Antipsychotic drugs have become indispensable in the treatment of schizophrenia since the introduction of first-generation antipsychotics (FGA; e.g., chlorpromazine and haloperidol) in the 1950s. Unfortunately, several of the commonly used drugs, particularly the second-generation antipsychotics (SGA) clozapine and olanzapine, induce metabolic disturbances, such as obesity, hypertriglyceridemia, glucose dysregulation, and in some studies, elevated serum cholesterol levels (1, 2). These adverse effects represent major challenges in the treatment of psychotic disorders, as they reduce compliance and contribute to increased cardiovascular mortality among patients (3). Despite these problems, the use of metabolically potent antipsychotic drugs is widespread, arguably due to superior therapeutic efficacy compared with antipsychotics with more favorable metabolic profiles (4).
Antipsychotic effect is related to blockage of dopamine D2 receptors in the brain. The molecular mechanisms mediating metabolic disturbances are incompletely understood. While antagonistic effects on serotonin 5HT2C and histamine H1 receptors in the hypothalamus seem to be relevant for antipsychotic-induced weight gain through stimulation of appetite (5), other mechanisms are also likely to contribute. In 1965, haloperidol was demonstrated to inhibit cholesterol biosynthesis (6). Later on, it was shown that other cationic amphiphilic drugs, such as the FGA chlorpromazine and the antidepressant imipramine, could increase lysosomal content of cholesterol and interfere with the sterol regulatory element binding protein (SREBP)-mediated cholesterol-sensing system in the endoplasmic reticulum (ER) (7–10). We and others, however, have demonstrated that antipsychotic drugs induce transcriptional activation of cholesterol and fatty acid biosynthesis genes controlled by the SREBP1 and SREBP2 transcription factors (11, 12). How these apparently divergent in vitro findings may be unified to form a common theory has not yet been sorted out, particularly with respect to their possible role in explaining antipsychotic-induced metabolic adverse effects in the clinical setting.
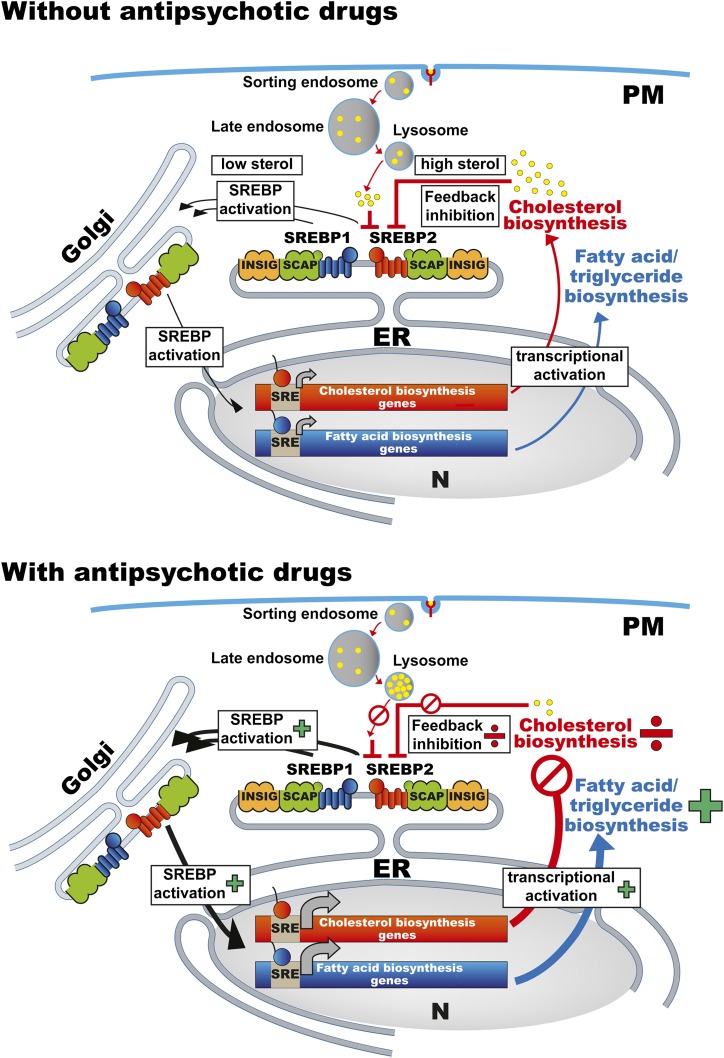
In this issue of Journal of Lipid Research, Canfrán-Duque et al. (13) contribute new, interesting data on the effect of antipsychotic drugs on cellular cholesterol biosynthesis and transport, elaborating previous work by others and themselves (6, 14–16). They demonstrate that the FGA haloperidol and the SGAs clozapine, risperidone, and ziprasidone reduce de novo cholesterol biosynthesis in three different types of cultured human cells (HepG2, SH-SY5Y, and HL-60). This occurs through inhibition of several enzymatic steps in the later part of the cholesterol biosynthesis pathway, such as Δ7-reductase, Δ8,7-isomerase, and Δ14-reductase, leading to accumulation of various cholesterol precursors. The resultant reduction in cholesterol biosynthesis may seem paradoxical in light of the well-established antipsychotic-related SREBP-controlled activation of lipid biosynthesis genes (11, 17). However, the authors suggest that transcriptional activation of cellular lipogenesis is, in fact, a homeostatic feedback mechanism triggered by reduced cholesterol biosynthesis, which is reinforced by antipsychotic-induced trapping of LDL-derived cholesterol within the endosomes/lysosomes, confirming formerly published results (16). In contrast to the reduced cholesterol production, biosynthesis of complex lipids (triglycerides and phospholipids) was increased in the cell cultures during exposure to antipsychotic drugs. The authors suggest that these opposite effects are indeed linked, as enzymes involved in triglyceride biosynthesis are not inhibited by the antipsychotics. The compensatory SREBP activation thus leads to elevated biosynthesis of free fatty acids, triglycerides, and phospholipids, as outlined in Fig. 1.
