Abstract
Effects of dioxins on cognitive functions were reported in previous studies conducted in humans and animals. In the present study, we investigated the influence of dioxin exposure during pregnancy on social interaction and on the activity of offspring, which are related to neurodevelopmental disturbances. In addition, we analyzed neurochemical alterations of the limbic system of rat brains to suggest one mechanism of dioxin effects on brain function. We believe that this manuscript is suitable for publication in “Environmental Health Insights” because it provides an interesting topic for a wide global audience.
To clarify the relationships between maternal dioxin exposure and socioemotional functions of rat offspring, dams were given TCDD (1.0 μg/kg) on gestational day 15. Social interactions and forced swimming time were compared between TCDD-exposed and control offspring in each gender. Frequency and duration of locomotion were higher, and durations per one behavior of proximity and social contact were significantly lower in the exposed males, while only the duration of proximity was lower in the exposed females. Forced swimming time on the first day was significantly longer in the exposed males. In the limbic system of the rat brain, the levels and/or activity of CaMKIIα were decreased in males and were increased in females in the exposed offspring. These results suggest that prenatal TCDD exposure induces hyperactivity and socioemotional deficits, particularly in the male offspring due to alterations in CaMKIIα activity in the limbic system of the brain.
Keywords: TCDD, maternal exposure, socio-emotional behavior, offspring rats, CaMKIIα
Introduction
The adverse impact of maternal exposure to dioxins during pregnancy on infant neurodevelopment has been suggested by Yu-Cheng children, whose mothers were accidentally exposed to high levels of polychlorinated biphenyls (PCBs) and polychlorinated dibenzo-p-dioxins (PCDDs)/polychlorinated dibenzo-furans (PCDFs).1,2 In a Dutch population exposed to PCBs and dioxins at a background level, Koopman-Esseboom et al3 reported an inverse association between prenatal dioxin exposure and psychomotor development at 7 months of age. In another epidemiological study of the general Japanese population, Nakajima et al4 reported that some PCDDs/PCDFs congeners exhibited significant inverse associations with the psychomotor and mental development of 6-month-old infants. Our group also found that infants whose maternal breast milk contained higher dioxins showed lower neurodevelopmental scores at 4 months and at 12 months of age in a hot spot of dioxin-contamination in Vietnam, and that several dioxin congeners exhibited significant inverse associations with communication skill scores, including expressive language and social emotional scales at 12 months of age.5
In animal studies, maternal exposure to TCDD induces deficits in higher brain functions, such as working memory in a radial arm maze,6 operant responding in running wheels or a two-lever chamber,7,8 and discrimination reversal learning.9 Nishijo et al10 also reported that TCDD exposure during pregnancy influenced emotional learning, such as active avoidance learning, in young offspring rats. However, the effects of prenatal exposure of TCDD on neurobehavioral development, especially socioemotional functions, remain unclear. Indeed, few previous TCDD studies have investigated social interactions of offspring.
The limbic system of the brain mediates learning and is also involved in various socioemotional behaviors such as social interactions and anxiety-like behaviors.11–13 Especially, Ca2+/calmodulin-dependent protein kinase IIα (CaMKIIα) in the limbic system plays an important role in learning and memory,14 and in socioemotional behaviors.15 Therefore, CaMKIIα analysis in the limbic system may clarify the mechanism of neurobehavioral alteration in offspring exposed to TCDD.
In the present study, the effects of prenatal exposure of TCDD on socioemotional behaviors, including social interaction and anxiety-like behavior, were investigated during development using rat offspring, and the level and/or activity of CaMKIIα was analyzed in the main structures of the limbic system including the orbital cortex, amygdala, and hippocampus.
Methods
Animals and exposure to chemicals
On gestation day (GD) 15, TCDD dissolved in corn oil (0.2 μg/mL) at a dose of 1.0 μg/kg body weight (bw) (1–1.2 mL) was gavaged to five pregnant Wistar rats (12 weeks of age) that served as a TCDD-exposed group. This protocol of TCDD exposure (a single oral dose of 1.0 μg/kg bw on GD 15) has been confirmed in previous studies to increase the level of a pup’s brain tissue at low levels, and to cause adverse developmental effects on endocrine function in the brain.16 The same volume of pure corn oil (MP Biomedicals LLC, Santa Ana, CA, USA) was gavaged to five pregnant Wister rats that served as a control group. All dams gave birth on GD 22. After birth, the litter size was adjusted to seven to eight pups per litter to ensure adequate nutrition. None of the offspring rats died in the control group, while five pups died in the TCDD group during the lactation period. A total of 38 pups in the control group and 35 pups in the TCDD group were available for behavioral tests. Offspring rats of the same gender from each dam were housed in the same cage after weaning with food and water ad libitum in a temperature-controlled room. Light/dark cycles were changed for the observation of behavior in the daytime (lights were turned on and off at 20:00 and 08:00, respectively).
All experimental procedures and study design were approved by Ethical Review Board at Kanazawa Medical University.
Behavior tests
Social interaction test
At 8–9 weeks of age, social interaction testing was performed to examine the offspring—14 males and 21 females in the dioxin group, and 17 males and 21 females in the control group. Mean weight (standard deviation, SD) at the time of testing were 319.0 (39.6) g for males and 216.8 (30.8) g for females in the TCDD group, and 343.0 (26.1) g for males and 218.0 (17.4) g for females in the control group. Two offspring rats were paired from the same exposure group and same gender, but from different house cages; 17 male and 23 female pairs in the control group and 18 male and 22 female pairs in the TCDD group were assessed for their social interaction behavior. Some rats were tested twice, but they were paired with a new rat each time.
The testing cage was a transparent plastic box (45 cm × 45 cm × 40 cm) with a black floor. Individual rats were allowed to acclimate to the testing cage for 20 minutes prior to the test. Pairs of rats from the same group were placed in opposite corners of the box. Their activities in the box were recorded using an overhead charge-coupled device (CCD) camera for 30 minutes. Frequencies (event times), durations (seconds), and duration/frequency [duration per one event (second/event)] of locomotor (locomotion and no movement) and social activities (ie, proximity behavior, approaching and leaving, following, social sniffing, active and passive contact, and mounting) were automatically analyzed using the Social Scan program (Clever Sys Inc, Reston, VA, USA). The definition of each social activity is shown in supplemental Table 1. The plastic box was wiped with 70% ethanol and was air-dried between the trials.
Forced swimming test (FST)
The offspring (11 males and 21 females in the exposed group, and 17 males and 21 females in the control group) were subjected to a forced swimming test at 11–12 weeks of age. Means (SD) of weight at the time of testing were 363.4 (33.9) g for males and 240.5 (20.0) g for females in the dioxin group, and 389.6 (27.3) g for males and 242.2 (11.9) g for females in the control group. One male rat in the exposed group was not tested because of skin trouble, and the data from two male rats were excluded from the analysis because of system trouble.
The FST used in this study was based on the original version used for rats by Porsolt, but was conducted with modifications. Rats were placed in a cylinder (20 cm × 50 cm; diameter × height) filled with water (30 cm high). The rats were not able to touch the bottom of the cylinder. The water temperature was set at 25 °C ± 1 °C. The cylinder was placed in a box with infrared cell sensors on the walls to detect swimming activity (SCANET, Melquest Inc, Toyama, Japan). On the first day, the rats were placed in water and forced to swim in a single trial of 15 minutes. On the second day, the rats were placed in water and forced to swim in a single trial of 5 minutes. Swimming time was continuously recorded every 1 minute of each trial for each animal. After the test, the animals were dried with towels and returned to their cages.
Neurochemistry study
CaMKIIα measurement
A total of eight pups (five males and three females) in the control group and a total of 14 pups (seven males and seven females) in the TCDD group were randomly selected for measurement of brain tissue CaMKIIα. The brain was removed after decapitation under pentobarbital anesthesia (50 mg/kg, intraperitoneally) at 19 weeks old. Then, the orbital cortex, amygdala, and hippocampus were quickly dissected from the brain slices on dry ice. Samples were immediately frozen in liquid nitrogen and kept in a −80 °C deep freezer until analysis.
