Abstract
It is important to clarify the distinct contributions of estrogen/estrogen receptor (ER) and androgen/androgen receptor (AR) signaling and their reciprocal effects on the regulation of hepatic lipid homeostasis. We studied the molecular mechanisms underlying the preventive effects of estradiol (E2), dihydrotestosterone (DHT), or E2+DHT on high-fat diet-induced nonalcoholic fatty liver disease (NAFLD) in an orchidectomized Sprague-Dawley (SD) rat model. E2 is shown to be associated with decreased fatty acid synthesis in hepatic zone 3-specific manner by increasing the phosphorylation of acetyl coenzyme-A carboxylase via an ERα-mediated pathway. DHT is shown to be associated with decreased lipid accumulation and cholesterol synthesis in a hepatic zone 1-specific manner by increasing expression of carnitine palmitotyltransferase1 and phosphorylation of 3-hydroxy-3-methyl-glutaryl-CoA reductase via an AR-mediated pathway. E2+DHT showed an additive positive effect and normalized all three impaired zones of the liver. Gene expression changes in human severe liver steatosis were similar to those of experimental rat NAFLD. Steroids reversed the histopathological NAFLD changes, likely by decreasing fatty acid and cholesterol synthesis and increasing β-oxidation. The diverse steroid effects (ER/AR) on NAFLD prevention in male rats indicate the potential applicability of ER/AR modulators for NAFLD treatment.
Keywords: nonalcoholic steatohepatitis, lipid metabolism, estrogen receptor, androgen receptor
Nonalcoholic fatty liver disease (NAFLD) is strongly linked to central obesity, insulin resistance (IR), and metabolic syndrome (1), and its increasing prevalence is estimated at 20–30% among the adult population in industrialized countries (2).
Although estrogen and androgen are important regulators of lipid homeostasis (3, 4), data on their possible influence on the prevention or treatment of NAFLD are scarce. Clinically, tamoxifen (Tam) is widely used for the treatment of estrogen-responsive breast cancer, but its frequent side effect is the development of NAFLD. Forty-three percent of patients with Tam-treated breast cancer develop steatosis within the first 2 years of treatment (5). Human estrogen receptor (ER) and aromatase deficiency are very rare clinical conditions (6, 7). Although men with ERα mutation developed severe steatosis (7), the three adult men reported with aromatase deficiency had impaired lipid metabolism, and only one presented with hepatic steatosis (8). Aromatase-knockout mice (3, 4), ERα-knockout (αERKO) mice (9), and the double ER α and β knockout mice (10) display elevated triglyceride (TG) levels (11). These clinical and experimental studies have shown the significant role of the estrogen signaling pathway in lipid homeostasis (3, 4).
Regardless of the importance of estrogen in NAFLD, testosterone treatment studies have showed visceral fat reduction in men (12, 13) and improvement of NAFLD (14). Androgen receptor knockout (ARKO) male mice develop late-onset obesity (15, 16). The liver-specific ARKO male mice have increased IR and steatosis, with decreased β-oxidation, upon being fed a high-fat diet (HFD) (17). Clinically, increased IR and impaired glucose tolerance have been observed in men with testosterone deficiency (18). However, the specific role of the androgen/androgen receptor (AR) signaling for the regulation of lipid metabolism in men is largely unknown.
Aromatase-knockout mouse male mice are characterized by a marked hepatic steatosis and consistently high serum testosterone levels (reviewed in Ref. 19). Accordingly, αERKO mice also have higher serum testosterone levels (reviewed in Ref. 20). In contrast, serum estradiol (E2) levels of ARKO mice are either elevated (21) or normal (15), implying an enhanced or sufficient supply of systemic estrogen action. It is thus anticipated that the estrogen/ER or androgen/AR pathways play key roles in lipid homeostasis. Clinical studies on the effects of androgens on lipid metabolism (12, 13) have used aromatizable androgens, resulting in a mixture of ER and AR signal actions (22). It is important to clarify the distinct contributions of estrogen/ERs and androgen/AR signaling in the regulation of hepatic lipid homeostasis.
