93.1 Introduction
In mammals, diurnal phagocytosis of shed photoreceptor outer segments (POS) by the retinal pigment epithelium (RPE) is important for retinal function. This process is under circadian regulation with a burst of rod shedding and RPE phagocytic activity occurring each morning. We previously showed that RPE cells use the integrin family adhesion receptor αvβ5 and its ligand MFG-E8, a secreted glycoprotein, to bind POS and stimulate their engulfment (Finnemann et al. 1997; Nandrot et al. 2004, 2007). Mice lacking either αvβ5 integrin or MFG-E8 lose the diurnal peak of phagocytosis and display similar numbers of POS phagosomes at all times of day. By contrast, wild-type RPE cells carry POS phagosomes only for a short period of time after light onset (LaVail 1976).
Like RPE cells, macrophages and dendritic cells rely on integrin receptors and secreted ligands including MFG-E8 for the recognition and phagocytosis of apoptotic cells (Savill et al. 2002). Studies of bone marrow-derived macrophages and dendritic cells in cell culture have shown that these cells express both αvβ5 integrin and the closely related αvβ3 integrin. However, while macrophages use dominantly αvβ3 for clearance phagocytosis (Finnemann and Rodriguez-Boulan 1999; Lucas et al. 2006), dendritic cells rely on αvβ5 (Albert et al. 1998). Much evidence suggests that these integrin/ligand complexes are essential for efficient phagocytosis and often assemble larger complexes in which additional proteins functionally interact (Chang and Finnemann 2007).
Transglutaminase 2 (TG2) is a multifunctional enzyme that is found intracellularly, in the cytoplasm and/or in the nucleus, as well as extracellularly associated with the cell surface and/or in the extracellular matrix (reviewed by Zemskov et al. 2006). In vitro and immunoprecipitation studies show that secreted TG2 binds to fibronectin with high affinity and mediates cell adhesion by forming complexes with β1 and β3 integrins that are present on the cell surface (Akimov et al. 2000; Akimov and Belkin 2001). TG2 has been found associated with the surface of macrophages, where it specifically forms a complex with β3 integrin and MFG-E8 (Toth et al. 2009a). The TG2/integrin complex has been implicated in the phagocytosis of apoptotic cells by macrophages because macrophages harvested from TG2−/− mice exhibit defective phagocytosis of apoptotic cells compared to wild-type cells. In this experimental system, lack of extracellular TG2 prevents αvβ3 integrin clustering likely causing the phagocytic defect (Toth et al. 2009a, b). TG2−/− mice generated by deleting the catalytic domain of TG2 are viable, fertile, and develop normally with no gross phenotypic abnormalities (De Laurenzi and Melino 2001). However, close examination found evidence for defective clearance phagocytosis in TG2−/− liver and thymus (Szondy et al. 2003).
The role of TG2 in phagocytosis by macrophages and its association with β integrins and MFG-E8 led us to investigate its potential role in the phagocytic activity of RPE cells. We hypothesized that TG2−/− retina may exhibit abnormalities as a consequence of impaired phagocytosis of POS by the RPE. Hence, we examined retinal structure and diurnal phagocytosis of POS by the RPE in TG2−/− mice to directly test this hypothesis.
93.2 Materials and Methods
93.2.1 Animals and Tissue Collection
TG2−/− mice were originally generated and characterized by De Laurenzi and Melino (2001). Mice were housed under cyclic 12:12 h light/dark-light conditions and fed ad libitum. Two- and twelve-month-old animals were sacrificed at 1 and 8 h after light onset by CO2 asphyxiation. Eyecups were enucleated and immediately fixed by immersion in formaldehyde/ethanol/acetic acid and embedded in paraffin. All procedures were approved by the local institutional animal care and use committees and adhered to the ARVO statement for the use of animals in ophthalmic and vision research.
93.2.2 Histology
Eight micrometer cross-sections of paraffin-embedded eyecups from TG2−/− mice were preheated to 50°C for 20 min and paraffin was removed using citrus clearing solvent (Richard-Allan Scientific) followed by rehydration with decreasing ethanol concentrations. To examine retinal layers, sections were stained with Hematoxylin followed by Eosin and subsequent dehydration by ethanol. Slides were coverslipped with Vectashield and imaged by brightfield microscopy.