For the CaMKIIα analysis, frozen samples were homogenizedinabuffercontaining0.5%TritonX-100, and insoluble material was removed by centrifugation after sonication. Samples of the supernatant, containing equivalent amounts of protein, were applied to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis. Western blotting analysis was carried out using polyclonal antibodies directed against CaMKIIα and phosphorylated (p)—CaMKIIα, as described by Fukunaga et al.17,18 For the samples that showed an increment in p-CaMKIIα or p/total ratio of CaMKIIα (p/t ratio), confirmation of increased neuronal activity was conducted by analyzing levels of N-methyl-D-aspartate (NMDA) type glutamate receptor subunit 1 (NR1) and α-amino 3-hydroxy 5-methyl 4-isoxazole propionate (AMPA) type glutamate receptor subunit 1 (GluR1), and synapsin 1 (Syn 1) that reflects presynaptic activity. These measurements were performed according to previous papers by Moriguchi et al19 using the following primary antibodies: anti-NR1 (EMD Millipore Corporation, Billerica, MA, USA), anti-GluR1, and anti-phospho-GluR1 (EMD Millipore Corporation, Billerica, MA, USA), anti-Syn1,20 anti-phospho-Syn 1 (EMD Millipore Corporation, Billerica, MA, USA), and anti-β-tubuline for a loading control (Sigma-Aldrich, St Louis, MO, USA).
Statistics
The Statistical Package for the Social Sciences (version 11.0) software package for Windows (SPSS; Chicago, IL, USA) was used for statistical analysis. The social interaction behavioral data were compared using the Student’s t-tests between the TCDD-exposed and control groups in each gender because the present study investigated the social interactions between rats of the same gender. FST was also compared between the TCDD-exposed and control groups using Student’s t-tests. The difference of CaMKIIα levels in the brain tissue between TCDD-exposed and control groups were tested using Student’s t-tests, and the means and standard deviations of the TCDD-exposed groups were expressed as a percentage of controls. The statistical significance level was set at P < 0.05.
Results
Effects of TCDD on locomotor activity and social interactions
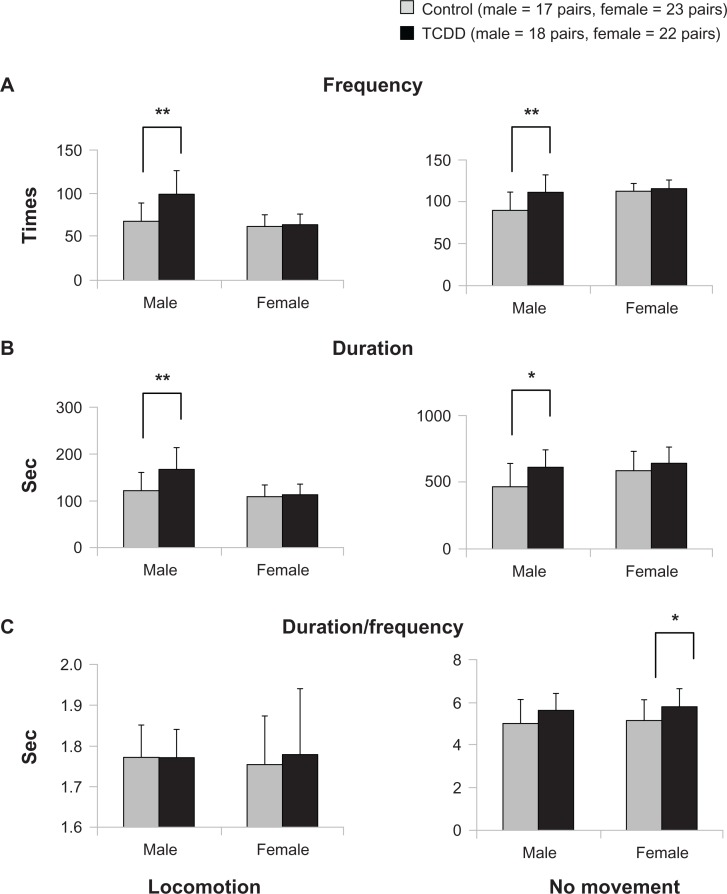
Figure 1 shows the locomotive activity of and being without movement (no movement) among the TCDD-exposed and control offspring rats in both sexes. For locomotion, the TCDD-exposed group of male rats showed higher frequency (P = 0.001) and duration (P = 0.003) than male control rats (Fig. 1A and B). However, there was no difference induration/frequency between the two exposed groups of male rats. In female offspring rats, the mean frequency and duration of locomotion were similar among the TCDD-exposed group and the control group, with no significant difference between them (Fig. 1A and B). For “no movement,” the mean frequency and duration were significantly higher (P = 0.003, P = 0.012, respectively) in male TCDD-exposed rats when compared with controls (Fig. 1A and B). However, there was no significant difference in the mean duration/frequency of “no movement” in the male TCDD-exposed group and controls (Fig. 1C). Although the increased frequency and duration of “no movement” in female rats were not significant, duration/frequency was higher in female TCDD-exposed rats (P = 0.048) than in controls (Fig. 1A–C).
Figure 1.
Effects of TCDD exposure on locomotion and no movement.
Notes: *P < 0.05, **P < 0.01; significant difference between exposure and control groups.
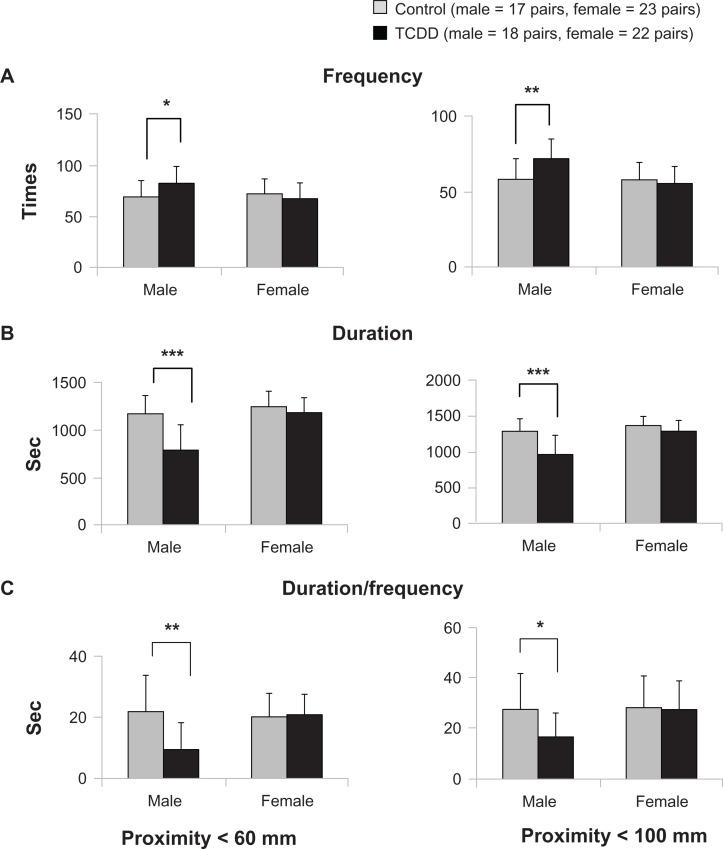
The frequency, duration, and duration/frequency of proximity behavior, which reflects affiliative social interaction, of the TCDD-exposed and control groups in each gender are shown in Figure 2. For proximity < 60 mm (inter-rat distance < 60 mm), mean frequency was significantly higher (P = 0.030) in male TCDD-exposed rats when compared with male controls (Fig. 2A). Similarly for proximity, <100 mm (inter-rat distance < 100 mm), mean frequency was significantly higher in male rats than in controls (P = 0.008) (Fig. 2A). However, the duration and duration/frequency of proximity < 60 mm were significantly lower in male TCDD-exposed rats than in control rats (P = 0.000, P = 0.001, respectively) (Fig. 2B and C), and the mean duration and duration/frequency of proximity < 100 mm were also significantly lower in the male TCDD-exposed group than in the control group (P = 0.000, P = 0.010, respectively) (Fig. 2B and C). In female rats, however, there was no significant difference in the mean frequency, duration, and duration/frequency of proximity < 60 mm and proximity < 100 mm between the TCDD-exposed and control groups (Fig. 2).
