90.1 Introduction
In the mammalian retina, rod photoreceptors shed the tips of their outer segments (POS) each morning following light onset (Young 1967). These POS are swiftly removed by the adjacent retinal pigment epithelium (RPE) via phagocytosis that involves coordinated activities of numerous membrane and cytoplasmic proteins (Young and Bok 1969). RPE cells in the healthy retina complete uptake and digestion of shed rod POS within a few hours after POS shedding. We previously found that RPE cells in culture fail to tether isolated POS for subsequent phagocytosis within about 12 h of a previous phagocytic challenge, although the principal tethering receptor for POS, αvβ5 integrin, remains abundant at the apical, phagocytic surface of these RPE cells (Finnemann 2003b). These data suggest that RPE cells may increase or decrease their phagocytic competence by altering the activity of the POS-binding receptor αvβ5 integrin. Indeed, RPE cells that lack αvβ5 do not phagocytose shed POS in a diurnal rhythm, but exhibit constant levels of POS uptake at all times of day (Nandrot et al. 2004).
The molecular mechanism of particle recognition, tethering, and phagocytosis of RPE cells is similar to the molecular mechanisms used by bone marrow-derived macrophages and dendritic cells for removal of apoptotic cells. Like RPE cells, dendritic cells employ αvβ5 integrin, while macrophages mainly use the related αvβ3 but can be stimulated to use αvβ5 as well (Finnemann et al. 1997; Albert et al. 1998; Finnemann and Rodriguez-Boulan 1999; Lucas et al. 2006). In all three cell types, the phagocytic particle binds to the integrin-tethering receptor indirectly via a soluble bridge protein, like the retina’s MFG-E8, that contains both phosphatidyl-serine (PS) and integrin receptor-binding domains (Hanayama et al. 2002; Nandrot et al. 2007). Exposure of PS as an “eat me” signal by cells undergoing apoptosis has been studied extensively and is known to be sufficient for their engulfment by phagocytic cells (Schlegel and Williamson 2001). Notably, the specific molecular changes designating shedding POS tips in the retina remain poorly understood in comparison.
Little is known about the regulation of integrin receptor activity in preparation for or subsequent to phagocytic particle binding. The multifunctional soluble protein transglutaminase 2 (TG2) may act as coreceptor of β1 or β3 integrins (Akimov et al. 2000). Studies exploring TG2−/− mice have recently shown that a complex of secreted TG2, MFG-E8, and αvβ3 promotes clearance of apoptotic cells in macrophages (Toth et al. 2009). Interestingly, TG2 has been found to be associated with β5 integrins on the surface of malignant melanoma cells, although its function in this system remains unknown (Fok et al. 2006). Depending on tissue and cell type, TG2 may localize intracellularly in the cytoplasm or nucleus, as well as extracellularly on the cell surface or in the extracellular matrix (Upchurch et al. 1991; Lesort et al. 1998; Verderio et al. 1998). Here, we report TG2 expression, localization, and relation to αvβ5 integrin in murine retina and RPE.
90.2 Materials and Methods
90.2.1 Animals and Tissue Collection
Wild-type Long Evans rats, wild-type 129T2/SvEmsJ mice, and β5−/− mice in the same background characterized previously (Nandrot et al. 2004, 2007) were housed under cyclic 12 h light: 12 h dark conditions and fed ad libitum. Three-month-old mice were sacrificed by CO2 asphyxiation for tissue collection. Briefly, eyes were enucleated immediately postmortem. Following opening of cornea and removal of the lens, whole eyecups were either lysed or fixed as described below. Neural retina and eyecups with remaining RPE and choroid were isolated from whole eyecups by first incubating whole eyecups in Hanks buffer without Ca 2+and Mg 2+ for 5 min before performing radial cuts toward the optic nerve, flattening eyecups in a dry petri dish, and removing the neural retina with a forceps with bent tip. Primary RPE was isolated from eyes of 10-day-old rat pups following a two-step protocol using hyaluronidase and trypsin described previously (Finnemann 2003a) and seeded on coverslips. All procedures were approved by the Fordham University Institutional Animal Care and Use Committee and adhered to the ARVO statement for the use of animals in ophthalmic and vision research.
