≥ PR respectively).76 These results were similar to other comparable trials, for example, the 75% ORR with 41% VGPR or better in the VCD arm of the Evolution trial, and displayed rapid reduction of M spike, translating to faster responses.77–81 While BDD appears to show rapid response in patients, any assessment of its clinicalutility needs further, larger cohort-based studies.
Bortezomib immunomodulatory combinations
With thalidomide
In one of the early trials, 38 newly diagnosed MM patients were treated with bortezomib, thalidomide, and dexamethasone (BTD). A rate of 87% RR was noted, together with 16% CR and improved responses in 25 patients (66%), who subsequently underwent transplantation and of which 3 with resistant disease achieved PR or CR and 7 with a previous PR upgraded to CR, resulting in an overall CR rate of 37%. With rapid median time to remission (0.4 months) and, more importantly, no mortality, this regimen showed potential for its addition to the treatment armamentarium for untreated MM patients.
Ghosh et al recently showed the benefits of using bortezomib with thalidomide in an upfront setting. This phase II trial of a steroid free regimen achieved high RR (≥PR = 81.5%) with 25.6% ≥ nCR and a 3 year OS of 74% (CI: 54%–89%) without SCT. Toxicity included a 22% Grade 3 PN, with resolution of symptoms on therapy discontinuation seen in 80%. Importantly, no thrombotic events or hyperglycemic complications were noted, a phenomenon which has previously been reported with thalidomide combinations.82 Comparable results were noted with bortezomib, pegylated doxorubicin and thalidomide combination in another trial with 78% ORR, of which 35% achieved CR + nCR. Along with reduction in the severity of sensory neuropathy, the overall toxicity profile was manageable and no treatment associated deaths.83 The promising results of such steroid-free regimens need further phase III verification for their clinical efficacy and relatively low toxicity.
The GIMEMA group conducted a Phase III study that evaluated the efficacy of VTD vs. TD as induction regimen, followed by tandem SCT and consolidation with the same regimens respectively. Significantly higher RR were noted in the VTD arm when compared to TD arm (30% vs. 11% CR respectively; P < 0.0001). The 3 year probabilities of relapse were 29% and 39% along with 68% and 56% PFS at 3 years, for VTD and TD arms, respectively. No significant differences were observed in OS. Patients with t(4;14) had an accelerated progression, relapse, or death in the TD arm with a 37% and 63% PFS at 3 years for the t(4;14) positive and negative patients, respectively. No such correlation could be drawn in the VTD arm. More Grade 1 and higher side effects were noted in the VTD arm, with constipation, neuropathy, and skin rash being the most common. Patients with high-risk prognostic features such as high lactate dehydrogenase levels (>190 U/L), age (>65 years of age), del3q, low bone marrow plasma cell count (<50%), t(4;14), +/−del17p, and high ISS stage had significantly improved outcome with VTD than TD arm.54
The PETHEMA group84 randomized 386 untreated patients to compare efficacy of BTD (130 patients), TD (127 patients), ands VBMCP/VBAD/B [vincristine, BCNU (bis-chloronitrosourea), melphalan, cyclophosphamide, prednisone/vincristine, BCNU, adriamycin, dexamethasone/bortezomib—129 patients] as induction regimens. Results showed the superiority of VTD over other treatment arms with significantly high CR (35%) on comparison with TD (14%) and VBMCP/VBAD/B(21%).In cytogenetically stratified high risk groups, high CR rates were seen with VTD when compared with TD (35% vs. 0%, P = 0.002) or with VBMCP/VBAD/B (35% vs. 22%, P = 0.02). In VBMCP/VBAD/B group the CR rate increased from 8% to 21% after completing 2 cycles of Bortezomib post 4 cycles of VBMCP/VBAD. While higher discontinuation within induction phase was noted with TD (n = 29) and VBMCP/VBAD (n = 15), only 9 discontinued in VTD due to progressive disease. A quarter of the 45% experiencing PN required dose reduction in VTD arm. This was much higher than the 8% and 15% in TD and VBMCP/VBAD/B arms, respectively, experiencing PN. PFS after a median of 33.1 months follow-up was also significantly higher in VTD than with VBMCP/VBAD/B or VT (56.2 vs. 35.3 vs. 28.2 months respectively) while no such differences were noted in OS. This trial’s results support the use of VTD as an induction regimen in MM patients.
Mateos et al57 took a less intense approach by using bortezomib once weekly instead of the usual twice weekly in combination with either MP or TP followed by maintenance with VP or VT. This randomized trial had 260 elderly patients, with patients receiving 6 cycles of induction and then up to 3 years of maintenance. The trial showed similar efficacies of both regimens in terms of CR, OS, and PFS. More importantly it showed improvement in the grade 3 or higher neurotoxicity seen with reduced dose of bortezomib (5% in VMP as compared to the 13% in the Vista trial). VTP was associated with higher cardiac adverse events and neurotoxicity while BMP had infection as a primary non-hematological toxicity warranting antibiotic treatment prophylaxis in future such combination. Also of note was the fact that despite a larger subgroup analyzed in comparison to the VISTA trial, no significant prognostic benefit was achieved in the high risk cytogenetic group (t4;17 and del17p), although significant benefits were observed for patients with hyperdiploidy over non-hyperdiploidy with BTP (P = 0.02).
Moreau et al examined the clinical efficacy of using low dose bortezomib in combination with thalidomide and bortezomib, when compared to the normal dosage of the VD combination. By inducing significantly deeper responses (VGPR being 49% vs. 38%) a marked reduction in neuropathy (14% vs. 34%) among the 199 patients enrolled was noted.85
More recently the multicentre study comparing VTD, VMP, and VD (each arm consisting 100 patients) showed deeper responses with the use of VTD when compared to VMP and VD. Of more value were the enhanced responses seen on bortezomib maintenance in all treatment arms and the absence of additional neuropathy.86
BTD has also been shown to induce persisting molecular remissions when used as consolidation therapy after autologous SCT. The results emphasized major tumor shrinkage as shown by the use of RQ-PCR for minimal residual disease detection after the consolidation, with no relapse after a median of 42 months; these are certainly promising results.87
With lenalidomide/revlimid
Lenalidomide (L) has shown favorable results when used as an induction agent in combination with bortezomib and dexamethasone by Richardson et al.58 A total of 66 patients were administered eight 3 week cycles of BLD with a 100% > = PR rate in treated patients. With 39% ≥ nCR and 67% ≥ VGPR, and despite the ASCT, PFS in both the transplant and non-transplanted group was 75% (95% CI: 63%, 84%). The 18 month OS rate was 97% (95% CI: 88%, 99%), irrespective of the transplant status. Sensory neuropathy (80%), fatigue (64%), and neuropathic pain (32%) were major adverse effects reported and, more importantly, no treatment related mortality was noted. More studies are needed to assess clinical feasibility of such combinations.
Multi-drug combinations
The Evolution phase I and II trials analyzed responses to various combinations of bortezomib (V) with cyclophosphamide (C), thalidomide (R) and dexamethasone (D) in terms of response, adverse events, overall survival, and minimal residual disease and assessed such differences achieved amongst the treatment arms.81,88
The Phase I trial showed feasibility of combining different groups of drugs (VDCR) and achieving high RR of 96% with 40% CR/nCR and 68% ≥ VGPR. Peripheral neuropathy was the major adverse event which improved/resolved in 85% patients on treatment completion. Additionally, no thrombotic events were noted. Such promising results led to the phase II multicentre randomized trial of VDCR, VRD, VDC, and modified VCD (with additional cyclophosphamide dose), using eight 21 days cycles for each regimen. Overall, 122 patients were enrolled, with 88%, 85%, 75%, and 100% in ORR (33%, 32%, 13%, and 41% having ≥VGPR respectively) among the VDCR, VDR, VDC, and VDC-modified arms respectively, prior to ASCT. PFS at one year for VDCR, VDR, VDC and VDC-mod was 83%, 68%, 97%, and 100% in non-ASCT patients and 86%, 83%, 93%, and 100% including the ASCT subgroup in each arm respectively; OS was 100% at one year in all arms. While no added advantage was noted in adding alkylating agent to VRD as initially speculated, better responses with lesser toxicities were seen in the VCD-mod and VDR arms.