Figure 2.
Effects of TCDD exposure on proximity.
Notes: *P < 0.05, **P < 0.01, ***P < 0.001; significant difference between exposure and control groups.
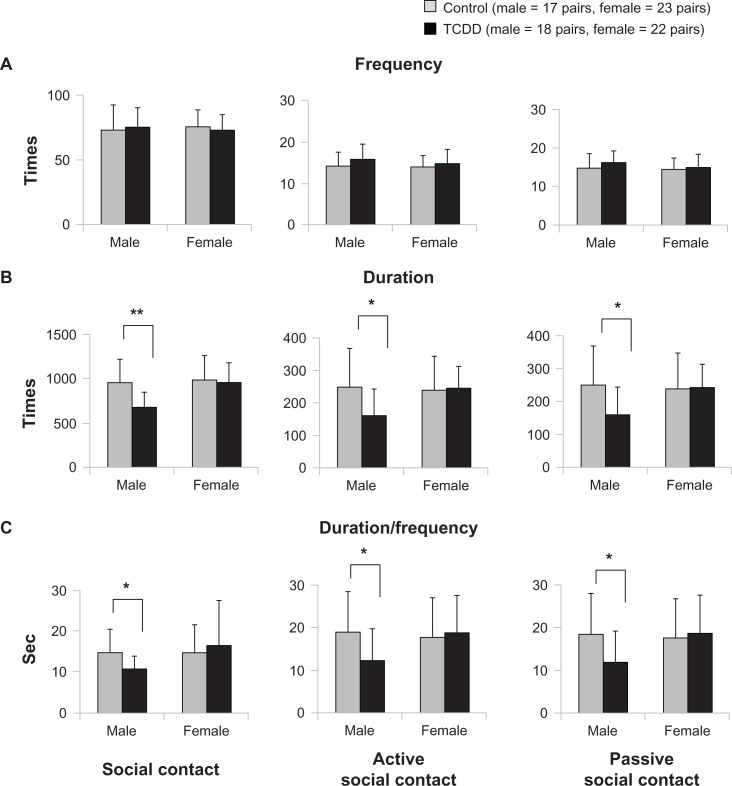
Figure 3 shows comparisons of social contact, active social contact, and passive social contact among the TCDD-exposed and control groups. No significant differences were found between the two exposed groups in the frequency of these three kinds of social contact (Fig. 3A). However, the mean duration and duration/frequency of social contact were significantly lower in the male TCDD-exposed group than in male controls (P = 0.001, P = 0.011, respectively) (Fig. 3B and C). In addition, the duration of active and passive social contact were significantly lower in the male TCDD-exposed group than in controls (P = 0.019, P = 0.018, respectively) (Fig. 3B). The mean duration/frequency of active and passive social contact were also significantly lower in the male TCDD-exposed rats than in controls (P = 0.024, P = 0.027, respectively) (Fig. 3C). However, in female rats, no differences were observed for frequency, duration, and duration/frequency across all three kinds of social contact.
Figure 3.
Effects of TCDD exposure on social contact.
Notes: *P < 0.05, **P < 0.01; significant difference between exposure and control groups.
Further, there was no significant difference in the duration, frequency, and duration/frequency of approach, leave, follow, sniff, and mount behaviors between the TCDD-exposed and control groups (data not shown).
Effects of TCDD on FST
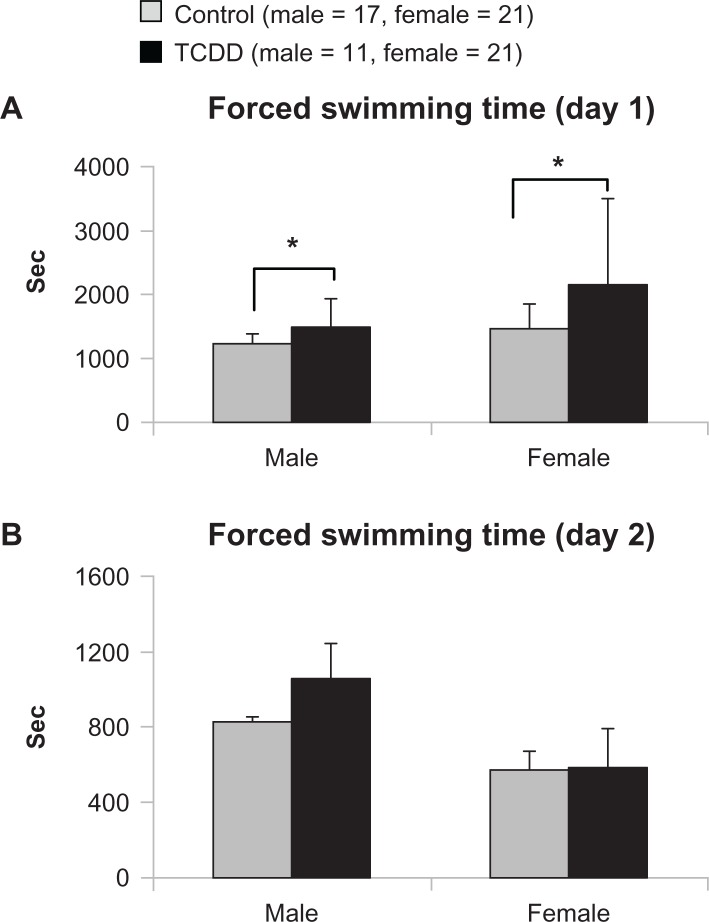
Figure 4 shows the results of the FST on the first and second days in both genders. Swimming time was significantly longer (P = 0.048) for the male TCDD-exposed group than for the male control group on the first day (Fig. 4A). In female rats, mean swimming time on the first day was also significantly longer in the TCDD-exposed group than in controls (P = 0.028) (Fig. 4A). No significant difference was also found between the exposed and control groups on the second day in both the male and female offspring rats (Fig. 4B).
Figure 4.
Effects of TCDD exposure on forced swimming time.
Notes: *P < 0.05; significant difference between TCDD-exposed and control groups.