90.2.2 Immunofluorescence Microscopy
Whole eyecups were incubated in 4% paraformaldehyde in 1X PBS for 10 min at RT. The lens was removed and the remaining retina and eyecup were incubated in 15% sucrose, 30% sucrose, and frozen in OCT. Primary rat RPE cells were fixed in ice-cold 95% ethanol, 5% acetic acid. 15 μm-thick eyecup cross-sections or primary RPE were labeled with TG2 antibody (Santa Cruz Biotechnology) and secondary antibody conjugated to AlexaFluor488. Primary RPE was further labeled with β-catenin antibody (Sigma) and secondary antibody conjugated to AlexaFluor568. Nuclei were stained with DAPI. Mounted coverslips or sections were imaged on a Leica TSP5 confocal microscopy system.
90.2.3 Immunoblotting
Tissue samples were solubilized in 50 mM HEPES (pH 7.4), 150 mM NaCl, 10% glycerol, 1.5 mM MgCl2, and 1% Triton X-100 with 1% of protease inhibitor cock-tail (Sigma). Cleared lysates of whole mouse eyecups, isolated mouse neural retina, or eyecup without neural retina were separated on 10% SDS-polyacrylamide gels and electroblotted. Immunoblots were probed with primary antibodies to TG2, β5 integrin (both Santa Cruz Biotechnology), or β-actin (Sigma), and processed for enhanced chemiluminescence detection.
90.3 Results
90.3.1 Comparison of TG2 Protein Levels of Wild-Type and β5−/− Mouse Eyecups
We first performed immunoblotting experiments to test if TG2 expression can be detected in the mouse eye. Figure 90.1 shows that TG2 antibody detects two bands in whole eyecup lysates migrating at ~80 kDa, the expected molecular size of mouse TG2, and an additional band at ~110 kDa that, to our knowledge, had not been reported previously. We subsequently incubated the same protein blots for β5 integrin and for β-actin. These controls confirmed the genotype of our samples and that both samples had approximately the same total protein content. These results demonstrate equal levels of TG2 in whole eye lysates obtained from wild-type and β5−/− mice, indicating that loss of the integrin does not affect steady state levels of TG2.
Fig. 90.1.
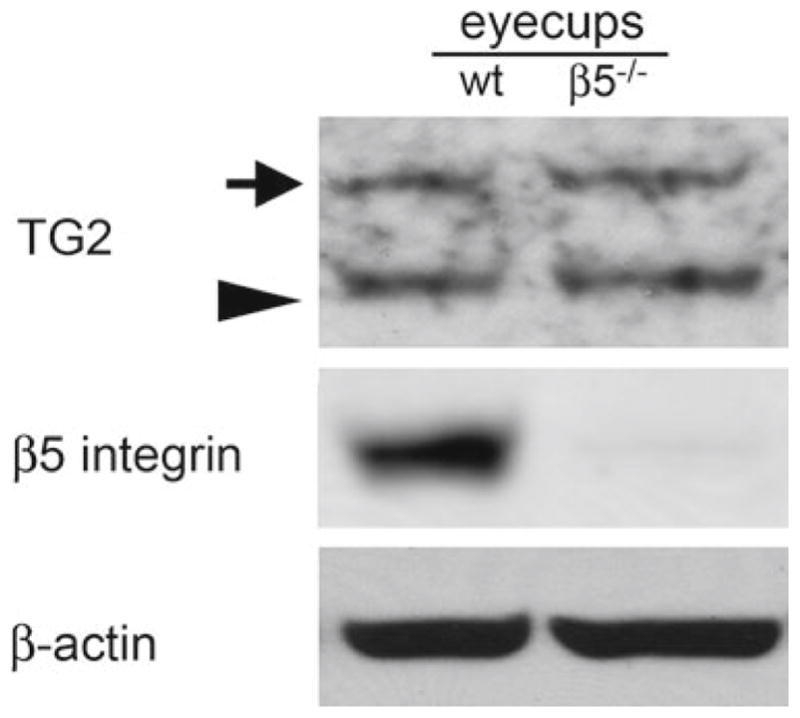
Comparison of TG2 levels in wild-type and β5−/− eyecups. TG2 immunoblotting of whole eyecup samples from wild-type (wt) and β5−/− yields two bands, at the expected molecular size of ~80 kDa (arrow head) and, unexpectedly, at ~110 kDa (arrow). Reprobing of the same blot for β5 integrin and β-actin blotting confirmed genotype and equal load of samples (lanes as indicated)
90.3.2 Localization of TG2 in Wild-Type Mouse Retina
To determine the localization of TG2 in the mouse retina, we next performed TG2 immunofluorescence labeling of wild-type mouse eye cryosections. We observed TG2 at the basal aspect of the RPE (arrow), but did not detect it in the photoreceptor outer segment region (Fig. 90.2a). We did not observe TG2 staining in other regions of the neural retina either (data not shown). To confirm this result, we analyzed isolated neural retina and eyecups without neural retina, but containing RPE and choroid of wild-type mice by immunoblotting. We found that TG2 at the expected molecular size of 72 kDa (arrow head) is concentrated in the RPE/choroid containing eyecups and not detected in the neural retina fraction (Fig. 90.2b). The unexpected 110 kDa band of the TG2 blot (arrow) was detected only in the neural retina fraction. Whether or not this band represents a form of TG2 remains unknown. Taken together, immunofluorescence microscopy and tissue fractionation experiments suggest that TG2 in the mouse eyecup is mostly expressed by the RPE.