A retrospective analysis, done at the Mayo clinic compared RD, CRD, and VCD and reported no conclusive PFS or OS advantage with any of the regimens.89 Higher responses were achieved with VCD but VCD had a higher incidence of neuropathy when compared to either RD or CRD.
The significance of maintenance was examined in the randomized phase III trial assessing VT maintenance used post-VMPT induction and its efficacy when compared to VMP induction without maintenance.90 Among the 511 patients enrolled, a first cohort of 139 patients received twice weekly bortezomib for 9 six weekly cycles while the remaining patients received bortezomib once a week for 9 five week cycles of treatment, intending to reduce PN. This trial was beneficialto evaluate appropriate dosage for transplant in eligible patients who experience diminished toxicity with reduced frequency of bortezomib administration. A remarkably decreased incidence of PN with uncompromised drug efficacy was noted with the reduced schedule of bortezomib. More importantly, the cumulative treatment dose was similar in both arms despite changes in frequency of administration, suggesting more dose reduction with the twice weekly schedule. Reduced PN also translated to better treatment compliance in the modified arm. VMPT-VT was more efficacious than VMP arm with an enhanced 3 year PFS (56% vs. 41% respectively) and time to therapy (72% vs. 60% respectively). CR and VGPR were also significantly better with VMPT (prior to maintenance) than VMP (38% and 59% vs. 24% and 50% respectively). Sixteen treatment-related deaths occurred in total, while in general more side effects were evident in VMPT than in VMP (38% vs. 28% respectively). Additionally, there were more cardiac adverse effects for VMPT than VMP (10% vs. 5% respectively). Neuropathy was more common in people undergoing VT maintenance post induction but treatment discontinuation was similar and even less frequent in the once weekly bortezomib cohort, highlighting the importance of considering the frequency of bortezomib administration in the treatment regimen. The trial provided the rationale for considering VMPT in elderly, transplant ineligible patients given the better tolerability of bortezomib with once a week infusion than the usual twice weekly dosage (16% vs. 3%).
Another combination approach91 examining sequential drug combinations has been tested using 3 cycles of VCD followed by another three of VTD as induction regimen with the goal of reducing toxicity related to prolonged drug exposure. Of the 42 evaluable patients ≥ 36% nCR, 19% sCR, and ≥57%VGPR were noted with an ORR of 95%. More importantly 82% patients completed all treatment cycles and no deaths related to treatment noted, highlighting the potential for larger trials in the future designed to test this regimen in larger randomized cohorts.
Varying degrees of bone involvement, from generalized osteopenia to lytic lesions and pathological-fractures, are a hallmark of the disease and affect a significant proportion of patients. Clinical trials have shown reduced frequency of skeletal events with bisphosphonate therapy and a recent randomized trial also demonstrated improved overall survival in the context of zoledronic acid therapy. Bortezomib treatment anabolically stimulates bone formation as indicated by the elevated levels of bone specific alkaline phosphatase and osteocalcin, results which are further complimented by radiologic evidence of improved bone structure in the same patients.92,93 Bortezomib is likely to add to the bone strengthening effect of the bisphosphonates directly by its effect on bone cells and indirectly through the disease control achieved with its use.94
Conclusion
Bortezomib has come a long way since its development as one of the most effective drugs currently available for treating Multiple Myeloma. With improved efficacy of combination regimens we have seen increasing usage of its therapeutic spectrum ranging from frontline induction to maintenance therapy. Bortezomib has been integrated into the treatment of myeloma at every disease stage. In the context of newly diagnosed disease, bortezomib has been combined with other drugs as induction regimens in both transplant eligible and ineligible patients. The most common regimens in use include the VCD, VTD, VRD, and MPV regimens. Bortezomib has been used as part of the conditioning regimens in clinical trials and the results certainly encourage future larger trials to ascertain benefit. Bortezomib as part of consolidation post-transplant, or as a maintenance agent both in the transplant and non-transplant setting, continues to be examined with the goal of inducing deeper responses, which can potentially translate to longer PFS and possibly OS. However, maintenance requires an understanding of drug action and the possible side effect profile on long-term basis,95 as elucidated by an increased risk of secondary malignancies reported with lenalidomide maintenance.96,97 Bortezomib has been shown to have a specific role in certain clinical situations. Based on several studies, it is clear that the drug should be part of any treatment regimen in the high-risk population and needs to be given for an extended period of time. The lack of renal excretion makes it an invaluable part of any regimen in patients presenting with renalin sufficiency. In the relapsed setting, bortezomib can be reused in combination with other myeloma drugs as well as with other experimental agents in clinical trials. Finally, the use of the subcutaneous administration and once weekly schedule has significantly decreased the neurological toxicity allowing for its extended use.
Table 1.
Potential bortezomib side effects.101
| Central nervous system |
| Motor and sensory neuropathy |
| Visualimpairment |
| Memory impairment |
| Speech impairment |
| Seizures |
| Hematological |
| Thrombocytopenia |
| Neutropenia |
| Anemia |
| Gastrointestinal |
| Nausea |
| Abdominal pain |
| Vomitting |
| Diarrhea |
| Constipation |
| Musculoskeletal |
| Bone/joint/muscle pain |
| Muscle cramps |
| Muscle weakness |
| Cardiovascular |
| Tachycardia |
| Hypotension |
| Dizziness |
| Lower limb swelling |
| Dermatological and immunological |
| Rash |
| Hives |
| Itchiness |
| Skin blistering |
| Non-specific |
| Sleep disturbances |
| Anxiety |
| Restlessness |
| Mood changes |
| Hallucinations |
| Tiredness |
| Thirst |
| Decreased urination |
| Hoarseness |
| Cough |
| Shortness of breath |
Table 2.