Fig. 1.
Cellular lipid homeostasis and regulation of SREBP-controlled cholesterol and fatty acid biosynthetic gene expression in the absence or presence of antipsychotic drugs.
Of note, the propensity of the individual antipsychotics to stimulate lipid biosynthesis changed under washout conditions in which growth medium containing antipsychotic drugs was replaced with drug-free medium for 4 h before cellular lipid concentrations were measured. In these experiments, clozapine came out as the most potent stimulator of fatty acid, triglyceride, and phospholipid biosynthesis. Removal of antipsychotics from the culturing medium also led to induction of cholesterol production, in agreement with previous findings (18). These data demonstrate how modest changes in experimental setup may lead to different conclusions, emphasizing the importance of careful planning of experimental design in future studies.
The in vivo significance of inhibited cholesterol biosynthesis as a causative mechanism for antipsychotic-induced triglyceride biosynthesis remains to be determined. Studies in rats have demonstrated that antipsychotic drugs indeed stimulate SREBP1-controlled gene expression and elevate lipid levels, both in acute (19, 20) and in subchronic experiments (21, 22). Although the washout conditions applied in the cell culture experiments may seem somewhat artificial, it should be noted that the half-life of antipsychotic drugs in rats is very short, resulting in oscillation of serum drug concentrations when the standard procedure of administering antipsychotics twice daily is followed. In female rats, increased lipogenic gene expression and elevated serum triglycerides were found 20 h after the last drug dose, when serum concentrations were negligible, implying that washout-like effects may have contributed to lipogenic activation in these conditions (22). In patients, however, serum levels of antipsychotics reach steady-state conditions after approximately one week at stable doses (23), and a direct parallel to the washout scenario observed in cell cultures seems less clear.
Among the antipsychotics examined, Canfrán-Duque et al. found that the FGA haloperidol and the SGAs risperidone and ziprasidone targeted the same enzymes in their inhibition of cholesterol biosynthesis, although with different relative activities: ziprasidone > haloperidol > risperidone. In contrast, clozapine mainly affected Δ24-reductase and Δ8,7-isomerase activity, although with weaker effects than the other antipsychotics examined. Clozapine is clearly the most metabolically unfavorable drug in patients, although ziprasidone and haloperidol have minor effects on serum lipids and other metabolic parameters (1, 24, 25). It seems puzzling, therefore, that depletion of cholesterol (with subsequent increase in triglyceride biosynthesis) in the cells was most pronounced during exposure to ziprasidone, whereas clozapine had limited effects. Whether the difference in enzymatic targets and degree of inhibition may be relevant with regard to this paradox remains to be elucidated. In this regard, it would be very interesting to include olanzapine in the group of drugs examined, since this antipsychotic is both highly metabolically potent and frequently used.