It was impossible to breed homozygous double knockout ER/AR mice for this study due to the infertility of ARKO male mice (23), the subfertility of ARKO female mice (23), and the sexual immaturity in both genders of homozygous αERKO mice. To analyze the actions of ER and AR signaling pathways on NAFLD, we depleted endogenous sex steroids by orchidectomized (ORX) male Sprague-Dawley (SD) adult rats on HFD and assessed the specific impacts of testosterone and estradiol deficiency and the effect of E2, 5α-dihydrotestosterone (DHT) (a nonaromatizable androgen), or E2+DHT supplementation in this inducible NAFLD model. To demonstrate this NAFLD model to human relevance, we studied the same molecular changes observed in this NAFLD rat model in samples of severe liver steatosis in men.
MATERIALS AND METHODS
Experimental design
Two-month-old, male, ORX SD rats (200 ± 20 g) (Vital-River, Beijing, China) were fed with normal diet (ND; n = 10) or high-fat diet (HFD; n = 30 per group). HFD ORX rats received vehicle (corn oil) or E2, DHT, or E2+DHT treatment once daily subcutaneously for 75 days starting 7 days after being orchidectomized (n = 30 per group). We also had wild-type, gonad-intact SD rats on HFD that received vehicle (corn oil) or E2, DHT, or E2+DHT (n = 6 per group) as an internal control group for the ORX rats. The China Agricultural University Ethics Committee approved the animal experiments. The treatment design is shown in Table 1.
TABLE 1.
Treatment groups for the estrogen and androgen application in castrated (ORX) and intact (non-ORX) SD male rats
| Group | Treatment | Diet | ORX (n) | Intact (n) |
| Vehicle | Oil | Normal diet | 10 | 6 |
| Vehicle | Oil | High-fat diet | 30 | 6 |
| E2 | 1.0 mg/kg | High-fat diet | 30 | 6 |
| DHT | 3.0 mg/kg | High-fat diet | 30 | 6 |
| E2+DHT | 1.0 mg/kg + 3.0 mg/kg | High-fat diet | 30 | 6 |
n, number of animals per group.
The rats were orchidectomized (ORX) under general anesthesia with 4.0% isoflurane. The testes were removed through a small incision in the scrotum, and the incision was sutured. The ORX had 7 days to recover from the surgery before the treatments started. Food and water were provided ad libitum, and animals were housed in a room with controlled light (12 h light 12 h darkness). The HFD was prepared according to our previous study (24). The 3.0 mg/kg/d dosage of DHT was chosen because it was shown that this dosage can restore the weight of the ventral prostate (VP) in ORX rats (control: 0.062 ± 0.13 g/VP; ORX: 0.02 ± 0.01 g/VP; DHT: 0.070 ± 0.32 g/VP). The 1.0 mg/kg/d dosage of E2 was chosen because this dosage was shown to prevent ORX-induced trabecular bone loss (Moverate-Skrtic et al., 25). Subcutaneous injections were given to all animals at the same time (16:00 h).
Chemicals
E2, ICI 182,780 (ICI, an ER antagonist), palmitic acid, oleic acid (OA), DHT, flutamide (Flu, an AR antagonist), and DMSO were purchased from Sigma-Aldrich (St. Louis, MO). 4,4,4′-(4-propyl-[1H]pyrazole-1,3,5-triyl) triphenol (PPT; an ERα selective agonist) and 2,3-bis(4-hydroxyphenyl) propionitrile (DPN; an ERβ selective agonist) were purchased from Tocris Biosciences (Ellisville, MO).