| Study author | Study type (phase) | Number of patients and groups | Regimen | Dosage | Usage | Cycles received | ORR (%) | Survival | Common toxicity | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| Scr | CR/nCR | VGPR | PR | PFS | OS | ||||||||
| Jagannath et al55,59 | II | 49 | B + BD | B—1.3 mg/m2 on days 1, 4, 8 and 11. D—40 mg/m2 if no PR ≤ 2 OR CR ≤ 4 weeks on B |
First-line | 6 | 3 | 9 | 14 | Reversible sensory neuropathy | |||
| Wang et al99 | Retrospective | 38 | BTD ± BMSCT | B—1.3 mg/m2 on days 1, 4, 8 and 11 T—100 mg/day increased to a maximum of 200 mg/day D—20 mg/2 on 1, 9 and 17 for 4 days |
First-line | 3-four week cycles | 16 | PR-71 ≥ PR-87 | Fatigue, GI AE’s, rash, edema, ↓ blood counts, neuropathy | ||||
| Popat et al47 | I/II | 41 21 (BAD1) 20 (BAD2) |
BAD (1) vs. BAD (2) | P—1.3 mg/m2 in (1) vs.1.0 mg/m2 in (2) on days 1, 4, 8 and 11 A—0–9 mg/m2 on days 1–4 D—40 mg/2 on days 1–4, 8–11 and 15–18 |
Front-line Induction | 4 | (1) 29 (2) 16 |
(1) 33 (2) 26 |
(1) 33 (2) 47 |
(1) 29 months (2) 24 months |
2 years (1) 95% (2) 73% |
Liver, GI, psychiatric, fatigue, neuropathy, skin, ↓ platlets | |
| Richardson et al63 | II | 64 | B | B—1.3 mg/m2 on days 1, 4, 8, 11 | First-line | 8 | 9 | 8 | 31 | 17 months | 2.5 years 79% |
Sensory neuropathy, GI AE’s, fatigue, rash, ↓ blood counts | |
| Vista trial56 | III | 682 337(BMP) 331(MP) |
BMP (a) vs. MP (b) | B—1.3 mg/m2 on days 1, 4, 8, 11, 22, 25, 29 and 32 M—9 mg/m2 6 weeks P—60 mg/m2 on days 1–4 |
First-line transplant ineligible | 9 | 33 (a) 4 (b) |
8 (a) 4 (b) |
33 (a) 31 (b) |
3.7 years (a) not 50% (b) 50% |
Peripheral neuropathy, ↓ blood counts, GI AE’s, pneumonia | ||
| IFM 2005-0153 | III | 482 VAD (A1-121) VAD + DCEP (A2-121) BD (B1-121) BD + DCEP (B2-119) |
A1 + A2 – (C-218) vs. B1 + B2 – (D-223) | Vin—0.4 mg/d four 4 week cycles Dox—9 mg/m2/d, on days 1–4. Dex—40 mg on days 1–4 B—1.3 mg/m2 four 3 weeks cycle on days 1, 4, 8 and 11 D—40 mg on days 1 to 4 all cycles and 9–12 (cycles 1 and 2) DCEP*– On days 1–4 |
Induction prior to auto-PBSCT | 6.4 (C) 14.8 (D) |
15.4 (C) 37.7 (D) |
(C) 29.7 months (D) 36 months |
2.10 years (C) not 50% (D) not 50% |
PN, Fatigue, ↓ blood counts, hemorrhages, infections, GI AE’s, Herpes zoster infection, Rash, | |||
| Mateos et al57,65 | Pilot and randomized trial | 260 260— Induction** 130—BMP (A) 130—BTP (B) 178— Maintenance# 87—BP (C) 91—BR (D) |
(A) vs.(B) and (C) vs.(D) | B—1.3 mg/m2 2x/week on days 1, 4, 8, 11, 22, 25, 29 and 32 (for one-6 week cycle) and same dosage for days 1,8, 15 and 22 (for five-5 week cycles) **M—9 mg/m2 on days 1–4 P—60 mg/m2 on days 1–4 T—100 mg/day B—1.3 mg/m2 on days 1, 4, 8 and 11 every 3 month P—50 mg every 48 hrs #R—50 mg/day |
Induction and maintenance | **(A) and (B) One, 6-week cycle each and five, 5–week cycles #≥ 3 yrs | (A) CR-20; nCR-12. (B) CR-28 nCR-8 |
(A)-48 (B)-45 |
At first randomization 31 months for all. (FU 32 months) At second randomization 32 months for (C) and 24 months for (D) (FU 22 months) |
At 3 years was 70% for all groups | ↓ blood counts, GI AE’s, Peripheral neuropathy, DVT/thromboembolism, cardiac events | ||
| Richardson et al58 | I/II | 66 | BRD | Planned eight-3 week cycles of-B—1.3 mg/m2 on days 1, 4, 8 and 11 R—25 mg on days 1–14 D—20 mg (cycles 1–4) and then 10 mg (for cycles 5–8) |
10-median number of cycles per drug including maintenance | Phase I— CR-29 nCR-11 Phase II— CR-37 nCR-20 |
Phase I—27 Phase II—17 |
Phase I—33 Phase II—26 (100% atleast PR) |
Fatigue, GI AE’s, NS AE’s, ↓ blood counts, ↓ K+, PE | ||||
| Cavo et al54 | III | 474 236—(A) 238—(B) |
BTD vs TD ± Double BMSCT | Induction B—1.3 mg/m2 on days 1, 4, 8 and 11 T—100 mg/day from 1–14 days and 200 mg/day thereafter D—in BTD (40 mg on days 1, 2, 4, 5, 8, 9 and 11) and in TD (40 mg on days 1–4 and 9–12). Consolidation B—1.3 mg/m2 on days 1, 8, 15 and 22 T—100 mg/day D—40 mg on days 1, 2, 8, 9, 15, 16, 22 and 23 for BTD and same dose on days 1–4 and 20–23 for TD Maintenance D—40 mg on days 1–4, every 28 days |
Induction and Consolidation Maintenance |
Three-21 days induction cycle of each Two-35 days consolidation cycles of each |
BTD CR-58 ≥nCR-71 TD CR-41 ≥nCR-54 |
BTD ≥VGPR −89 TD ≥VGPR −74% |
BTD ≥PR-96 TD ≥PR-89 |
At 3 years BTD—68% TD—56% |
At 3 year BTD—86% TD—84% |
Constipation, neuropathy, rash, fever, infection, oedema, GI AE’s, hematological AE’s | |
| Palumbo et al90 | III | 511 BMPT (254) + BT* (A) (maintenance)* BMP (257) (B) | (A) vs.(B) No maintenance for (B) | B—1.3 mg/m2 on days 1, 8, 15 and 22 M—9 mg/m2 on days 1–4 P—60 mg/m2 on days 1–4 T—50 mg/day continuously |
Induction and maintenance for (A) only | 9-five week cycles | (A) 38 (B) 24 |
(A) 21 (B) 26 |
(A) 30 (B) 31 |
At 3 years 56% (A) 41% (B) |
At 3 years 89% (A) 87% (B) |
↓ blood counts, cardiac AE’s, NS AE’s, infections, vascular events, fatigue, rash | |
| Ghosh et al82 | II | 27 | BT | B—1.3 mg/m2 on days 1, 4, 8 and 11 every 21 days T—150 mg/day |
Frontline | 8 weeks | CR-10 nCR-13.3 |
6.6 | 43.3 | 16.8 months | At 3 years 74% | GI AE’s, NS AE’s, infection, fatigue, rash, ↓ blood counts | |
| Reeder et al75 | II | 28 | CBD ± BMSCT | C—300 mg/m2 on days 1, 8, 15 and 22 B—1.3 mg/m2 on days 1, 4, 8 and 11 D—40 mg on days 1–4,9–12, 17–20 and 28 |
Induction | 4-four week cycles | 39 | ≥VGPR-61 | ≥PR-88 | ↓ blood counts, hyperglycemia, diarrhea, ↓ K+, neuropathy, ↓ blood counts, infections, fatigue, GI AE’s, PN | |||
| Moreau et al85 | III | 199 100 btD (A) 99 BD (B) |