Effects of TCDD on CaMKIIα levels and/or activity in the limbic system of the rat brain
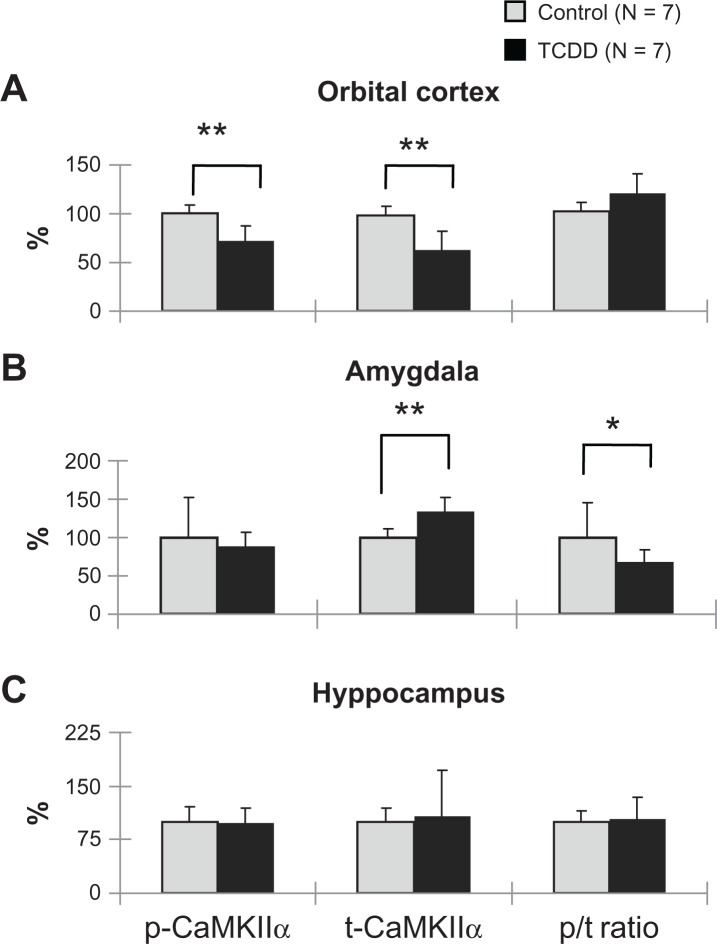
The levels of p-CaMKIIα and t-CaMKIIα in the orbital cortex were significantly decreased (P = 0.001, P = 0.002, respectively) in the male TCDD-exposed group when compared with controls (Fig. 5A), indicating lower levels of CamKIIα in the TCDD-exposed male offspring. In the amygdala, the level oft-CaMKIIα was significantly increased (P = 0.003) in the exposed males (Fig. 5B), while the ratio of p-CaMKIIα to t-CaMKIIα (p/t ratio) was significantly decreased (P = 0.029) in the TCDD-exposed group (Fig. 5B), suggesting decreased enzyme activity of CaMKIIα with secondary increased synthesis of the enzyme. However, no significant difference in p-CaMKIIα, t-CaMKIIα, and the p/t ratio in the hippocampus was found when comparing the TCDD-exposed and the control groups in male rats (Fig. 5C).
Figure 5.
Effects of dioxin exposure on CaMKIIα in the orbital cortex, amygdala, and hippocampus in the male rat offspring.
Notes: *P < 0.05, **P < 0.01; significant difference between TCDD-exposed and control groups.
Abbreviations: CaMKIIα, Ca2+/calmodulin-dependent protein kinase IIα; t-, total; p-, phosphorylated; p/t ratio, phosphorylated/total ratio of CaMKIIα.
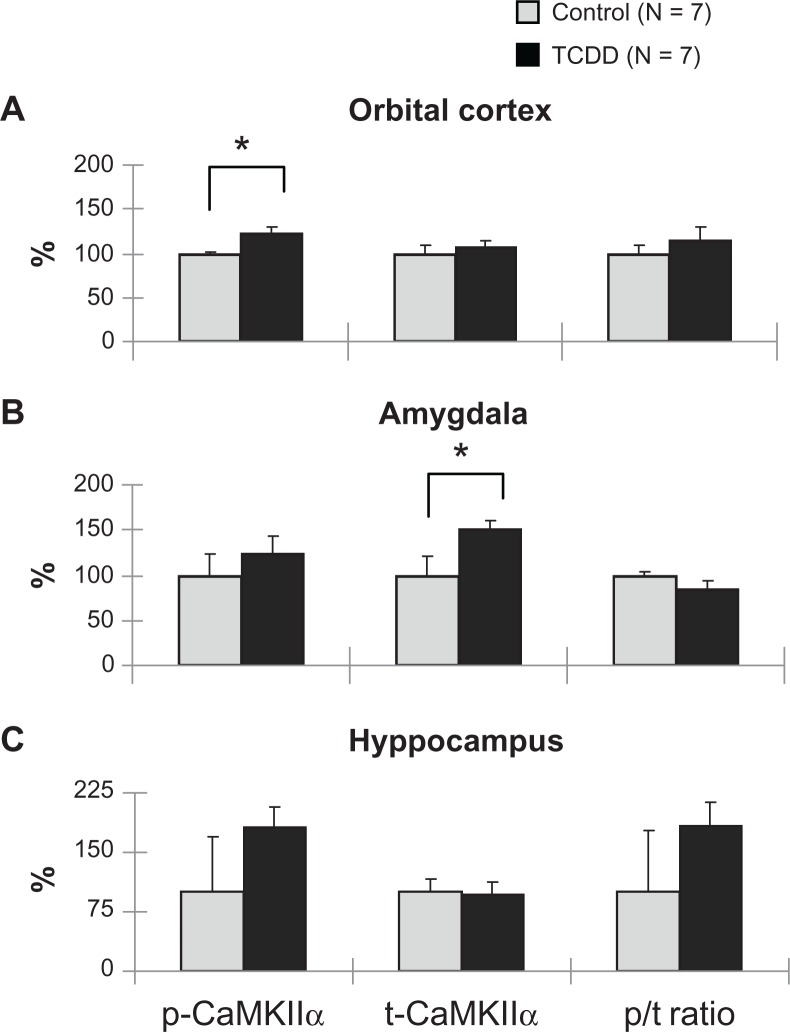
In the female rat offspring, the level of p-CaMKIIα in the orbital cortex was significantly increased (P = 0.012) in the TCDD-exposed group (Fig. 6A), suggesting that TCDD may increase neuronal activity. The level of t-CaMKIIα in the amygdala was also significantly increased (P = 0.011) in the female exposed group (Fig. 6B). In the hippocampus (Fig. 6C), the level of p-CaMKIIα was increased in the female TCDD-exposed group when compared with controls, but the difference was not significant (P = 0.092).
Figure 6.
Effects of dioxin exposure on CaMKIIα in the orbital cortex, amygdala, and hippocampus in the female rat offspring.
Notes: *P < 0.05; significant difference between TCDD-exposed and control groups.
Abbreviations; CaMKIIα, Ca2+/calmodulin-dependent protein kinase IIα; t-, total, p-, phosphorylated; p/t ratio, phosphorylated/total ratio of CaMKIIα.
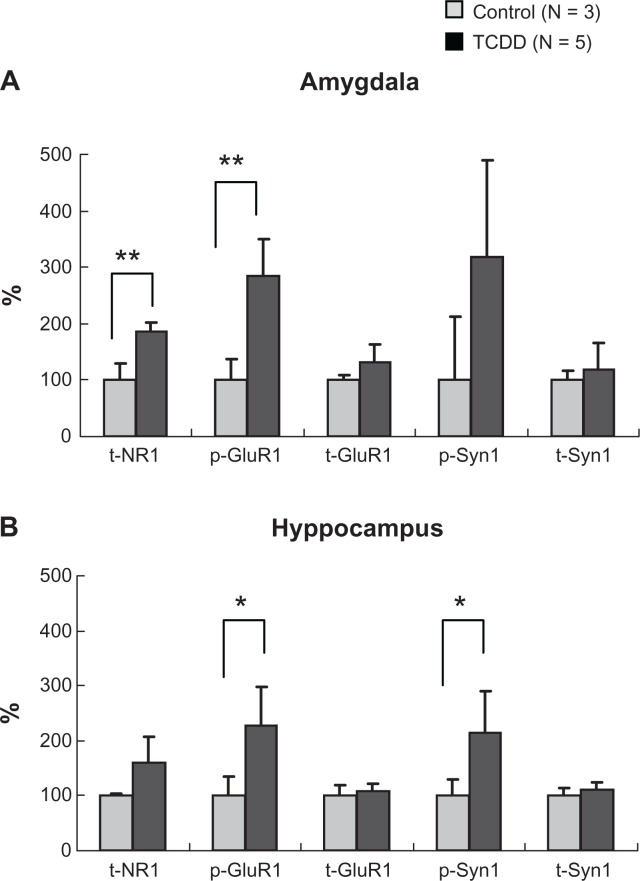
Furthermore, the levels of NR1, GluR1, and Syn 1 were analyzed in three areas of the limbic system in the female offspring rats that showed an increase in CaMKIIα levels. The levels of t-NR1 and p-GluR1 in the amygdale (Fig. 7A; P = 0.002, P = 0.004, respectively), and p-GluR1 and p-Syn1 in the hippocampus (Fig. 7B; P = 0.030, P = 0.049, respectively) were significantly increased in the female TCDD-exposed group when compared with the female control group, while no significant increase was noted in the orbital cortex. These results suggest that activity in the pre- and post-synaptic membrane was increased in the amygdala and hippocampus of the limbic system in female offspring rats.