In summary, the findings by Canfrán-Duque and colleagues provide interesting new insight into the effects of antipsychotic drugs on lipid biosynthesis in vitro. The authors launch a new, integrative theory on how inhibition of cholesterol biosynthesis may be relevant for the antipsychotic-induced increase in serum triglyceride levels observed both in rodent models and in patients. Once the basic mechanisms have been firmly established, it is time to investigate the role of antipsychotic-induced inhibition of cellular cholesterol production and homeostatic lipogenic activation in dyslipidemia and obesity.
REFERENCES
- 1.Meyer J. M., Koro C. E. 2004. The effects of antipsychotic therapy on serum lipids: a comprehensive review. Schizophr. Res. 70: 1–17 [DOI] [PubMed] [Google Scholar]
- 2.Newcomer J. W., Meyer J. M., Baker R. A., Eudicone J. M., Pikalov A., Vester-Blokland E., McQuade R. D., Crandall D. T., Carson W. H., Marcus R. N., et al. 2008. Changes in non-high-density lipoprotein cholesterol levels and triglyceride/high-density lipoprotein cholesterol ratios among patients randomized to aripiprazole versus olanzapine. Schizophr. Res. 106: 300–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newcomer J. W., Hennekens C. H. 2007. Severe mental illness and risk of cardiovascular disease. JAMA. 298: 1794–1796 [DOI] [PubMed] [Google Scholar]
- 4.Buchanan R. W., Kreyenbuhl J., Kelly D. L., Noel J. M., Boggs D. L., Fischer B. A., Himelhoch S., Fang B., Peterson E., Aquino P. R., et al. 2010. The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr. Bull. 36: 71–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds G. P., Kirk S. L. 2010. Metabolic side effects of antipsychotic drug treatment–pharmacological mechanisms. Pharmacol. Ther. 125: 169–179 [DOI] [PubMed] [Google Scholar]
- 6.Summerly R., Yardley H. 1965. The effect of a substituted fluorobutyrophenone (haloperidol) on the metabolism of sterols in rat skin. Biochem. J. 96: 30 [Google Scholar]
- 7.Adams C. M., Goldstein J. L., Brown M. S. 2003. Cholesterol-induced conformational change in SCAP enhanced by Insig proteins and mimicked by cationic amphiphiles. Proc. Natl. Acad. Sci. USA. 100: 10647–10652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lange Y., Steck T. L. 1994. Cholesterol homeostasis. Modulation by amphiphiles. J. Biol. Chem. 269: 29371–29374 [PubMed] [Google Scholar]
- 9.Lange Y., Ye J., Rigney M., Steck T. L. 1999. Regulation of endoplasmic reticulum cholesterol by plasma membrane cholesterol. J. Lipid Res. 40: 2264–2270 [PubMed] [Google Scholar]
- 10.Lange Y., Ye J., Steck T. L. 1998. Circulation of cholesterol between lysosomes and the plasma membrane. J. Biol. Chem. 273: 18915–18922 [DOI] [PubMed] [Google Scholar]
- 11.Yang L. H., Chen T. M., Yu S. T., Chen Y. H. 2007. Olanzapine induces SREBP-1-related adipogenesis in 3T3-L1 cells. Pharmacol. Res. 56: 202–208 [DOI] [PubMed] [Google Scholar]
- 12.Fernø J., Raeder M. B., Vik-Mo A. O., Skrede S., Glambek M., Tronstad K. J., Breilid H., Lovlie R., Berge R. K., Stansberg C., et al. 2005. Antipsychotic drugs activate SREBP-regulated expression of lipid biosynthetic genes in cultured human glioma cells: a novel mechanism of action? Pharmacogenomics J. 5: 298–304 [DOI] [PubMed] [Google Scholar]