(A) vs.(B) ± BMSCT | B—1.3 mg/m2 on days 1, 4, 8 and 11 D—40 mg on days 1–4 (all cycle) and 9–12 (on cycle 1 and 2) b—1.0 mg/m2 on days 1, 4, 8 and 11 t—100 mg/day |
Induction | 4-three week cycles | ≥nCR (A)-31 (B)-22 CR (A)-13 (B)-12 |
≥VGPR (A)-49 (B)-36 |
PR (A)-88 (B)-81 |
A—30 months B—26 months |
No difference | ||
| Lee et al100 | II | 31 | BAD ± BMSCT and T | B—1.3 mg/m2 on days 1, 4, 8 and 11 A—9 mg/m2 on days 1–4 D—40 mg on days 1–4 and 8–11 T-100–200 mg/day post-transplant (Maintenance) |
Induction | 2-three week cycles | Induction CR-19.3 Maintenance CR-70 |
Induction −16.1 Maintenance −23.3 |
Induction −45.2 Maintenance −6.7 |
At 5 years 23.5% | At 5 years 71.1% | ↓ blood counts, nausea, vomiting, PN, hepatotoxicity | |
| Roussel et al69 | II | 54 | B-HDM | B—1.0 mg/m2 on days −6, −3, +1 and +4 HDM—200 mg/m2 on days −2. |
Conditioning | Pre-transplant only | 32 | 38 | 24 | At 2 years 96% | Mucositis, Skin rash, GI AE’s, PN | ||
| Kim et al73 | II | Total 71; 65 evaluated | VAD + *BTD + BMSCT + B# | Vin—0.4 mg/day continuous on A—same as Vincristine days 1–4 D—40 mg on days 1–4 and 9–12 B—1.3 mg/m2 on days 1, 4, 8 and 11 T—100 mg/day #1.3 mg/m2 weekly |
*Induction #Consolidation |
VAD and BTD 2-three week cycles each #4 cycles on 4 of every 6 week |
*nCR-27 CR-17 After # ≥nCR-75 CR-72 |
*≥VGPR-54 After #≥VGPR-81 |
*≥PR-97 After #≥PR-94 |
29.4 months | Not reached at 29.4 months | ↓ blood counts, peripheral neuropathy, DM, infection, thrombosis | |
| Gasparetto et al68 | II | 44 24 (BMP only) 20 (BMSCT) |
BMP (only)* ± BMSCT # | B—1.3 mg/m2 on days 1, 4, 8 and 11 M—6 mg/m2 on days 1–7 P—6 mg/m2 on days 1–7 |
Frontline or as Induction | 6-four week cycles | *8 #10 |
*CR-17 #CR-20 |
*17 #65 |
*50 #5 |
*19.8 months #27.9 months |
At 1 year *82% #95% |
PN, GI AE’s infection, ↓ blood counts, DVT, orthostatic hypotension |
| PETHEMA64 | II | 40 | BD ± BMSCT* | B—1.3 mg/m2 on days 1, 4, 8 and 11 (cycles 1,3 and 5) D—40 mg on days 1 to 4, 9 to 12 and 17 to 20 (cycles 2, 4 and 6) |
Induction | 3-four week cycles | 12.5* 33 |
7.5* 22 |
40 * 33 | Thrombocytopenia, fatigue neutropenia, PN, rash, | |||
| PETHEMA84 | III | 266 TB—90 (A) T—89 (B) α2-INF— 87 (C) |
Maintenance | For 3 years | Post SCT | ||||||||
| A vs.B vs. C Post-SCT |
T—100 mg/day B—1.3 mg/m2 on days 1, 4, 8 and 11 every 3 months α2-INF—3MU thrice/week |
CR-51 | VGPR-23 | PR-24 | At 2 years (A) 78% (B) 63% (C) 49% |
No significant difference between the three arms | 266 TB—90 (A) T—89 (B) α2-INF —87 (C) |
||||||
| Improved CR after maintenance by (A) 21 (B) 11 (C) 19 | |||||||||||||
| Sahebi et al98 | II | 45 | BD then TD and then T Post-ASCT | B—1.3 mg/m2 on days 1,8 and 28 D—40 mg on days 1 through 4 T—50 mg to a maximum of 200 mg/day for 28 days |
Maintenance | Six-28 days cycle of BD then TD and then T until progression or toxicity | At 6 months CR post B-52.5 At 1 year CR post T-50 |
At 6 months B-17.5 At 1 year T-5 |
At 6 months B-20 At 1 year T-7.5 |
At 1 year was 88% | At 1 year was 95% | PN, ↓ blood counts, fatigue, GI AE’s, hyperglycemia, insomnia | |
| Landau et al72 | II | 42 | BLDD then either TD or BTD depending on response |
BLDD– B-1.3 mg/m2 on days 1, 4, 8 and 11 LD-30 mg/m2 on day 4D-20 mg on days 1, 2, 4, 5, 8, 9, 11 and 12 TD—If ≥ PR T-50 mg to 200 mg max on days 1–28 D-40 mg on days 1–4, 9–12 and 17–20 BTD—If SD or PD B-1.3 mg/m2 on days 1, 4, 8 and 11 T-50 mg to 200 mg max on days 1–28 D-20 mg on days 1, 2, 4, 5, 8, 9, 11 and 12 and 40 mg on days 17–20 |
Induction | Three-21 days cycle of BLDD, followed by either two-28 days of TD or two-28 days cycles of BTD | ncr/CR-43 | ≥VGPR-60 | ≥PR-60 | At 1 year 88% At 2 years 83% OS was better if ≥ PR post BLDD with either TD or BTD | Fatigue, rash GI AE’s, neuropathy, ↓ blood counts, thrombosis, | ||
| Evolution Study81,88 | I and II | 140 BDCR(48)-(A) BDR(42)-(B) BDC(33)-(C) BDC-Mod(17)-(D) |
A vs.B vs. C vs. D | B—1.3 mg/m2 on days 1, 4, 8 and 11. D—40 mg/m2 on days 1, 8 and 15. C—500 mg/m2 on days 1 and 8 only for (D) on day 15 as well R—Days 1–4 for (A) 15 mg and (B) 25 mg |
Frontline | 12 | (A) 15 (B) 17 (C) 9 (D) 29 |
(A) 25 (B) 24 (C) 22 (D) 47 |
(A) 58 (B) 51 (C) 41 (D) 53 |
At 1 year (A) 86% (B) 83% (C) 93% (D) 100% |
Neutropenia, peripheral neuropathy, GI AE’s | ||
| HOVON74 | III | 827 414 (VAD + HDM + T) −(A) 413(BAD + HDM +B) − (B) |
(A) vs.(B) | Induction Vin— 0.4 mg/day continuous on A—same as Vincristine days 1–4 D—40 mg on days 1–4 and 9–12 B—1.3 mg/m2 on days 1, 4, 8 and 11 A—9 mg/m2 on days 1–4 D—40 mg on days 1–4, 9–12 and 17–20 Maintenance B—1.3 mg/m2, two-weekly T—50 mg/day |
(A) and (B) have components for Induction, pre-transplant and maintenance | 3 cycles of VAD or BAD 1 or 2 doses of HDM 2 years of maintenance (B or T) | (A) 38 (B) 50 |
(A) 61 (B) 75 |
(A) 87 (B) 92 |
At 3 years (A) 42 (B) 48 |
At 3 years (A) 71 (B) 78 |
Infection, GI AE’s, PN | |
Footnotes
Author Contributions
Analysed the data: UP, SKK. Wrote the first draft of the manuscript: UP. Contributed to the writing of the manuscript: SKK. Agree with manuscript results and conclusions: UP, SKK. Jointly developed the structure and arguments for the paper: UP, SKK. Made critical revisions and approved final version: UP, SKK. All authors reviewed and approved of the final manuscript.
Funding
Author(s) disclose no funding sources.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest. Provenance: the authors were invited to submit this paper.