Figure 7.
Effects of dioxin exposure on NR1, Glu1, and Syn1 in the amygdala and hippocampus in the female rat offspring.
Notes: *P < 0.05, **P < 0.01; significant difference between TCDD-exposed and control groups.
Abbreviations: NR1, N-methyl-D-aspartate (NMDA) type glutamate receptor subunit 1; GluR1, α-amino 3-hydroxy 5-methyl 4-isoxazole propionate (AMPA) type glutamate receptor subunit 1; Syn 1, synapsin I, t-, total; p-, phosphorylated; p/t ratio, phosphorylated/total ratio of CaMKIIα.
Discussion
Effects of TCDD on locomotion and FST
In the present study, duration and frequency of locomotive activity in the open field were higher, and the swimming time on the first day was significantly longer in male and female offspring whose mothers were exposed to TCDD during pregnancy. A previous study reported that an animal model of attention deficit hyperactivity disorder (ADHD) with high locomotor activity displayed longer swimming time in the FST.21 These findings suggest that hyperactivity might be induced by maternal exposure to TCDD in the present rat offspring. In a human survey,22 background levels of exposure to dioxins and PCB increase the risk of developing learning disorders and ADHD in the general population. Yu-Cheng children perinatally exposed to PCBs in Taiwan were more active and had more behavior problems as compared with controls.23 In animal studies, ADHD-like symptoms such as hyperactivity and impulsiveness were observed in perinatally-exposed rat offspring to PCBs.24 Holene et al25–27 found a gender-specific behavior effect of dioxin-like (dl) PCB 153 exposure, which showed similar symptoms of ADHD in male rats. Administration of bisphenol A, one of the endocrine disruptors, to male infant rats induced hyperactivity in childhood, which was caused by a reduction of tyrosine hydroxylase activity in the midbrain.28 In addition, ADHD patients displayed abnormalities in the limbic system, including the amygdala, hippocampus, and prefrontal cortex.29,30 In an animal model of ADHD, ADHD symptoms are suggested to result from impaired dopamine function related to neurotransmission disturbance, such as impaired Ca2+ signaling and poor regulation of norepinephrine release in the prefrontal cortex.31 Consistent with these findings from previous studies, the alteration of activity or levels of CaMKIIα in the amygdala, hippocampus, and orbital frontal cortex (one part of prefrontal cortex) in the offspring rat brain was observed in the present study. Furthermore, CaMKII has also been implicated in the regulation of catecholamine biosynthesis through its impact on tyrosine hydroxylase.32,33 Therefore, these findings suggest that maternal exposure to TCDD might induce ADHD-like behaviors by directly affecting the limbic system or through its effects on tyrosine hydroxylase.
Effects of TCDD exposure on social interaction
The present results showed that the duration of proximity and social contact was significantly lower in the male TCDD-exposed offspring; however, frequency (time) of proximity was significantly higher in the TCDD-exposed group. It is possible that this increase in frequency of proximity may not be related to an increase in social interaction, but rather could be ascribed to an increase in hyperactivity in the TCDD-exposed group, especially in male rats. Consistent with this idea, the duration per one event was markedly decreased in the male TCDD-exposed group, suggesting that the male TCDD-exposed offspring often approached the other rat, but quickly withdrew from that rat as if they were overly cautious. This trend is similar to that seen in platelet-derived growth factor receptor-β gene knockout mice, which have deficits in social interaction and which display an increase in approaching behavior.34 These results suggest that maternal exposure to TCDD decreased affiliative social interaction in male rats. Perinatal exposure to PCBs is also reported to impair the context- or experience-dependent modulation of social approaches and investigation, which is disturbed in autism and other pervasive developmental disorders in rats.35 These results suggested that polychlorinated compounds such as dioxins and PCBs may influence the development of emotional and motivational systems involved in social interactions.
Yamasaki et al36 reported that the genetic alteration of CaMKIIα induces behavioral deficits similar to psychiatric disorders that present with social deficits, such as schizophrenia and autism. Furthermore, chronic treatment with phencyclidine, which induces behavioral deficits similar to schizophrenia, also induces alterations of CaMKII in the limbic part of the prefrontal cortex.37 These findings suggest that maternal exposure to TCDD might induce social deficits through its effects on CaMKIIα in the limbic system. Mitsui et al38 reported that prenatal TCDD exposure impaired activation of cyclic AMP response element-binding protein (CREB) in the hippocampal CA1 region in rat offspring with deficits in learning of fear conditioning. Their results are consistent with our results (which found decreased CaMKIIα activity due to TCDD exposure) because CREB activity is regulated by CaMKIIα.
Recent studies suggest that impairments in gamma-aminobutyric acid (GABAergic) neurotransmission are associated with social disorders in schizophrenia and autism.39–42 Further, CaMKII regulates GABAergic neurotransmission by phosphorylation of GABAergic receptors.43,44 These findings suggest that deficits in GABAergic neurotransmission may also contribute to alteration of CaMKIIα leading to the social abnormalities in the TCDD-exposed rat offspring.
TCDD effects on levels and/or activity of CaMKIIα
In the present study, prenatal exposure to TCDD decreased the levels and/or activity of CaMKIIα in the limbic system of the male offspring brain, but increased CaMKIIα activity in this area of the female offspring. We also confirmed that the levels of phosphorylated synapsin 1, which reflects pre-synaptic activity, and those of phosphorylated NR1 and GluR1, which reflect postsynaptic activity of the limbic system, were increased in the female TCDD-exposed group. These results are consistent with the idea that synaptic activity as well as CaMKIIα activity was increased in the limbic system of female rat brains in response to TCDD exposure.
In a previous study,14 over-phosphorylation of CaMKIIα in the limbic system of the rat brain induced saturation of long-term potentiation, consequently resulting in learning deficits. Furthermore, the effects of CaMKIIα levels on behaviors are complex in that both downregulation and upregulation of CaMKIIα resulted in an increased frequency of aggressive behaviors.15 However, Mizuno and Giese45 reported that male CaMK-kinase knock-out mice show impaired behavior in water maze tests and contextual fear conditioning, but this was not observed in female mice. Therefore, increased CaMKIIα might lead to only slight behavior alterations in the female offspring that were exposed to TCDD in the present study.
Gender difference in effects of dioxin
Adverse effects of maternal exposure to TCDD on social behavior were more evident in the male offspring in both the present study as well as in our previous study, indicating that there is poor learning behavior in TCDD-exposed rat offspring.10 Holene et al25,26 also reported that the male dl-PCB-exposed offspring had an increased frequency of lever press, but not female offspring, suggesting a gender-dependent endocrine disruption effect of TCDD and dl-PCB in rat offspring. Previously, Ikeda et al46 reported that prenatal and lactational TCDD exposure affected intraneuronal conversion of androgen to estrogen in the hypothalamic preoptic area of the fetal brain, and induced demasuculinization in male offspring. Clements et al47 reported that intra-uterine TCDD exposure decreased gonadotropin release in the mediobasal-hypothalamus/preoptic area of male offspring rats. Furthermore, a reduction of metabolic activity in the hypothalamus, but not the pituitary, of male fetal rats was reported by metabolome profile analysis, suggesting the reduced synthesis of gonadotropins.48 These findings suggest that there are gender-dependent adverse effects of TCDD on steroidogenic processes in both prenatal and postnatal periods. On the other hand, sex steroids exert multiple influences on the development of the brain (eg, neurite outgrowth, synaptogenesis, myelination, and so on).49,50 Furthermore, CaMKII, of which activity is modulated by estradiol,51 was reported to be involved in the development of male-specific neural circuitry.52 Taken together, these findings suggest that maternal exposure to TCDD affects genders differently, not only in terms of development of the sexually dimorphic brain regions in the hypothalamus, but also in terms of the development of other brain regions including the amygdala, hippocampus, and the cortical regions with estrogen/androgen receptors and aromatase.53–55 It is important to note that these processes are mediated partly through CaMKII.