Sample collection and histological analysis
After overnight fasting for 12 h, rats were anesthetized with intraperitoneal pentobarbital (50 mg/kg) and euthanized by cardiac puncture. All the animals were euthanized in the morning (08:00–9:00 h). The serum samples were stored at −20°C. To minimize the variability of the treatment response for rat liver lobes, we followed the optimal sampling of rat liver tissue methodology validated by Foley et al. (26). Briefly, the left lateral lobe of rat liver was divided into three parts: one third was used for histopathological and immunohistochemical analyses, one third was homogenized for RNA and protein analyses, and one third was used for biochemical and steroid measurement analyses. Liver tissues were fixed with 4% paraformaldehyde, with portions snap-frozen in liquid nitrogen and stored at −80°C. For histological examination, paraformaldehyde-fixed liver tissues were embedded in paraffin and stained with hematoxylin and eosin, or the frozen sections of liver were stained with Oil Red O (ORO) as described previously (n = 30 for all the HF diet groups; n = 10 per ND group) (24). The histology of liver zonation was determined according to Monga (27). Briefly, hepatic acinus represents a liver lobule that is divided into three regions based on their proximity to the distributing veins: zone 1, cells closest to the vessels (portal triad); zone 2, cells in between portal triad and central vein; and zone 3, cells near the central vein.
Human liver specimens
Samples from male patients with liver cancer (ages 50–70 years) were collected with informed consent in the 306th Hospital of PLA, China. The Ethics Committee of the 306th Hospital of PLA approved the study. Samples consisted of five individual patient cases of milder/subnormal liver steatosis and five individual patient cases of severe liver steatosis. Due to a lack of normal human liver samples, the milder/subnormal liver steatosis specimens were used as controls.
Serum concentrations of E2, DHT, aminotransferase, and serum static lipid profile
Serum E2 and DHT were measured as described in previous studies (28, 29). Serum lipid parameters were measured with a lipid-measuring kit (Wako Pure Chemical Industries Ltd, Osaka, Japan) according to the protocol provided with kit. Serum levels of alanine aminotransferase (ALT) were measured as a biochemical marker of hepatic function using a multiparametric analyzer (AU 5400; Olympus, Japan). The cholesterol ester for serum was measured with a Cholesterol/Cholesteryl Ester Quantitation Kit (cat. KA0829; Abnova, Walnut, CA) according to the manufacturer's protocol.
Liver gene expression analysis by RT-PCR
Total RNA was extracted from the frozen liver tissues using the acid guanidinium method (30). RT-PCR was performed as described previously (31); primer pairs are shown in supplementary Table I. Briefly, all the samples were run individually in RT reactions in triplicate and normalized to β-actin. Transcripts were measured using RT-PCR, and the data were presented with densitometric analysis as a mean of the three times in triplicate. The relative values were quantified using the NIH Image J 1.34 program (http://rsb.info.nih.gov/ij/download.html).
Western blot analysis
Protein was extracted from the frozen liver tissue samples using the RIPA method and quantified by a Bio-Rad protein assay kit (cat. 500-0002; BioRad, San Diego, CA). Protein samples were separated by SDS-PAGE and then transferred to a PVDF membrane as described previously (24). Antibodies and dilutions are presented in supplementary Table II. After washing, the membranes were incubated with HRP-conjugated secondary antibodies for 20 min. Antibody binding was visualized by using a Supersignal West Pico detection kit (Pierce, Rockford, IL). Peroxidase-conjugated secondary antibodies included goat anti-rabbit IgG diluted at 1:2,000 (Zymed, San Francisco, CA), goat anti-mouse IgG diluted at 1:4,000 (Pierce, Rockford, IL), or rabbit anti-sheep IgG diluted 1:2,000 (Sigma, Beijing, China). Each reaction was performed in triplicate in three independent experiments Western blotting assays. The films were scanned and quantified using the NIH Image 1.34 program (http://rsb.info.nih.gov/ij/download.html).
Fatty acid treatment and preparation of conditioned medium: in vitro treatment on BRL3A rat hepatocyte cell line
All the in vitro experiments in this study were carried on in the rat hepatocyte BRL3A cell line (32) purchased from the Chinese Academy of Sciences Cell Bank (under the license of ATCC, cat. CRL-1442) and cultured using DMEM/Ham's F-12 (Sigma, Beijing, China) with 10% FBS (Sigma). The treatment groups are shown in Table 2. Cells were treated for 24 h. The individual experiments were repeated three times. Cells were exposed to medium containing 1% free fatty acid-free BSA (Sigma-Aldrich) and fat loaded with 1 mM palmitic acid and OA or DMSO (vehicle control).
TABLE 2.