References
- 1.Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111(6):2962–72. doi: 10.1182/blood-2007-10-078022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet. 2009;374(9686):324–39. doi: 10.1016/S0140-6736(09)60221-X. [DOI] [PubMed] [Google Scholar]
- 3.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survivalin multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516–20. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Combination chemotherapy versus melphalan plus prednisone as treatment for multiple myeloma: an overview of 6,633 patients from 27 randomized trials. Myeloma Trialists’ Collaborative Group. J Clin Oncol. 1998;16(12):3832–42. doi: 10.1200/JCO.1998.16.12.3832. [DOI] [PubMed] [Google Scholar]
- 5.Brenner H, Gondos A, Pulte D. Expected long-term survival of patients diagnosed with multiple myeloma in 2006–2010. Haematologica. 2009;94(2):270–5. doi: 10.3324/haematol.13782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) National Cancer Institute; Available at http://seer.cancer.gov/csr/1975_2009_pops09/. Published Apr 2012. Updated Aug 20, 2012. Accessibility verified Jan 13, 2013. [Google Scholar]
- 7.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23(1):3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121(5):749–57. [PubMed] [Google Scholar]
- 9.Durie BG, Kyle RA, Belch A, et al. Myeloma management guidelines: a consensus report from the Scientific Advisors of the International Myeloma Foundation. T Hematol J. 2003;4(6):379–98. [PubMed] [Google Scholar]
- 10.Kyle RA, Remstein ED, Therneau TM, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356(25):2582–90. doi: 10.1056/NEJMoa070389. [DOI] [PubMed] [Google Scholar]
- 11.Willard WR. A present for Alabama. J Med Assoc State Ala. 1978;47(7):18–9. [PubMed] [Google Scholar]
- 12.Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36(3):842–54. doi: 10.1002/1097-0142(197509)36:3<842::aid-cncr2820360303>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 13.Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412–20. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 14.Ludwig H, Durie BG, McCarthy P, et al. IMWG consensus on maintenance therapy in multiple myeloma. Blood. 2012;119(13):3003–3015. doi: 10.1182/blood-2011-11-374249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gertz MA, Lacy MQ, Dispenzieri A, et al. Clinical implications of t(11;14) (q13;q32), t(4;14)(p16.3;q32), and −17p13 in myeloma patients treated with high-dose therapy. Blood. 2005;106(8):2837–40. doi: 10.1182/blood-2005-04-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avet-Loiseau H, Attal M, Moreau P, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood. 2007;109(8):3489–95. doi: 10.1182/blood-2006-08-040410. [DOI] [PubMed] [Google Scholar]
- 17.Herve AL, Florence M, Philippe M, et al. Molecular heterogeneity of multiple myeloma: pathogenesis, prognosis, and therapeutic implications. J Clin Oncol. 2011;29(14):1893–7. doi: 10.1200/JCO.2010.32.8435. [DOI] [PubMed] [Google Scholar]
- 18.Fonseca R, Debes-Marun CS, Picken EB, et al. The recurrent IgH translocations are highly associated with nonhyperdiploid variant multiple myeloma. Blood. 2003;102(7):2562–7. doi: 10.1182/blood-2003-02-0493. [DOI] [PubMed] [Google Scholar]
- 19.Ciechanover A. Intracellular protein degradation: from a vague idea, through the lysosome and the ubiquitin-proteasome system, and onto human diseases and drug targeting (Nobel lecture) Angewandte Chemie. 2005;44(37):5944–67. doi: 10.1002/anie.200501428. [DOI] [PubMed] [Google Scholar]
- 20.Hershko A. The ubiquitin system for protein degradation and some of its roles in the control of the cell-division cycle (Nobel lecture) Angewandte Chemie. 2005;44(37):5932–43. doi: 10.1002/anie.200501724. [DOI] [PubMed] [Google Scholar]
- 21.Kloetzel PM. Antigen processing by the proteasome. Nat Rev Mol Cell Biol. 2001;2(3):179–87. doi: 10.1038/35056572. [DOI] [PubMed] [Google Scholar]
- 22.Almond JB, Cohen GM. The proteasome: a novel target for cancer chemotherapy. Leukemia. 2002;16(4):433–43. doi: 10.1038/sj.leu.2402417. [DOI] [PubMed] [Google Scholar]
- 23.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–79. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 24.Adams J. Development of the proteasome inhibitor PS-341. Oncologist. 2002;7(1):9–16. doi: 10.1634/theoncologist.7-1-9. [DOI] [PubMed] [Google Scholar]
- 25.Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 2004;4(5):349–60. doi: 10.1038/nrc1361. [DOI] [PubMed] [Google Scholar]
- 26.Cardozo C. Catalytic components of the bovine pituitary multicatalytic proteinase complex (proteasome) Enzyme and protein. 1993;47(4–6):296–305. doi: 10.1159/000468687. [DOI] [PubMed] [Google Scholar]
- 27.Fang S, Jensen JP, Ludwig RL, Vousden KH, Weissman AM. Mdm2 is a RING finger-dependent ubiquitin protein ligase for itself and p53. J Biol Chem. 2000;275(12):8945–51. doi: 10.1074/jbc.275.12.8945. [DOI] [PubMed] [Google Scholar]
- 28.Adams J, Palombella VJ, Sausville EA, et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59(11):2615–22. [PubMed] [Google Scholar]
- 29.Drexler HC. Activation of the cell death program by inhibition of proteasome function. Proc Natl Acad Sci U S A. 1997;94(3):855–60. doi: 10.1073/pnas.94.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.An B, Goldfarb RH, Siman R, Dou QP. Novel dipeptidyl proteasome inhibitors overcome Bcl-2 protective function and selectively accumulate the cyclin-dependent kinase inhibitor p27 and induce apoptosis in transformed, but not normal, human fibroblasts. Cell Death Differ. 1998;5(12):1062–75. doi: 10.1038/sj.cdd.4400436. [DOI] [PubMed] [Google Scholar]
- 31.Vinitsky CC A, Sepp-Lorenzino L, Michaud C, Orlowski M. Inhibition of the proteolytic activity of the multicatalytic proteinase complex (proteasome) by substrate-related peptidyl aldehydes. J Biol Chem. 1994;269:29860–6. [PubMed] [Google Scholar]
- 32.Craiu A, Gaczynska M, Akopian T, et al. Lactacystin and clasto-lactacystin beta-lactone modify multiple proteasome beta-subunits and inhibit intracellular protein degradation and major histocompatibility complex class I antigen presentation. J Biol Chem. 1997;272(20):13437–45. doi: 10.1074/jbc.272.20.13437. [DOI] [PubMed] [Google Scholar]
- 33.Adams J SR. Novel inhibitors of the proteasome and their therapeutic use in inflammation. Annu Rep Med Chem. 1996;31:279–88. [Google Scholar]
- 34.Seemuller E, Lupas A, Stock D, Lowe J, Huber R, Baumeister W. Proteasome from Thermoplasma acidophilum: a threonine protease. Science. 1995;268(5210):579–82. doi: 10.1126/science.7725107. [DOI] [PubMed] [Google Scholar]
- 35.Stein RL, Melandri F, Dick L. Kinetic characterization of the chymotryptic activity of the 20S proteasome. Biochemistry. 1996;35(13):3899–908. doi: 10.1021/bi952262x. [DOI] [PubMed] [Google Scholar]
- 36.Adams J, Behnke M, Chen S, et al. Potent and selective inhibitors of the proteasome: dipeptidyl boronic acids. Bioorg Med Chem Lett. 1998;8(4):333–8. doi: 10.1016/s0960-894x(98)00029-8. [DOI] [PubMed] [Google Scholar]
- 37.Frankel A, Man S, Elliott P, Adams J, Kerbel RS. Lack of multicellular drug resistance observed in human ovarian and prostate carcinoma treated with the proteasome inhibitor PS-341. Clin Cancer Res. 2000;6(9):3719–28. [PubMed] [Google Scholar]
- 38.Bold RJ, Virudachalam S, McConkey DJ. Chemosensitization of pancreatic cancer by inhibition of the 26S proteasome. J Surg Res. 2001;100(1):11–7. doi: 10.1006/jsre.2001.6194. [DOI] [PubMed] [Google Scholar]
- 39.Cusack JC, Jr, Liu R, Houston M, et al. Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: implications for systemic nuclear factor-kappaB inhibition. Cancer Res. 2001;61(9):3535–40. [PubMed] [Google Scholar]
- 40.Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61(7):3071–6. [PubMed] [Google Scholar]
- 41.Hideshima T, Chauhan D, Richardson P, et al. NF-kappa B as a therapeutic target in multiple myeloma. J Biol Chem. 2002;277(19):16639–47. doi: 10.1074/jbc.M200360200. [DOI] [PubMed] [Google Scholar]