Possible molecular mechanisms of gender-specific neuronal and developmental toxicity of TCDD
Gestational and lactational exposure of TCDD has been reported to differentially affect genders differently across survival gene expression of certain brain regions during brain development such as the dioxin-responsive gene CYP1A1 (cytochrome P4501A1), the apoptotic gene Bax, and the antiapoptotic genes Bcl-2 and Bcl-xL.56 Recently, CaMKII is reported to control these apoptotic processes,57 which play significant roles both in normal and neuropathological brain development.58 These findings suggest that TCDD exposure might adversely affect development and differentiation of different brain regions depending on the genders through the alteration of CaMKII activity.
A previous study reported that a single oral dose of TCDD treatment induced AhR, Arnt (aryl hydrocarbon receptor nuclear translocator), and CYP mRNA expression in various brain regions, including the hippocampus and the cortex in rats.59 Normally, AhR and CYP isozymes are involved in the regulation of neurogenesis and differentiation during brain development and during the metabolism of neurotransmitters, endogenous steroids, and neurosteriods in the brain.60–62 Furthermore, AhR mRNA is co-localized with glutamic acid decarboxylase (GAD) 67, which is an enzyme specific for the production of GABA (a marker of GABAergicneurons). Therefore, maternal exposure to TCDD, which reduced GAD67 mRNA expression in the preoptic area of neonatal rat brains in a gender-dependent fashion,63 may affect the development of GABAergic neurons through AhR in a gender-specific manner. These findings suggest that TCDD may also exert a direct adverse effect on the central nervous system, especially on GABAergic neurons in the brain.
Conclusions
TCDD exposure during pregnancy affected the activity and socioemotional behavioral development of offspring rats. This alteration may be partly mediated through the changes in CaMKIIα activity in the limbic system. More studies using animal offspring are necessary to confirm the effects of TCDD exposure on social development (which can lead to the communicative deficits shown in developmental disorders), and to clarify the underlying neural mechanisms.
Supplementary Data
Table S1.
Definition of social behavior in the social interaction test
| Social behavior | Definition and detected criteria |
|---|---|
| Proximity | Two rats stay together, with their inter distance < 60 mm or <100 mm. |
| Approach | The approaching rat moves towards the other rat, with the angle < 45° and at the speed > 30 mm/s and the other rat moves at the speed < 100 mm/s. They travels at least 30 mm and for 2.0 sec continuously, with the distance between the 2 rats to be < 200 mm. |
| Leave | One rat leaves the other rat first, with the angle > 100° and at the speed > 30 mm/s. They travels at least 30 mm for 0.5 sec, with the distance between the 2 rats to be < 200 mm. |
| Social contact | Two rats stay together, with their inter distance < 20 mm. |
| Active social contact | Inter distance needs to be < 20 mm. One rat moves at the speed > 40 mm/s, with the ratio of the “movement” of the active rat vs. the passive rat to be greater than 2.0. |
| Passive social contact | The “movement” of the passive rat in active social contact. |
| Follow | One rat follows the other rat continuously at least for 0.5 sec, with the angle between their direction < 90°, the angle between the direction of the following rat and the line from him/her to the leaving rat < 30°, and the angle between the direction of the leaving rat and the line from him/her to the following rat > 100°. Their distance needs to be < 300 mm. Both rats need to move at least 5 mm, and the following rat needs to travel at least 30 mm. |
| Sniff | The distance between the nose of the sniffing rat and the body of the other rat needs to be < 30 mm, and the rat sniffs continuously at least for 0.27 sec. |
| Mount | Two-thirds of the head/body of 1 rat needs to ride on top of the other rat at least for 0.6s, with joint shape change of two rats. |
Acknowledgments
The authors thank Dr. Jun-ichi Kuriwaki for his valuable advice surrounding the experimental methods, and Miss Akane Hashimoto for her collaboration in the neurochemical analysis.
Footnotes
Funding
This work was supported partly by project research from the Japan Society for the Promotion of Science (Grant-in-Aid for Scientific Research (C) (22590556), Grant-in-Aid for Scientific Research (A) (22240051)), and JSPS (Japan Society for the Promotion of Science) Asian Core Program.
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of manuscript.
Author Contributions
Conceived and designed the experiments: MN, HNi. Analyzed the data: EH, TTP, KF. Wrote the first draft of the manuscript: ATNN. Contributed to the writing of the manuscript: ATNN, NMN, HNa, AHT, HNi. Jointly developed structure and arguments for the paper: AHT, HNa, TTP. Made critical revisions and approved final version: ATNN, MN, EH. All authors reviewed and approved of the final manuscript.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributor-ship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Rogan WJ, Gladen BC, Hung KL, et al. Congenital poisoning by polychlorinated biphenyls and their contaminations in Taiwan. Science. 1988;241(4863):334–6. doi: 10.1126/science.3133768. [DOI] [PubMed] [Google Scholar]
- 2.Chen YJ, Guo YL, Hsu CC. Cognitive development of children prenatally exposed to polychlorinated biphenyls (Yu-Cheng children) and their siblings. J Formos Med Assoc. 1992;91(7):704–7. [PubMed] [Google Scholar]
- 3.Koopman-Esseboom C, Weisglas-Kuperus N, deRidder MA, VanderPaauw CG, Tuinstra LG, Sauer PJ. Effects of polychlorinated biphenyl/dioxin exposure and feeding type on infants’ mental and psychomotor development. Pediatrics. 1996;97(5):700–6. [PubMed] [Google Scholar]
- 4.Nakajima S, Saijo Y, Kato S, et al. Effects of prenatal exposure to polychlorinated biphenyls and dioxins on mental and motor development in Japanese children at 6 months of age. Environ Health Perspect. 2006;114(5):773–8. doi: 10.1289/ehp.8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pham TT, Nishijo M, Nakagawa H, et al. Associations of perinatal dioxin exposure on neurodevelopment of Vietnamese infants. Am J Epidemiol. 2013. (in press).