In vitro treatment on hepatocytes
| Group | Dose | Pretreatment |
| Control | DMSO | Non-fatty acid |
| Control | 1 mM (PA:OA = 1:2) | Fatty acid |
| E2 | 10 nM | Fatty acid/non-fatty acid |
| E2+ICI | 10 nM + 1 μM | Fatty acid/non-fatty acid |
| DHT | 10 nM | Fatty acid/non-fatty acid |
| DHT+Flu | 10 nM + 10 nM | Fatty acid/non-fatty acid |
| E2+DHT | 10 nM + 10 nM | Fatty acid/non-fatty acid |
| E2+DHT+ICI+Flu | 10 nM + 10 nM + 1 μM + 10 nM | Fatty acid/non-fatty acid |
| PPT | 10 nM | Fatty acid/non-fatty acid |
| DPN | 10 nM | Fatty acid/non-fatty acid |
Mitochondrial membrane potential measurement
A mitochondrial staining kit allowing detection of mitochondrial potential changes was purchased from Sigma (catalog #CS0390), and the mitochondrial membrane potential (MMP) was measured. JC-1 dye was used as an internal control, and the experiments were performed in triplicate. The cells were suspended (0.6–0.8 × 106 cells per ml) in complete medium (the medium used for cell growth containing the required supplements) with 1 ml of staining solution and incubated for 20 min at 37°C in a CO2 incubator. The cells were collected and washed with 5 ml of the ice-cold 1×JC-1 staining buffer. Fluorescence intensity was determined by fluorescence spectrophotometer. The red fluorescence of the aggregated JC-1 represents intact mitochondria, and the green fluorescence of the monomeric JC-1 represents disrupted mitochondria. The ratio of red and green fluorescence intensity can reflect the level of mitochondrial membrane potential. The individual experiments were repeated three times.
Static ATP level measurements
Static ATP levels in total liver homogenate were determined using a commercially available bioluminescence assay (Sigma). Homogenate (20 µg protein) was added to a reaction mix containing 1.25 µg/ml luciferase and 0.1 mM luciferin. Luminescence was determined using the SpectraMax 5 luminometer (Molecular Devices, Sunnyvale, CA). The amount of ATP in the experimental samples was calculated. Duplicates were performed for each sample. The results are expressed as nmol of ATP per mg of protein.
Measurement of static levels of fatty acids from liver using GS for lipogenesis analysis
Due to difficulties with experimental equipment for measuring the dynamic levels of free fatty acid of rat liver samples, we could only measure the static levels of fatty acids by GC. Briefly, about 0.2–0.3 g of frozen liver samples were placed in glass test tubes. Four milliliters of acetyl chloride and methanol mixture (1:10) were added to the test tube with 1 ml hexane and 1 ml stearic acid (1 mg/ml as internal standard). The test tubes were sealed and placed in a water bath (80°C) for 2 h. After that, the samples were cooled to room temperature, and 5 ml of potassium carbonate solution (7%) was added to the reaction mixture. The mixture was then centrifuged at 1,200 rpm for 5 min, and the supernatant was collected for GC analysis. An Agilent 6890 GC (Agilent, Santa Clara, CA) with HP-88 column (100 m × 0.25 mm × 0.20 µm; Agilent) and flame ionization detector was used for quantitative analysis of the fatty acids. The GC inlet temperature was set at 260°C, and the detector temperature was set at 270°C. The temperature program raised the oven temperature from 100°C to 220°C in 1 h. The fatty acids were identified based on retention times of fatty acid standards. Quantification was achieved by comparing the integrated peak area of each fatty acid with that of the internal standard.
Measurement of de novo lipogenesis
The measurement of lipogenesis was performed as described previously (33). Briefly, BRL3A hepatocytes were cultured and treated as described above. For the final 4 h or 6 h of treatment, cells were labeled with 1 μCi 1-14C acetic acid (PerkinElmer Inc., Waltham, MA) and incubated in a shaker. Cells were washed four times with PBS before lysis in 0.5% Triton X-100. The lipid fraction was extracted by the addition of 10 times the volume of chloroform, methanol, and water (8:4:3 v/v). Samples were centrifuged (3,000 rpm, 20 min), and the lower chloroform phase was collected. The chloroform phase was evaporated to dryness. The residue was redissolved in scintillation cocktail. 14C incorporation was measured by a Beckman LS6500 scintillation counter.