- 42.Li ZW, Chen H, Campbell RA, Bonavida B, Berenson JR. NF-kappaB in the pathogenesis and treatment of multiple myeloma. Curr Opin Hematol. 2008;15(4):391–9. doi: 10.1097/MOH.0b013e328302c7f4. [DOI] [PubMed] [Google Scholar]
- 43.Annunziata CM, Davis RE, Demchenko Y, et al. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12(2):115–30. doi: 10.1016/j.ccr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bharti AC, Shishodia S, Reuben JM, et al. Nuclear factor-kappaB and STAT3 are constitutively active in CD138+ cells derived from multiple myeloma patients, and suppression of these transcription factors leads to apoptosis. Blood. 2004;103(8):3175–84. doi: 10.1182/blood-2003-06-2151. [DOI] [PubMed] [Google Scholar]
- 45.Millennium Pharmaceuticals Inc . VELCADE® (bortezomib) for Injection. Prescribing information. Cambridge, MA, USA: 2008. Issued Jun 2008, Rev 9 2009. [Google Scholar]
- 46.Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12(5):431–40. doi: 10.1016/S1470-2045(11)70081-X. [DOI] [PubMed] [Google Scholar]
- 47.Popat R, Oakervee HE, Hallam S, et al. Bortezomib, doxorubicin and dexamethasone (PAD) front-line treatment of multiple myeloma: updated results after long-term follow-up. Br J Haematol. 2008;141(4):512–6. doi: 10.1111/j.1365-2141.2008.06997.x. [DOI] [PubMed] [Google Scholar]
- 48.Reece DE, Sullivan D, Lonial S, et al. Pharmacokinetic and pharmacodynamic study of two doses of bortezomib in patients with relapsed multiple myeloma. Cancer Chemother Pharmacol. 2011;67(1):57–67. doi: 10.1007/s00280-010-1283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moreau P, Coiteux V, Hulin C, et al. Prospective comparison of subcutaneous versus intravenous administration of bortezomib in patients with multiple myeloma. Haematologica. 2008;93(12):1908–11. doi: 10.3324/haematol.13285. [DOI] [PubMed] [Google Scholar]
- 50.Uttamsingh V, Lu C, Miwa G, Gan LS. Relative contributions of the five major human cytochromes P450, 1A2, 2C9, 2C19, 2D6, and 3A4, to the hepatic metabolism of the proteasome inhibitor bortezomib. Drug Metab Dispos. 2005;33(11):1723–8. doi: 10.1124/dmd.105.005710. [DOI] [PubMed] [Google Scholar]
- 51.Pekol T, Daniels JS, Labutti J, et al. Human metabolism of the proteasome inhibitor bortezomib: identification of circulating metabolites. Drug Metab Dispos. 2005;33(6):771–7. doi: 10.1124/dmd.104.002956. [DOI] [PubMed] [Google Scholar]
- 52.Leveque D, Carvalho MC, Maloisel F. Review. Clinical pharmacokinetics of bortezomib. In vivo. 2007;21(2):273–8. [PubMed] [Google Scholar]
- 53.Harousseau JL, Attal M, Avet-Loiseau H, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial. J Clin Oncol. 2010;28(30):4621–9. doi: 10.1200/JCO.2009.27.9158. [DOI] [PubMed] [Google Scholar]
- 54.Cavo M, Tacchetti P, Patriarca F, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2010;376(9758):2075–85. doi: 10.1016/S0140-6736(10)61424-9. [DOI] [PubMed] [Google Scholar]
- 55.Jagannath S, Durie BG, Wolf J, et al. Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. Br J Haematol. 2005;129(6):776–83. doi: 10.1111/j.1365-2141.2005.05540.x. [DOI] [PubMed] [Google Scholar]
- 56.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359(9):906–17. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 57.Mateos MV, Oriol A, Martinez-Lopez J, et al. Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. Lancet Oncol. 2010;11(10):934–41. doi: 10.1016/S1470-2045(10)70187-X. [DOI] [PubMed] [Google Scholar]
- 58.Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116(5):679–86. doi: 10.1182/blood-2010-02-268862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jagannath S, Durie BG, Wolf JL, et al. Extended follow-up of a phase 2 trial of bortezomib alone and in combination with dexamethasone for the frontline treatment of multiple myeloma. Br J Haematol. 2009;146(6):619–26. doi: 10.1111/j.1365-2141.2009.07803.x. [DOI] [PubMed] [Google Scholar]
- 60.Gay F, Hayman SR, Lacy MQ, et al. Lenalidomide plus dexamethasone versus thalidomide plus dexamethasone in newly diagnosed multiple myeloma: a comparative analysis of 411 patients. Blood. 2010;115(7):1343–50. doi: 10.1182/blood-2009-08-239046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rajkumar SV, Hayman S, Gertz MA, et al. Combination therapy with thalidomide plus dexamethasone for newly diagnosed myeloma. J Clin Oncol. 2002;20(21):4319–23. doi: 10.1200/JCO.2002.02.116. [DOI] [PubMed] [Google Scholar]
- 62.Rajkumar SV, Rosinol L, Hussein M, et al. Multicenter, randomized, double-blind, placebo-controlled study of thalidomide plus dexamethasone compared with dexamethasone as initialtherapy for newly diagnosed multiple myeloma. J Clin Oncol. 2008;26(13):2171–7. doi: 10.1200/JCO.2007.14.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Richardson PG, Xie W, Mitsiades C, et al. Single-agent bortezomib in previously untreated multiple myeloma: efficacy, characterization of peripheral neuropathy, and molecular correlations with response and neuropathy. J Clin Oncol. 2009;27(21):3518–25. doi: 10.1200/JCO.2008.18.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosinol L, Oriol A, Mateos MV, et al. Phase II PETHEMA trial of alternating bortezomib and dexamethasone as induction regimen before autologous stem-cell transplantation in younger patients with multiple myeloma: efficacy and clinical implications of tumor response kinetics. J Clin Oncol. 2007;25(28):4452–8. doi: 10.1200/JCO.2007.12.3323. [DOI] [PubMed] [Google Scholar]
- 65.Mateos MV, Hernandez JM, Hernandez MT, et al. Bortezomib plus melphalan and prednisone in elderly untreated patients with multiple myeloma: results of a multicenter phase 1/2 study. Blood. 2006;108(7):2165–72. doi: 10.1182/blood-2006-04-019778. [DOI] [PubMed] [Google Scholar]
- 66.Mateos MV, Hernandez JM, Hernandez MT, et al. Bortezomib plus melphalan and prednisone in elderly untreated patients with multiple myeloma: updated time-to-events results and prognostic factors for time to progression. Haematologica. 2008;93(4):560–5. doi: 10.3324/haematol.12106. [DOI] [PubMed] [Google Scholar]
- 67.Mateos MV, Richardson PG, Schlag R, et al. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol. 2010;28(13):2259–66. doi: 10.1200/JCO.2009.26.0638. [DOI] [PubMed] [Google Scholar]
- 68.Gasparetto C, Gockerman JP, Diehl LF, et al. “Short course” bortezomib plus melphalan and prednisone as induction prior to transplant or as frontline therapy for nontransplant candidates in patients with previously untreated multiple myeloma. Biol Blood Marrow Transplant. 2010;16(1):70–7. doi: 10.1016/j.bbmt.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 69.Roussel M, Moreau P, Huynh A, et al. Bortezomib and high-dose melphalan as conditioning regimen before autologous stem cell transplantation in patients with de novo multiple myeloma: a phase 2 study of the Intergroupe Francophone du Myelome (IFM) Blood. 2010;115(1):32–7. doi: 10.1182/blood-2009-06-229658. [DOI] [PubMed] [Google Scholar]
- 70.Oakervee HE, Popat R, Curry N, et al. PAD combination therapy (PS-341/bortezomib, doxorubicin and dexamethasone) for previously untreated patients with multiple myeloma. Br J Haematol. 2005;129(6):755–62. doi: 10.1111/j.1365-2141.2005.05519.x. [DOI] [PubMed] [Google Scholar]
- 71.Jakubowiak AJ, Kendall T, Al-Zoubi A, et al. Phase II trialof combination therapy with bortezomib, pegylated liposomal doxorubicin, and dexamethasone in patients with newly diagnosed myeloma. J Clin Oncol. 2009;27(30):5015–22. doi: 10.1200/JCO.2008.19.5370. [DOI] [PubMed] [Google Scholar]
- 72.Landau H, Pandit-Taskar N, Hassoun H, et al. Bortezomib, liposomaldoxorubicin and dexamethasone followed by thalidomide and dexamethasone is an effective treatment for patients with newly diagnosed multiple myeloma with Internatinal Staging System stage II or III, or extramedullary disease. Leuk Lymphoma. 2012;53(2):275–81. doi: 10.3109/10428194.2011.606943. [DOI] [PubMed] [Google Scholar]