Fig. 90.2.
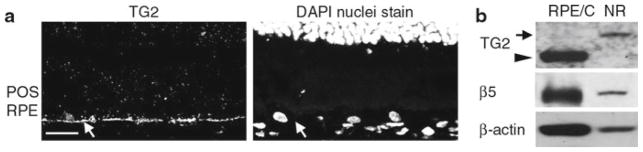
Expression and localization of TG2 in mouse RPE in situ. (a) Image shows a representative cross-section of 3-month-old wild-type mouse retina labeled for TG2 (left panel) and counter-stained with DAPI (right panel). Staining for TG2 is visible in the RPE (arrow), but not in the outer segment region. Scale bar equals 20 μm. (b) Comparison of TG2 levels in wild-type mouse eyecup fractions containing either neural retina (NR) or RPE and choroid (RPE/C). TG2 of expected molecular size of 80 kDa is detected only in the RPE and choroid (arrowhead), while the unidentified band at 110 kDa is detected only in the NR (arrow)
90.3.3 Localization of TG2 in Wild-Type Rat RPE in Primary Culture
RPE cells in primary culture may differ from RPE cells in the eye with respect to protein expression or subcellular localization. To determine if this was the case for TG2, we seeded isolated patches of wild-type rat RPE on coverslips and studied their TG2 expression using immunofluorescence microscopy. Figure 90.3A1 shows cytoplasmic TG2 protein distribution in primary RPE that is markedly enriched at lateral membranes. There, TG2 partially overlaps with the cytoplasmic protein β-catenin that is part of the lateral adherens junction complex (compare Fig. 90.3A1 with Fig. 90.3A2). TG2 staining was specific as incubation of fixed cells with secondary antibody alone did not produce labeling (Fig. 90.3B1).
Fig. 90.3.
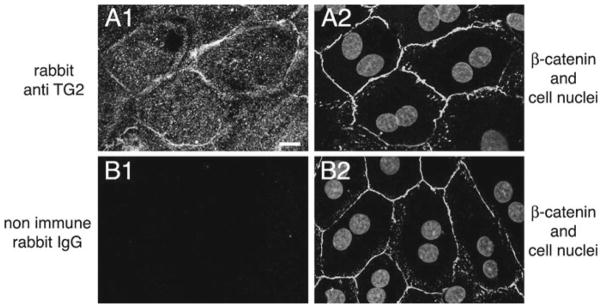
Localization of TG2 in rat primary RPE. A1, A2: images show a representative field labeled with TG2 (A1) and β-catenin (adherens junctions) antibodies and DAPI (nuclei) (overlay of both in A2). Scale bar equals 10 μm. B1, B2: images show a representative field labeled with nonimmune rabbit IgG (B1), β-catenin antibody, and DAPI (overlay of both in B2) demonstrating specificity of the TG2 signal in A1
90.4 Discussion
The association of TG2 with β integrins and their ligands and its role in the phagocytosis of apoptotic cells by macrophages led us to speculate that TG2 may also play a role in the avβ5 integrin-dependent phagocytosis of POS by RPE cells. Here, we determined TG2 expression in whole eyecup lysates from β5−/− mice and found that they do not differ from wild-type mouse eyecups in TG2 expression levels. Furthermore, we examined TG2 expression in the mouse eye by immunohistochemistry and by immunoblotting and found that TG2 is present in the RPE but is absent from its apical, phagocytic surface and from photoreceptor outer segments. Finally, RPE cells in primary culture, which possess apical αvβ5 integrin and vigorous phagocytic activity toward experimental particles, retain basolateral TG2 expression. Taken together, these results suggest that TG2 is unlikely to form a direct complex with the phagocytic machinery. Rather, TG2 may participate in processes specific to the basolateral aspect of the RPE. TG2 has been shown to regulate adhesion to fibronectin by acting as coreceptor of β3 and β1 integrins in fibroblasts (Akimov et al. 2000). RPE cells possess a number of basolateral integrins that mediate adhesion to Bruch’s membrane components (Finnemann et al. 1997). It is thus possible that TG2 regulates RPE interactions with its extracellular matrix. Alternately, TG2 in the RPE may function independently of integrins.