- 6.Seo BW, Sparks AJ, Medora K, Amin S, Schantz SL. Learning and memory in rats gestationally and lactationally exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Neurotoxicol Teratol. 1999;21(3):231–9. doi: 10.1016/s0892-0362(98)00049-x. [DOI] [PubMed] [Google Scholar]
- 7.Markowski VP, Zareba G, Stern S, Cox C, Weiss B. Altered operant responding to motor reinforcement and the determination of benchmark dosed following perinatal exposure to low-level 2,3,7,8-tetrachlorodibenzo-p-dioxin. Environ Health Perspect. 2001;109(6):621–7. doi: 10.1289/ehp.01109621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markowski VP, Cox C, Preston R, Weiss B. Impaired cued delayed alternation behavior in adult rat offspring following exposure to 2,3,7,8-tet-rachlorodibenzo-p-dioxin on gestation day 15. Neurotoxicol Teratol. 2002;24(2):209–18. doi: 10.1016/s0892-0362(02)00186-1. [DOI] [PubMed] [Google Scholar]
- 9.Schantz SL, Bowman RE. Learning in monkeys exposed perinatally to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Neurotoxicol Teratol. 1989;11(1):13–9. doi: 10.1016/0892-0362(89)90080-9. [DOI] [PubMed] [Google Scholar]
- 10.Nishijo M, Kuriwaki J, Hori E, Tawara K, Nakagawa H, Nishijo H. Effects of maternal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin on fetal brain growth and motor and behavioral development in offspring rats. Toxicol Lett. 2007;173(1):41–7. doi: 10.1016/j.toxlet.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Brunet-Gouet E, Decety J. Social brain dysfunctions in schizophrenia: a review of neuroimaging studies. Psychiatry Res. 2006;148(2–3):75–92. doi: 10.1016/j.pscychresns.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Blakemore SJ. The social brain in adolescence. Nat Rev Neurosci. 2008;9(4):267–77. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- 13.Canteras NS, Resstel LB, Bertoglio LJ, Carobrez Ade P, Guimarães FS. Neuroanatomy of anxiety. Curr Top Behav Neurosci. 2010;2:77–96. doi: 10.1007/7854_2009_7. [DOI] [PubMed] [Google Scholar]
- 14.Kaitsuka T, Fukunaga K, Soeda F, Shirasaki T, Miyamoto E, Takahama K. Changes in Ca(2+)/calmodulin-dependent protein kinase II activity and its relation to performance in passive avoidance response and long-term potentiation formation in mice prenatally exposed to diethylstilbestrol. Neurosci. 2007;144(4):1415–24. doi: 10.1016/j.neuroscience.2006.10.051. [DOI] [PubMed] [Google Scholar]
- 15.Hasegawa S, Furuichi T, Yoshida T, et al. Transgenic up-regulation of alpha-CaM-KII in forebrain leads to increased anxiety and aggression. Mol Brain. 2009;2:6. doi: 10.1186/1756-6606-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kakeyama M, Tohyama C. Developmental neurotoxicity of dioxin and its related compounds. Ind Health. 2003;41(3):215–30. doi: 10.2486/indhealth.41.215. [DOI] [PubMed] [Google Scholar]
- 17.Fukunaga K, Goto S, Miyamoto E. Immunohistochemical localization of Ca2+/calmodulin-dependent protein kinase II in rat brain and various tissues. J Neurochem. 1988;51(4):1070–8. doi: 10.1111/j.1471-4159.1988.tb03070.x. [DOI] [PubMed] [Google Scholar]
- 18.Fukunaga K, Horikawa K, Shibata S, Takeuchi Y, Miyamoto E. Ca2+/calmodulin-dependent protein kinase II-dependent long-term potentiation in the rat suprachiasmatic nucleus and its inhibition by melatonin. J Neurosci Res. 2002;70(6):799–807. doi: 10.1002/jnr.10400. [DOI] [PubMed] [Google Scholar]
- 19.Moriguchi S, Shioda N, Yamamoto Y, Tagashira H, Fukunaga K. The T-type voltage-gated calcium channel as a molecular target of the novel cognitive enhancer ST101: enhancement of long-term potentiation and CaMKII auto-phosphorylation in rat cortical slices. J Neurochem. 2012;121(1):44–53. doi: 10.1111/j.1471-4159.2012.07667.x. [DOI] [PubMed] [Google Scholar]
- 20.Fukunaga K, Soderling TR, Miyamoto E. Activation of Ca2+/calmodulin-dependent protein kinase II and protein kinase C by glutamate in cultured rat hippocampal neurons. J Biol Chem. 1992;267(31):22527–33. [PubMed] [Google Scholar]
- 21.Sterley TL, Howells FM, Russell VA. Effects of early life trauma are dependent on genetic predisposition: a rat study. Behav Brain Funct. 2011;7:11. doi: 10.1186/1744-9081-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee DH, Jacobs DR, Porta M. Association of serum concentrations of persistent organic pollutants with the prevalence of learning disability and attention deficit disorder. J Epidemiol Community Health. 2007;61(7):591–6. doi: 10.1136/jech.2006.054700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen YC, Yu ML, Rogan WJ, Gladen BC, Hsu CC. A 6-year follow-up of behavior and activity disorders in the Taiwan Yu-cheng children. Am J Public Health. 1994;84(3):415–21. doi: 10.2105/ajph.84.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daly GB, Hertzler DR, Sargent DM. Ingestion of environmentally contaminated Lake Ontario salmon by laboratory rats increases avoidance of unpredictable aversive nonreward and mild electric shock. Behav Neurosci. 1989;103(6):1356–65. doi: 10.1037//0735-7044.103.6.1356. [DOI] [PubMed] [Google Scholar]
- 25.Holene E, Nafstad I, Skaare JU, Bernholf A, Engen P, Sagvolden T. Behavioral effects of pre- and postnatal exposure to individual polychlorinated biphenyl congeners in rats. Environ Toxicol Chem. 1995;14(6):967–76. [Google Scholar]
- 26.Holene E, Nafstad I, Skaare JU, Sagvolden T. Behavioural hyperactivity in rats following postnatal exposure to sub-toxic doses of polychlorinated biphenyl congeners 153 and 126. Behav Brain Res. 1998;94(1):213–24. doi: 10.1016/s0166-4328(97)00181-2. [DOI] [PubMed] [Google Scholar]
- 27.Holene E, Nafstad I, Skaare JU, Krogh H, Sagvolden T. Behavioural effects in female rats of postnasal exposure to sub-toxic doses of polychlorinated biphenyls congener 153. Acta Paediatr Suppl. 1999;88(429):55–63. doi: 10.1111/j.1651-2227.1999.tb01291.x. [DOI] [PubMed] [Google Scholar]
- 28.Ishido M, Masuo Y, Kunimoto M, Oka S, Morita M. Bisphenol A causes hyperactivity in the rat concomitantly with impairment of tyrosine hydroxylase immunoreactivity. J Neurosci Res. 2004;76(3):423–33. doi: 10.1002/jnr.20050. [DOI] [PubMed] [Google Scholar]
- 29.Shaw P, Rabin C. New insights into attention-deficit/hyperactivity disorder using structural neuroimaging. Curr Psychiatry Rep. 2009;11(5):393–8. doi: 10.1007/s11920-009-0059-0. [DOI] [PubMed] [Google Scholar]
- 30.Frodl T, Stauber J, Schaaff N, et al. Amygdala reduction in patients with ADHD compared with major depression and healthy volunteers. Acta Psychiatr Scand. 2010;121(2):111–8. doi: 10.1111/j.1600-0447.2009.01489.x. [DOI] [PubMed] [Google Scholar]
- 31.Russell VA, Sagvolden T, Johansen EB. Animal models of attention-deficit hyperactivity disorder. Behav Brain Funct. 2005;1:9. doi: 10.1186/1744-9081-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamauchi T, Fujisawa H. Evidence for three distinct forms of calmodulin-dependent protein kinases from rat brain. FEBS Lett. 1980;116(2):141–4. doi: 10.1016/0014-5793(80)80628-4. [DOI] [PubMed] [Google Scholar]
- 33.Yamauchi T, Nakata H, Fujisawa H. A new activator protein that activates tryptophan 5-monooxygenase and tyrosine 3-monooxygenase in the presence of Ca2+-, calmodulin-dependent protein kinase. Purification and characterization. J Biol Chem. 1981;256(11):5404–9. [PubMed] [Google Scholar]
- 34.Nguyen PT, Nakamura T, Hori E, et al. Cognitive and socio-emotional deficits in platelet-derived growth factor receptor-β gene knockout mice. PLoS One. 2011;6(3):e18004. doi: 10.1371/journal.pone.0018004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joluous-Jamshidi B, Cromwell HC, McFarland AM, Meserve LA. Perinatal exposure to polychlorinated biphenyls alters social behaviors in rats. Toxicol Lett. 2010;199(2):136–43. doi: 10.1016/j.toxlet.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamasaki N, Maekawa M, Kobayashi K, et al. Alpha-CaMKII deficiency causes immature dentate gyrus, a novel candidate endophenotype of psychiatric disorders. Mol Brain. 2008;1:6. doi: 10.1186/1756-6606-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murai R, Noda Y, Matsui K, et al. Hypofunctional glutamatergic neurotransmission in the prefrontal cortex is involved in the emotional deficit induced by repeated treatment with phencyclidine in mice: implications for abnormalities of glutamate release and NMDA-CaMKII signaling. Behav Brain Res. 2007;180(2):152–60. doi: 10.1016/j.bbr.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Mitsui T, Sugiyama N, Maeda S, Tohyama C, Arita J. Perinatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin suppresses contextual fear conditioning-accompanied activation of cyclic AMP response element-binding protein in the hippocampal CA1 region of male rats. Neurosci Lett. 2006;398(3):206–10. doi: 10.1016/j.neulet.2005.12.087. [DOI] [PubMed] [Google Scholar]