- 73.Kim HJ, Yoon SS, Lee DS, et al. Sequential vincristine, adriamycin, dexamethasone (VAD) followed by bortezomib, thalidomide, dexamethasone (VTD) as induction, followed by high-dose therapy with autologous stem cell transplant and consolidation therapy with bortezomib for newly diagnosed multiple myeloma: results of a phase II trial. Ann Hematol. 2012;91(2):249–56. doi: 10.1007/s00277-011-1298-9. [DOI] [PubMed] [Google Scholar]
- 74.Sonneveld P, Schmidt-Wolf IG, van der Holt B, et al. Bortezomib Induction and Maintenance Treatment in Patients With Newly Diagnosed Multiple Myeloma: Results of the Randomized Phase III HOVON-65/GMMG-HD4 Trial. J Clin Oncol. 2012;30(24):2946–55. doi: 10.1200/JCO.2011.39.6820. [DOI] [PubMed] [Google Scholar]
- 75.Reeder CB, Reece DE, Kukreti V, et al. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia. 2009;23(7):1337–41. doi: 10.1038/leu.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kropff M, Liebisch P, Knop S, et al. DSMM XI study: dose definition for intravenous cyclophosphamide in combination with bortezomib/dexamethasone for remission induction in patients with newly diagnosed myeloma. Ann Hematol. 2009;88(11):1125–30. doi: 10.1007/s00277-009-0726-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ahn JS, Yang DH, Jung SH, et al. A comparison of bortezomib, cyclophosphamide, and dexamethasone (Vel-CD) chemotherapy without and with thalidomide (Vel-CTD) for the treatment of relapsed or refractory multiple myeloma. Ann Hematol. 2012;91(7):1023–30. doi: 10.1007/s00277-012-1420-7. [DOI] [PubMed] [Google Scholar]
- 78.Fu W, Delasalle K, Wang J, et al. Bortezomib-cyclophosphamide-dexamethasone for relapsing multiple myeloma. AmJClinOncol. 2012;35(6):562–5. doi: 10.1097/COC.0b013e31822043f6. [DOI] [PubMed] [Google Scholar]
- 79.Mele G, Giannotta A, Pinna S, et al. Frail elderly patients with relapsed-refractory multiple myeloma: efficacy and toxicity profile of the combination of bortezomib, high-dose dexamethasone, and low-dose oral cyclophosphamide. Leuk Lymphoma. 2010;51(5):937–40. doi: 10.3109/10428191003695660. [DOI] [PubMed] [Google Scholar]
- 80.Davies FE, Wu P, Jenner M, Srikanth M, Saso R, Morgan GJ. The combination of cyclophosphamide, velcade and dexamethasone induces high response rates with comparable toxicity to velcade alone and velcade plus dexamethasone. Haematologica. 2007;92(8):1149–50. doi: 10.3324/haematol.11228. [DOI] [PubMed] [Google Scholar]
- 81.Kumar S, Flinn I, Richardson PG, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood. 2012;119(19):4375–82. doi: 10.1182/blood-2011-11-395749. [DOI] [PubMed] [Google Scholar]
- 82.Ghosh N, Ye X, Ferguson A, Huff CA, Borrello I. Bortezomib and thalidomide, a steroid free regimen in newly diagnosed patients with multiple myeloma. Br J Haematol. 2011;152(5):593–9. doi: 10.1111/j.1365-2141.2010.08534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sher T, Ailawadhi S, Miller KC, et al. A steroid-independent regimen of bortezomib, liposomal doxorubicin and thalidomide demonstrate high response rates in newly diagnosed multiple myeloma patients. Br J Haematol. 2011;154(1):104–10. doi: 10.1111/j.1365-2141.2011.08703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rosinol L, Oriol A, Teruel AI, et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood. 2012;120(8):1589–96. doi: 10.1182/blood-2012-02-408922. [DOI] [PubMed] [Google Scholar]
- 85.Moreau P, Avet-Loiseau H, Facon T, et al. Bortezomib plus dexamethasone versus reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment before autologous stem cell transplantation in newly diagnosed multiple myeloma. Blood. 2011;118(22):5752–8. doi: 10.1182/blood-2011-05-355081. [DOI] [PubMed] [Google Scholar]
- 86.Niesvizky R, Flinn I, Rifkin R, et al. Efficacy and safety of three bortezomib-based combinations in elderly, newly diagnosed multiple myeloma patients: results from all randomized patients in the community-based, phase 3b UPFRONT Study. 2010. Available at http://myeloma.org/pdfs/ASH2011_Niesvizky_3599.pdf. Accessed Jan 13, 2013.
- 87.Ladetto M, Pagliano G, Ferrero S, et al. Major tumor shrinking and persistent molecular remissions after consolidation with bortezomib, thalidomide, and dexamethasone in patients with autografted myeloma. J Clin Oncol. 2010;28(12):2077–84. doi: 10.1200/JCO.2009.23.7172. [DOI] [PubMed] [Google Scholar]
- 88.Kumar SK, Flinn I, Noga SJ, et al. Bortezomib, dexamethasone, cyclophosphamide and lenalidomide combination for newly diagnosed multiple myeloma: phase 1 results from the multicenter EVOLUTION study. Leukemia. 2010;24(7):1350–6. doi: 10.1038/leu.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Khan ML, Reeder CB, Kumar SK, et al. A comparison of lenalidomide/dexamethasone versus cyclophosphamide/lenalidomide/dexamethasone versus cyclophosphamide/bortezomib/dexamethasone in newly diagnosed multiple myeloma. Br J Haematol. 2012;156(3):326–33. doi: 10.1111/j.1365-2141.2011.08949.x. [DOI] [PubMed] [Google Scholar]
- 90.Palumbo A, Bringhen S, Rossi D, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initialtreatment of multiple myeloma: a randomized controlled trial. J Clin Oncol. 2010;28(34):5101–9. doi: 10.1200/JCO.2010.29.8216. [DOI] [PubMed] [Google Scholar]
- 91.Bensinger WI, Jagannath S, Vescio R, et al. Phase 2 study of two sequential three-drug combinations containing bortezomib, cyclophosphamide and dexamethasone, followed by bortezomib, thalidomide and dexamethasone as frontline therapy for multiple myeloma. Br J Haematol. 2010;148(4):562–8. doi: 10.1111/j.1365-2141.2009.07981.x. [DOI] [PubMed] [Google Scholar]
- 92.Heider U, Kaiser M, Muller C, et al. Bortezomib increases osteoblast activity in myeloma patients irrespective of response to treatment. Eur J Haematol. 2006;77(3):233–8. doi: 10.1111/j.1600-0609.2006.00692.x. [DOI] [PubMed] [Google Scholar]
- 93.Ozaki S, Tanaka O, Fujii S, et al. Therapy with bortezomib plus dexamethasone induces osteoblast activation in responsive patients with multiple myeloma. Int J Hematol. 2007;86(2):180–5. doi: 10.1532/IJH97.07030. [DOI] [PubMed] [Google Scholar]
- 94.Delforge M, Terpos E, Richardson PG, et al. Fewer bone disease events, improvement in bone remodeling, and evidence of bone healing with bortezomib plus melphalan-prednisone vs. melphalan-prednisone in the phase III VISTA trialin multiple myeloma. Eur J Haematol. 2011;86(5):372–84. doi: 10.1111/j.1600-0609.2011.01599.x. [DOI] [PubMed] [Google Scholar]
- 95.Kyle RA. Role of maintenance therapy after autologous stem cell transplant for multiple myeloma: lessons for cancer therapy. Mayo Clin Proc. 2011;86(5):419–20. doi: 10.4065/mcp.2011.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Attal M, Cances Lauwers V, Marit G, et al. Maintenance treatment with lenalidomide after transplantation for MYELOMA: final analysis of the IFM 2005-02 [abstract 310] Blood (ASH Annual Meeting Abstracts) 2010;116(21):310. [Google Scholar]
- 97.McCarthy PL, Owzar K, Anderson KC, et al. Phase III intergroup study of lenalidomide versus placebo maintenance therapy following single autologous hematopoietic stem cell transplantation (AHSCT) for multiple myeloma: CALGB 100104 [abstract 37] Blood (ASH Annual Meeting Abstracts) 2010;116(21):37. [Google Scholar]
- 98.Sahebi F, Frankel PH, Farol L, et al. Sequential bortezomib, dexamethasone, and thalidomide maintenance therapy after single autologous peripheral stem cell transplantation in patients with multiple myeloma. Biol Blood Marrow Transplant. 2012;18(3):486–92. doi: 10.1016/j.bbmt.2011.12.580. [DOI] [PubMed] [Google Scholar]
- 99.Wang M, Giralt S, Delasalle K, Handy B, Alexanian R. Bortezomib in combination with thalidomide-dexamethasone for previously untreated multiple myeloma. Hematology. 2007;12(3):235–9. doi: 10.1080/10245330701214236. [DOI] [PubMed] [Google Scholar]
- 100.Lee JH, Kim DY, Kim SD, et al. Two cycles of the PS-341/bortezomib, adriamycin, and dexamethasone combination followed by autologous hematopoietic cell transplantation in newly diagnosed multiple myeloma patients. Eur J Haematol. 2012;88(6):478–84. doi: 10.1111/j.1600-0609.2012.01771.x. [DOI] [PubMed] [Google Scholar]
- 101.2010. Bortezomib. Pubmed health.