93.2.3 Quantification of POS Phagosomes in the RPE
Opsin-positive inclusions in the RPE were quantified in 8 μm cross-sections from TG2−/− mice as a measure of RPE phagocytic activity (Nandrot et al. 2007). Paraffin was removed from sections as described above, and the tissue was bleached with 1% sodium borohydrate and labeled with rhodopsin antibody B6–30 (a kind gift by P. Hargrave, University of Florida, Gainesville), antimouse IgG-AlexaFluor 488 (Invitrogen), and DAPI. Sections were mounted in Vectashield, and x–y image stacks were acquired at 0.24-μm intervals on a Leica TSP5 confocal microscopy system. Opsin-labeled phagosomes in the RPE were counted on maximal projections of 5-μm-thick stacks. Phagosome counts were normalized to length of retina and averaged (for details please see Nandrot et al. 2007).
93.3 Results
93.3.1 TG2−/− Mice Display Normal Retinal Architecture
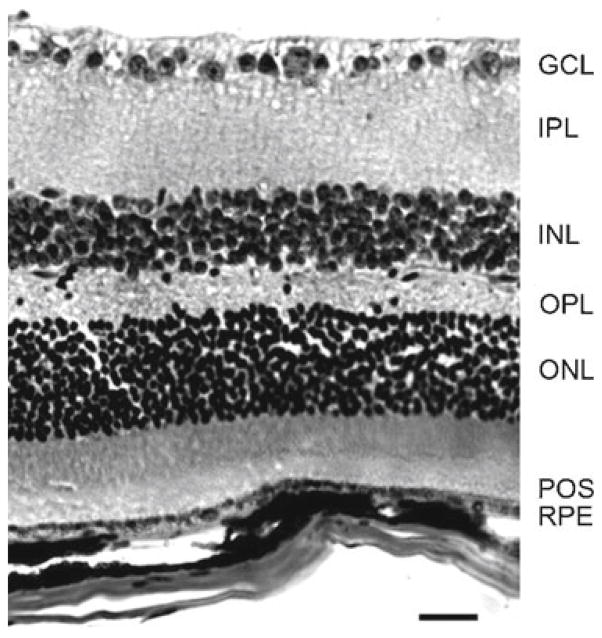
We examined cross-sections of eyecups taken from TG2−/− mice and stained by H&E for obvious alterations in morphology of the neural retina or the RPE. We found that all retinal layers, namely the POS, photoreceptor outer nuclear layer (ONL), outer plexiform layer (OPL), inner nuclear layer (INL), inner plexiform layer (IPL), ganglion cell layer (GCL), as well as the RPE, were present and intact, with no obvious disruption (Fig. 93.1). Appearance of the RPE was normal as well. This was true for young adult mice at 2 months of age (not shown) and for aged mice at 1 year of age (Fig. 93.1).
Fig. 93.1.
Analysis of morphology of TG2−/− retinal tissue. Representative H&E stained cross-section of an eyecup from a 1-year-old TG2−/− mouse showing intact RPE, photoreceptor outer segments (POS), outer nuclear layer (ONL), outer plexiform layer (OPL), inner nuclear layer (INL), inner plexiform layer (IPL), and ganglion cell layer (GCL). Scale bar equals 25 μm
93.3.2 The RPE in TG2−/− Mice Displays a Normal Diurnal Peak of Rod POS Phagocytosis
Phagocytosis of POS by the vertebrate RPE is synchronized to the light/dark cycle in wild-type animals, with a daily burst of phagocytosis of shed rod POS shortly after the onset of light. To examine whether lack of TG2 alters rod POS phagocytosis by the RPE, we counted rhodopsin-labeled phagosomes that were present in the RPE of TG2−/− mice at the expected peak and off peak times of phagocytosis. Phagosomes were highly abundant in the RPE of TG2−/− mice 1 h after light onset (Fig. 93.2a). As characteristic for wild-type mouse RPE, TG2−/− mouse RPE carries a significantly higher number of phagosomes in the morning (1 h after light onset), and a decline in phagosome number later in the day (8 h after light onset). This was true of both young adult and aged mice (Fig. 93.2b).
Fig. 93.2.