- 13.Canfrán-Duque A., Casado M., Pastor Ó., Sánchez-Wandelmer J., Peña G., Lerma M., Mariscal P., Bracher P., Lasunción M., Busto R. 2013. Atypical antipsychotics alter cholesterol and fatty acid metabolism in vitro. J. Lipid Res. 54: 310–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sánchez-Wandelmer J., Hernandez-Pinto A. M., Cano S., Davalos A., de la Pena G., Puebla-Jimenez L., Arilla-Ferreiro E., Lasuncion M. A., Busto R. 2009. Effects of the antipsychotic drug haloperidol on the somastostatinergic system in SH-SY5Y neuroblastoma cells. J. Neurochem. 110: 631–640 [DOI] [PubMed] [Google Scholar]
- 15.Sánchez-Wandelmer J., Davalos A., de la Pena G., Cano S., Giera M., Canfran-Duque A., Bracher F., Martin-Hidalgo A., Fernandez-Hernando C., Lasuncion M. A., et al. 2010. Haloperidol disrupts lipid rafts and impairs insulin signaling in SH-SY5Y cells. Neuroscience. 167: 143–153 [DOI] [PubMed] [Google Scholar]
- 16.Kristiana I., Sharpe L. J., Catts V. S., Lutze-Mann L. H., Brown A. J. 2010. Antipsychotic drugs upregulate lipogenic gene expression by disrupting intracellular trafficking of lipoprotein-derived cholesterol. Pharmacogenomics J. 10: 396–407 [DOI] [PubMed] [Google Scholar]
- 17.Fernø J., Skrede S., Vik-Mo A. O., Havik B., Steen V. M. 2006. Drug-induced activation of SREBP-controlled lipogenic gene expression in CNS-related cell lines: marked differences between various antipsychotic drugs. BMC Neurosci. 7: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauressergues E., Staels B., Valeille K., Majd Z., Hum D. W., Duriez P., Cussac D. 2010. Antipsychotic drug action on SREBPs-related lipogenesis and cholesterogenesis in primary rat hepatocytes. Naunyn Schmiedebergs Arch. Pharmacol. 381: 427–439 [DOI] [PubMed] [Google Scholar]
- 19.Fernø J., Vik-Mo A. O., Jassim G., Havik B., Berge K., Skrede S., Gudbrandsen O. A., Waage J., Lunder N., Mork S., et al. 2009. Acute clozapine exposure in vivo induces lipid accumulation and marked sequential changes in the expression of SREBP, PPAR, and LXR target genes in rat liver. Psychopharmacology (Berl.). 203: 73–84 [DOI] [PubMed] [Google Scholar]
- 20.Jassim G., Skrede S., Vazquez M. J., Wergedal H., Vik-Mo A. O., Lunder N., Dieguez C., Vidal-Puig A., Berge R. K., Lopez M., et al. 2012. Acute effects of orexigenic antipsychotic drugs on lipid and carbohydrate metabolism in rat. Psychopharmacology (Berl.). 219: 783–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minet-Ringuet J., Even P. C., Valet P., Carpene C., Visentin V., Prevot D., Daviaud D., Quignard-Boulange A., Tome D., de Beaurepaire R. 2007. Alterations of lipid metabolism and gene expression in rat adipocytes during chronic olanzapine treatment. Mol. Psychiatry. 12: 562–571 [DOI] [PubMed] [Google Scholar]
- 22.Skrede S., Fernø J., Vazquez M. J., Fjaer S., Pavlin T., Lunder N., Vidal-Puig A., Dieguez C., Berge R. K., Lopez M., et al. 2012. Olanzapine, but not aripiprazole, weight-independently elevates serum triglycerides and activates lipogenic gene expression in female rats. Int. J. Neuropsychopharmacol. 15: 163–179 [DOI] [PubMed] [Google Scholar]
- 23.Callaghan J. T., Bergstrom R. F., Ptak L. R., Beasley C. M. 1999. Olanzapine. Pharmacokinetic and pharmacodynamic profile. Clin. Pharmacokinet. 37: 177–193 [DOI] [PubMed] [Google Scholar]
- 24.Bushe C. J., Slooff C. J., Haddad P. M., Karagianis J. L. 2012. Weight change from 3-year observational data: findings from the worldwide schizophrenia outpatient health outcomes database. J. Clin. Psychiatry. 73: e749–e755 [DOI] [PubMed] [Google Scholar]
- 25.Rummel-Kluge C., Komossa K., Schwarz S., Hunger H., Schmid F., Lobos C. A., Kissling W., Davis J. M., Leucht S. 2010. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr. Res. 123: 225–233 [DOI] [PMC free article] [PubMed] [Google Scholar]