- 102.Dispenzieri, et al. Mayo Clin Proc. 2007;82:323–41. doi: 10.4065/82.3.323. [DOI] [PubMed] [Google Scholar]; Kumar, et al. Mayo Clin Proc. 2009;84:1095–110. doi: 10.4065/mcp.2009.0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Landowski TH, Megli CJ, Nullmeyer KD, Lynch RM, Dorr RT. Mitochondrial-mediated disregulation of Ca2+ is a critical determinant of Velcade (PS-341/bortezomib) cytotoxicity in myeloma cell lines. Cancer Res. 2005;65(9):3828–36. doi: 10.1158/0008-5472.CAN-04-3684. [DOI] [PubMed] [Google Scholar]
- 104.Ling YH, Liebes L, Zou Y, Perez-Soler R. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic response to Bortezomib, a novel proteasome inhibitor, in human H460 non-small cell lung cancer cells. J Biol Chem. 2003;278(36):33714–23. doi: 10.1074/jbc.M302559200. [DOI] [PubMed] [Google Scholar]
- 105.Pei XY, Dai Y, Grant S. The proteasome inhibitor bortezomib promotes mitochondrial injury and apoptosis induced by the small molecule Bcl-2 inhibitor HA14-1 in multiple myeloma cells. Leukemia. 2003;17(10):2036–45. doi: 10.1038/sj.leu.2403109. [DOI] [PubMed] [Google Scholar]
- 106.Perez-Galan P, Roue G, Villamor N, Montserrat E, Campo E, Colomer D. The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status. Blood. 2006;107(1):257–64. doi: 10.1182/blood-2005-05-2091. [DOI] [PubMed] [Google Scholar]
- 107.Mitsiades N, Mitsiades CS, Poulaki V, et al. Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proc Natl Acad Sci U S A. 2002;99(22):14374–9. doi: 10.1073/pnas.202445099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shi J, Tricot GJ, Garg TK, et al. Bortezomib down-regulates the cell-surface expression of HLA class I and enhances natural killer cell-mediated lysis of myeloma. Blood. 2008;111(3):1309–17. doi: 10.1182/blood-2007-03-078535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Spisek R, Charalambous A, Mazumder A, Vesole DH, Jagannath S, Dhodapkar MV. Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood. 2007;109(11):4839–45. doi: 10.1182/blood-2006-10-054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shin DH, Chun YS, Lee DS, Huang LE, Park JW. Bortezomib inhibits tumor adaptation to hypoxia by stimulating the FIH-mediated repression of hypoxia-inducible factor-1. Blood. 2008;111(6):3131–6. doi: 10.1182/blood-2007-11-120576. [DOI] [PubMed] [Google Scholar]
- 111.Ma MH, Yang HH, Parker K, et al. The proteasome inhibitor PS-341 markedly enhances sensitivity of multiple myeloma tumor cells to chemotherapeutic agents. Clin Cancer Res. 2003;9(3):1136–44. [PubMed] [Google Scholar]
- 112.Mitsiades N, Mitsiades CS, Richardson PG, et al. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood. 2003;101(6):2377–80. doi: 10.1182/blood-2002-06-1768. [DOI] [PubMed] [Google Scholar]
- 113.Gomez-Bougie P, Wuilleme-Toumi S, Menoret E, et al. Noxa up-regulation and Mcl-1 cleavage are associated to apoptosis induction by bortezomib in multiple myeloma. Cancer Res. 2007;67(11):5418–24. doi: 10.1158/0008-5472.CAN-06-4322. [DOI] [PubMed] [Google Scholar]
- 114.Podar K, Gouill SL, Zhang J, et al. A pivotalrole for Mcl-1 in Bortezomib-induced apoptosis. Oncogene. 2008;27(6):721–31. doi: 10.1038/sj.onc.1210679. [DOI] [PubMed] [Google Scholar]
- 115.Obeng EA, Carlson LM, Gutman DM, Harrington WJ, Jr, Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107(12):4907–16. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dong H, Chen L, Chen X, et al. Dysregulation of unfolded protein response partially underlies proapoptotic activity of bortezomib in multiple myeloma cells. Leuk Lymphoma. 2009;50(6):974–84. doi: 10.1080/10428190902895780. [DOI] [PubMed] [Google Scholar]
- 117.Hideshima T, Bradner JE, Wong J, et al. Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma. Proc Natl Acad Sci U S A. 2005;102(24):8567–72. doi: 10.1073/pnas.0503221102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fennell DA, Chacko A, Mutti L. BCL-2 family regulation by the 20S proteasome inhibitor bortezomib. Oncogene. 2008;27(9):1189–97. doi: 10.1038/sj.onc.1210744. [DOI] [PubMed] [Google Scholar]
- 119.Qin JZ, Ziffra J, Stennett L, et al. Proteasome inhibitors trigger NOXA-mediated apoptosis in melanoma and myeloma cells. Cancer Res. 2005;65(14):6282–93. doi: 10.1158/0008-5472.CAN-05-0676. [DOI] [PubMed] [Google Scholar]
- 120.Hideshima T, Mitsiades C, Akiyama M, et al. Molecular mechanisms mediating antimyeloma activity of proteasome inhibitor PS-341. Blood. 2003;101(4):1530–4. doi: 10.1182/blood-2002-08-2543. [DOI] [PubMed] [Google Scholar]
- 121.Chauhan D, Uchiyama H, Akbarali Y, et al. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood. 1996;87(3):1104–12. [PubMed] [Google Scholar]
- 122.Hideshima T, Chauhan D, Hayashi T, et al. Proteasome inhibitor PS-341 abrogates IL-6 triggered signaling cascades via caspase-dependent downregulation of gp130 in multiple myeloma. Oncogene. 2003;22(52):8386–93. doi: 10.1038/sj.onc.1207170. [DOI] [PubMed] [Google Scholar]
- 123.Roccaro AM, Hideshima T, Raje N, et al. Bortezomib mediates antiangiogenesis in multiple myeloma via direct and indirect effects on endothelial-cells. Cancer Res. 2006;66(1):184–91. doi: 10.1158/0008-5472.CAN-05-1195. [DOI] [PubMed] [Google Scholar]
- 124.Salmena L, Lam V, McPherson JP, Goldenberg GJ. Role of proteasomal-degradation in the cell cycle-dependent regulation of DNA topoisomerase II alpha expression. Biochem Pharmacol. 2001;61(7):795–802. doi: 10.1016/s0006-2952(01)00580-9. [DOI] [PubMed] [Google Scholar]
- 125.Hideshima T, Richardson PG, Anderson KC. Mechanism of action of proteasome inhibitors and deacetylase inhibitors and the biological basis of synergy in multiple myeloma. Mol Cancer Ther. 2011;10(11):2034–42. doi: 10.1158/1535-7163.MCT-11-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]