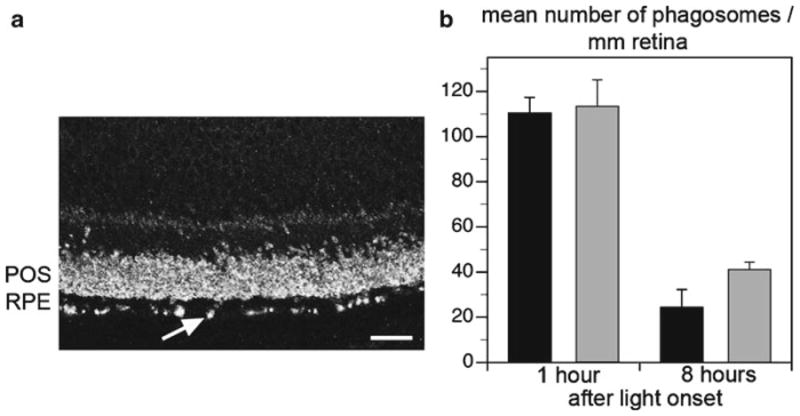
Analysis of RPE phagocytosis of POS in TG2−/− mouse retina. (a) Representative image of a rhodopsin-labeled retinal cross-section indicating the presence of numerous phagosome inclusions (arrow shows example) in the RPE layer of a 1-year-old TG2−/− mouse at 1 h after light onset. Scale bar equals 20 μm. (b) Average number of phagosomes detected in RPE of 2-month-old (black bars) and 1-year-old (gray bars) TG2−/− mice sacrificed 1 or 8 h after light onset as indicated. Bars represent mean ± s. e. m, n= 4
93.4 Discussion
The experimental results presented here demonstrate that complete and constitutive absence of TG2 does not affect the overall retinal architecture in either young or aged mice as TG2−/− mice exhibit the presence and normal morphology of all retinal layers. Based on the lack of any obvious difference in morphology of TG2−/− and wild-type retina, we decided not to expand the characterization of the TG2−/− retina to assess whether it is functionally normal, e.g., by recording electroretinograms. We can thus not exclude that lack of TG2 may cause subtle changes in retinal neuronal or RPE functions that our postmortem tissue analysis failed to detect. Additionally, loss of TG2 does not affect the diurnal pattern of phagocytosis of POS by the RPE since TG2−/− mice exhibit the expected burst of phagosome number shortly after light onset and a decline 8 h later. Given that TG2 in the mouse eye is mainly expressed by the RPE (cf. Ruggiero and Finnemann in this issue) our data strongly suggest that TG2 expression is not essential for the development or maintenance of any retinal cell type or retinal cell interactions.
Synchronized diurnal phagocytosis of rod POS by the RPE requires the ligand receptor pair αvβ5 integrin and MFG-E8. Mice lacking αvβ5 integrin or MFG-E8 lose the peak of phagocytosis that occurs at the onset of light in wild-type mice. In this study, we examined the potential role of TG2 in phagocytosis of POS by RPE by quantifying phagosome accumulation in the RPE at light onset and 8 h later in TG2−/− mice. If TG2 was a necessary component for the recognition and engulfment of POS by RPE through interactions with αvβ5 integrin and/or MFG-E8, we would expect similar numbers of phagosomes in TG2−/− RPE at both time points, like in β5−/− and MFG-E8−/− RPE. Instead, we found a pronounced diurnal rhythm of rod POS phagocytosis in TG2−/− mice similar to the rhythm in wild-type mice. We conclude that the avβ5 integrin-MFG-E8-dependent pathway does not require TG2 to function suggesting that it may utilize a different cofactor with similar functions as TG2 that remains to be identified.
Macrophages and RPE cells share expression of many proteins involved in clearance phagocytosis. All proteins known thus far to be involved in RPE phagocytosis have also been implicated in macrophage phagocytosis at least in in vitro settings. This study shows that RPE cells do not necessarily utilize the same proteins for phagocytosis as macrophages even if they express them. Macrophages use αvβ3 to dispose of apoptotic cells in vitro, and TG2 has been shown to be functionally related only to αvβ3 in this process. Dendritic cells and RPE cells, on the other hand, rely on αvβ5. Although dendritic cells also express high amounts of TG2 on their cell surface (Hodrea et al. 2010), we found that their phagocytosis is also independent of TG2 (our unpublished observation). These data indicate that while αvβ3 requires TG2 as a coreceptor for its function in the phagocytosis of apoptotic cells, αvβ5 does not. These observations are in agreement with the findings that TG2 acts as a coreceptor for β1 and β3, but not for all β integrins (Akimov et al. 2000). RPE cells also express αvβ3 integrin but they are unable to employ this alternate recognition receptor for POS uptake because αvβ3 is restricted to the basolateral surface in the RPE where it likely mediates specific interactions with Bruch’s membrane proteins. Thus, the highly polarized phenotype of the RPE may limit their repertoire of proteins available for phagocytosis. Whether TG2 physically or functionally interacts with αvβ3 in RPE cells will require further investigation.
Acknowledgments
These studies were supported by NIH grant EY-13295 to S.C.F. and Hungarian Research Fund OTKA 77587 to Z.S.