- 39.Pollack MH, Jensen JE, Simon NM, Kaufman RE, Renshaw PF. High-field MRS study of GABA, glutamate and glutamine in social anxiety disorder: response to treatment with levetiracetam. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(3):739–43. doi: 10.1016/j.pnpbp.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 40.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nature Rev. 2005;6(4):312–24. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 41.Gogolla N, Leblanc JJ, Quast KB, Südhof TC, Fagiolini M, Hensch TK. Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J Neurodev Disord. 2009;1(2):172–81. doi: 10.1007/s11689-009-9023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29(8):2344–54. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Houston CM, He Q, Smart TG. CaMKII phosphorylation of the GABA(A) receptor: receptor subtype- and synapse-specific modulation. J Physiol. 2009;587(Pt 10):2115–25. doi: 10.1113/jphysiol.2009.171603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guetg N, Abdel Aziz S, Holbro N, et al. NMDA receptor-dependent GABAB receptor internalization via CaMKII phosphorylation of serine 867 in GABAB1. Proc Natl Acad Sci U S A. 2010;107(31):13924–9. doi: 10.1073/pnas.1000909107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mizuno K, Giese KP. Towards a molecular understanding of sex differences in memory formation. Trends Neurosci. 2010;33(6):285–91. doi: 10.1016/j.tins.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Ikeda M, Mitsui T, Setani K, et al. In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats disrupts brain sexual differentiation. Toxicol Appl Pharmacol. 2005;205(1):98–105. doi: 10.1016/j.taap.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 47.Clements RJ, Lawrence RC, Blank JL. Effects of intrauterine 2,3,7,8-tetrachlorodibenzo-p-dioxin on the development and function of the gonadotrophin releasing hormone neuronal system in the male rat. Reprod Toxicol. 2009;28(1):38–45. doi: 10.1016/j.reprotox.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 48.Matsumoto Y, Ishida T, Takeda T, et al. Maternal exposure to dioxin reduced hypothalamic but not pituitary metabolome in fetal rats: a possible mechanism for fetus-specific reduction in steroidogenesis. J Toxicol Sci. 2010;35(3):365–73. doi: 10.2131/jts.35.365. [DOI] [PubMed] [Google Scholar]
- 49.McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88(1):91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peper JS, van den Heuvel MP, Mandl RC, Hulshoff Pol HE, van Honk J. Sex steroids and connectivity in the human brain: a review of neuroimaging studies. Psychoneuroendocrinology. 2011;36(8):1101–13. doi: 10.1016/j.psyneuen.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 51.Sawai T, Bernier F, Fukushima T, Hashimoto T, Ogura H, Nishizawa Y. Estrogen induces a rapid increase of calcium-calmodulin-dependent protein kinase II activity in the hippocampus. Brain Res. 2002;950(1–2):308–11. doi: 10.1016/s0006-8993(02)03186-4. [DOI] [PubMed] [Google Scholar]
- 52.Hein AM, Sridharan A, Nordeen KW, Nordeen EJ. Characterization of CaMKII-expressing neurons within a striatal region implicated in avian vocal learning. Brain Res. 2007;1155:125–33. doi: 10.1016/j.brainres.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 53.Wagner C, Morrell JI. Distribution and steroid hormone regulation of aromatase mRNA expression in the forebrain of adult male and female rats: a cellular-level analysis using in situ hybridization. J Comp Neurology. 1995;370(1):71–84. doi: 10.1002/(SICI)1096-9861(19960617)370:1<71::AID-CNE7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 54.Wagner CK, Morrell JI. Neuroanatomical distribution of aromatase MRNA in the rat brain: indications of regional regulation. J Steroid Biochem Mol Biol. 1997;61(3–6):307–14. [PubMed] [Google Scholar]
- 55.Handa RJ, Price RH, Jr, Wilson ME. Steroid hormone receptors in the developing brain. Biomedical reviews. 1997;7:51–66. [Google Scholar]
- 56.Chang SF, Sun YY, Yang LY, et al. Bcl-2 gene family expression in the brain of rat offspring after gestational and lactational dioxin exposure. Ann N Y Acad Sci. 2005;1042:471–80. doi: 10.1196/annals.1338.040. [DOI] [PubMed] [Google Scholar]
- 57.Kajihara R, Fukushige S, Shioda N, Tanabe K, Fukunaga K, Inui S. CaMKII phosphorylates serine 10 of p27 and confers apoptosis resistance to HeLa cells. Biochem Biophys Res Commun. 2010;40(3):350–5. doi: 10.1016/j.bbrc.2010.09.051. [DOI] [PubMed] [Google Scholar]
- 58.Roth KA, D’Sa C. Apoptosis and brain development. Ment Retard Dev Disabil Res Rev. 2001;7(4):261–6. doi: 10.1002/mrdd.1036. [DOI] [PubMed] [Google Scholar]
- 59.Huang P, Rannug A, Ahlbom E, Håkansson H, Ceccatelli S. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the expression of cytochrome P4501A1, the aryl hydrocarbon receptor, and the aryl hydrocarbon receptor nuclear translocator in rat brain and pituitary. Toxicol Appl Pharmacol. 2000;169(2):159–67. doi: 10.1006/taap.2000.9064. [DOI] [PubMed] [Google Scholar]
- 60.Miksys SL, Tyndale RF. Drug-metabolizing cytochrome P450 s in the brain. J Psychiatry Neurosci. 2002;27(6):406–15. [PMC free article] [PubMed] [Google Scholar]
- 61.Williamson MA, Gasiewicz TA, Opanashuk LA. Aryl hydrocarbon receptor expression and activity in cerebellar granule neuroblasts: implications for development and dioxin neurotoxicity. Toxicol Sci. 2005;83(2):340–8. doi: 10.1093/toxsci/kfi031. [DOI] [PubMed] [Google Scholar]
- 62.Gohlke JM, Stockton PS, Sieber S, Foley J, Portier CJ. AhR-mediated gene expression in the developing mouse telencephalon. Reprod Toxicol. 2009;28(3):321–8. doi: 10.1016/j.reprotox.2009.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hays LE, Carpenter CD, Petersen SL. Evidence that GABAergic neurons in the preoptic area of the rat brain are targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin during development. Environ Health Perspect. 2002;110(Suppl 3):369–76. doi: 10.1289/ehp.02110s3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Definition of social behavior in the social interaction test
| Social behavior | Definition and detected criteria |
|---|---|
| Proximity | Two rats stay together, with their inter distance < 60 mm or <100 mm. |
| Approach | The approaching rat moves towards the other rat, with the angle < 45° and at the speed > 30 mm/s and the other rat moves at the speed < 100 mm/s. They travels at least 30 mm and for 2.0 sec continuously, with the distance between the 2 rats to be < 200 mm. |
| Leave | One rat leaves the other rat first, with the angle > 100° and at the speed > 30 mm/s. They travels at least 30 mm for 0.5 sec, with the distance between the 2 rats to be < 200 mm. |
| Social contact | Two rats stay together, with their inter distance < 20 mm. |
| Active social contact | Inter distance needs to be < 20 mm. One rat moves at the speed > 40 mm/s, with the ratio of the “movement” of the active rat vs. the passive rat to be greater than 2.0. |
| Passive social contact | The “movement” of the passive rat in active social contact. |
| Follow | One rat follows the other rat continuously at least for 0.5 sec, with the angle between their direction < 90°, the angle between the direction of the following rat and the line from him/her to the leaving rat < 30°, and the angle between the direction of the leaving rat and the line from him/her to the following rat > 100°. Their distance needs to be < 300 mm. Both rats need to move at least 5 mm, and the following rat needs to travel at least 30 mm. |
| Sniff | The distance between the nose of the sniffing rat and the body of the other rat needs to be < 30 mm, and the rat sniffs continuously at least for 0.27 sec. |
| Mount | Two-thirds of the head/body of 1 rat needs to ride on top of the other rat at least for 0.6s, with joint shape change of two rats. |