Acknowledgments
These studies were supported by NIH grant EY-13295 to S.C.F.
References
- Akimov SS, Krylov D, Fleischman LF, et al. Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin. J Cell Biol. 2000;148:825–838. doi: 10.1083/jcb.148.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert ML, Pearce SFA, Francisco LM, et al. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann SC. Focal adhesion kinase signaling promotes phagocytosis of integrin-bound photoreceptors. EMBO J. 2003a;22:4143–4154. doi: 10.1093/emboj/cdg416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann SC. Role of αvβ5 integrin in regulating phagocytosis by the retinal pigment epithelium. Adv Exp Med Biol. 2003b;533:337–342. doi: 10.1007/978-1-4615-0067-4_42. [DOI] [PubMed] [Google Scholar]
- Finnemann SC, Rodriguez-Boulan E. Macrophage and retinal pigment epithelium phagocytosis: apoptotic cells and photoreceptors compete for αvβ3 and αvβ5 integrins, and protein kinase C regulates αvβ5 binding and cytoskeletal linkage. J Exp Med. 1999;190:861–874. doi: 10.1084/jem.190.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann SC, Bonilha VL, Marmorstein AD, et al. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires αvβ5 integrin for binding but not for internalization. Proc Natl Acad Sci USA. 1997;94:12932–12937. doi: 10.1073/pnas.94.24.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fok JY, Ekmekcioglu S, Mehta K. Implications of tissue transglutaminase expression in malignant melanoma. Mol Cancer Ther. 2006;5:1493–1503. doi: 10.1158/1535-7163.MCT-06-0083. [DOI] [PubMed] [Google Scholar]
- Hanayama R, Tanaka M, Miwa K, et al. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- Lesort M, Attanavanich K, Zhang J, et al. Distinct nuclear localization and activity of tissue transglutaminase. J Biol Chem. 1998;273:11991–11994. doi: 10.1074/jbc.273.20.11991. [DOI] [PubMed] [Google Scholar]
- Lucas M, Stuart LM, Zhang A, et al. Requirements for apoptotic cell contact in regulation of macrophage responses. J Immunol. 2006;177:4047–4054. doi: 10.4049/jimmunol.177.6.4047. [DOI] [PubMed] [Google Scholar]
- Nandrot EF, Kim Y, Brodie SE, et al. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking αvβ5 integrin. J Exp Med. 2004;200:1539–1545. doi: 10.1084/jem.20041447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandrot EF, Anand M, Almeida D, et al. Essential role for MFG-E8 as ligand for αvβ5 integrin in diurnal retinal phagocytosis. Proc Natl Acad Sci USA. 2007;104:12005–12010. doi: 10.1073/pnas.0704756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel RA, Williamson P. Phosphatidylserine, a death knell. Cell Death Differ. 2001;8:551–563. doi: 10.1038/sj.cdd.4400817. [DOI] [PubMed] [Google Scholar]
- Toth B, Garabuczi E, Sarang Z, et al. Transglutaminase 2 is needed for the formation of an efficient phagocyte portal in macrophages engulfing apoptotic cells. J Immunol. 2009;182:2084–2092. doi: 10.4049/jimmunol.0803444. [DOI] [PubMed] [Google Scholar]
- Upchurch HF, Conway E, Patterson MK, Jr, et al. Localization of cellular transglutaminase on the extracellular matrix after wounding: characteristics of the matrix bound enzyme. J Cell Physiol. 1991;149:375–382. doi: 10.1002/jcp.1041490304. [DOI] [PubMed] [Google Scholar]
- Verderio E, Nicholas B, Gross S, et al. Regulated expression of tissue transglutaminase in Swiss 3 T3 fibroblasts: effects on the processing of fibronectin, cell attachment, and cell death. Exp Cell Res. 1998;239:119–138. doi: 10.1006/excr.1997.3874. [DOI] [PubMed] [Google Scholar]
- Young RW. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967;33:61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol. 1969;42:392– 403. doi: 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]