Contributor Information
Linda Ruggiero, Department of Biological Sciences, Fordham University, Bronx, NY 10458, USA.
Zsolt Sarang, Department of Biochemistry and Molecular Biology, Apoptosis and Genomics Research Group, Hungarian Academy of Sciences, University of Debrecen, Debrecen, Hungary.
Zsuzsa Szondy, Department of Biochemistry and Molecular Biology, Apoptosis and Genomics Research Group, Hungarian Academy of Sciences, University of Debrecen, Debrecen, Hungary.
Silvia C. Finnemann, Email: finnemann@fordham.edu, Department of Biological Sciences, Fordham University, Bronx, NY 10458, USA
References
- Akimov SS, Krylov D, Fleischman LF, et al. Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin. J Cell Biol. 2000;148:825–838. doi: 10.1083/jcb.148.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimov SS, Belkin AM. Cell-surface transglutaminase promotes fibronectin assembly via interaction with the gelatin-binding domain of fibronectin: a role in TGFβ-dependent matrix deposition. J Cell Sci. 2001;114:2989–3000. doi: 10.1242/jcs.114.16.2989. [DOI] [PubMed] [Google Scholar]
- Albert M, Pearce SFA, Francisco LM, et al. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Finnemann SC. Tetraspanin CD81 is required for the αvβ5 integrin-dependent particle-binding step of RPE phagocytosis. J Cell Sci. 2007;120:3053–3063. doi: 10.1242/jcs.006361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Laurenzi V, Melino G. Gene disruption of tissue transglutaminase. Mol Cell Biol. 2001;21:148–155. doi: 10.1128/MCB.21.1.148-155.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann SC, Rodriguez-Boulan E. Macrophage and retinal pigment epithelium phagocytosis: apoptotic cells and photoreceptors compete for αvβ3 and αvβ5 integrins, and protein kinase C regulates αvβ5 binding and cytoskeletal linkage. J Exp Med. 1999;190:861–874. doi: 10.1084/jem.190.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann SC, Bonilha VL, Marmorstein AD, et al. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires αvβ5 integrin for binding but not for internalization. Proc Natl Acad Sci USA. 1997;94:12932–12937. doi: 10.1073/pnas.94.24.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodrea J, Demeny MA, Majai G, et al. Transglutaminase 2 is expressed and active on the surface of human monocyte-derived dendritic cells and macrophages. Immunol Lett. 2010;130:74–81. doi: 10.1016/j.imlet.2009.12.010. [DOI] [PubMed] [Google Scholar]
- LaVail MM. Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science. 1976;194:1071–1074. doi: 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- Lucas M, Stuart LM, Zhang A, et al. Requirements for apoptotic cell contact in regulation of macrophage responses. J Immunol. 2006;177:4047–4054. doi: 10.4049/jimmunol.177.6.4047. [DOI] [PubMed] [Google Scholar]
- Nandrot EF, Kim Y, Brodie SE, et al. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking αvβ5 integrin. J Exp Med. 2004;200:1539–1545. doi: 10.1084/jem.20041447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandrot EF, Anand M, Almeida D, et al. Essential role for MFG-E8 as ligand for αvβ5 integrin in diurnal retinal phagocytosis. Proc Natl Acad Sci USA. 2007;104:12005–12010. doi: 10.1073/pnas.0704756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J, Dransfield I, Gregory C, et al. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- Szondy Z, Sarang Z, Molnar P, et al. Transglutaminase 2−/− mice reveal a phagocytosis-associated crosstalk between macrophages and apoptotic cells. Proc Natl Acad Sci USA. 2003;100:7812–7817. doi: 10.1073/pnas.0832466100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth B, Garabuczi E, Sarang Z, et al. Transglutaminase 2 is needed for the formation of an efficient phagocyte portal in macrophages engulfing apoptotic cells. J Immunol. 2009a;182:2084–2092. doi: 10.4049/jimmunol.0803444. [DOI] [PubMed] [Google Scholar]
- Toth B, Sarang Z, Vereb G, et al. Over-expression of integrin β3 can partially overcome the defect of integrin β3 signaling in transglutaminase 2 null macrophages. Immunol Lett. 2009b;126:22–28. doi: 10.1016/j.imlet.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Zemskov EA, Janiak A, Hang J, et al. The role of tissue transglutaminase in cell-matrix interactions. Front Biosci. 2006;11:1057–1076. doi: 10.2741/1863. [DOI] [PubMed] [Google Scholar]