Immnunohistochemical and Immunofluorescence visualization assay
For immunohistochemical detection of acetyl coenzyme-A carboxylase (P-ACC), the streptavidin-biotin-peroxidase complex method was used. Sections (5 μm thick) from paraffin-embedded liver tissues were deparaffinized and rehydrated in xylene and ethanol, placed in 10 mmol/l citrate buffer (pH 6.0), and boiled in a microwave oven for antigen retrieval; four periods of 4 min each were used. The sections were treated with 3% H2O2 in PBS (pH 7.2 to ∼7.4) for 10 min and blocked with normal goat serum (1:10 dilution) for 30 min. The sections were then incubated in 37°C for 2 h with P-ACC antibody (rabbit polyclonal IgG; Cell Signaling Technology, Inc., #3661, at 1:400 dilution). The primary antibody bound was detected by using biotinylated goat anti-rabbit IgG (1:200 dilution) followed by incubation with HRP streptavidin. After staining with diaminobenzidine, the slides were counterstained with hematoxylin, dehydrated, and mounted.
The frozen liver sections were fixed in 4% paraformaldehyde in PBS for 10 min, rinsed in PBS, and then blocked for 60 min at room temperature in 10% normal rabbit serum (TNF-α) or goat serum (IL-6) in PBS. The sections were then incubated overnight at 4°C with TNF-α antibody (goat polyclonal IgG; Santa Cruz Biotechnology, Inc., sc-1348, at 1:100 dilution) or IL-6 antibody (Rabbit polyclonal IgG; Abcam, Inc., ab6672, at 1:500 dilution). The primary antibody bound was detected by using FITC-conjugated rabbit anti-goat IgG (1:50 dilution) or FITC-conjugated goat anti-rabbit IgG (1:50 dilution). The sections were slightly counterstained with 4,6-diamidino-2-phenylindole (DAPI) (1:1,000 in PBS) and mounted with a cover glass.
Confocal laser microscopy
Confocal laser microscope setup (Nikon, Japan) was used to detect emission light emitted from FITC or DAPI. The sections were exposed to a 488 nm (FITC) or 408 nm (DAPI) excitation wavelength. Four images with 2 μm intervals in the A-axis were collected with a confocal scanner equipped with ECLIPSE C1Si laser system (Nikon) coupled with a Nikon EZ-C1 program.
Semiquantification of ORO staining and immunofluorescence by Image ProPlus6.0 software
The semi-quantification of staining of the ORO for liver tissue and hepatic cell lines and immunofluorescence of TNF-α and IL-6 for liver tissues were analyzed by using Image Pro plus 6.0 (Media Cybernetics, Rockville, MD). Integrated optical density of positive staining area and total areas of sections were calculated. The ratio of integrated optical density to total area is determined as a protein (TNF-α or IL-6) expression semiquantification or ORO staining quantification. To eliminate variations, the sections were captured under the same conditions for all the tissues or cells.
Statistical analysis
One-way ANOVA and Dunnett's post hoc tests were performed for statistical analyses using the SPSS12.0.1 Package (SPSS Inc., Chicago, IL). P values <0.05 were regarded as statistically significant. Values are presented as mean ± SEM.
RESULTS
Body and liver weights of ORX SD rats on HFD after different treatments
ORX SD male rats on HFD received vehicle, E2, DHT, or E2+DHT (n = 30 per group). An additional control group consisted of ORX-SD male rats on ND (n = 10). A 2.5-month HFD increased the body and liver weights compared with normal diet ORX controls (P < 0.05) (Table 3). E2 and E2+DHT significantly decreased the body and liver weights (P < 0.05), whereas DHT induced only a mild decrease in these weights, as compared with HFD-treated controls (Table 3). Relative peri-kidney fat weight did not differ between the studied groups (Table 3). We also checked wild-type, gonad-intact SD rats on ND or HFD receiving identical treatments of ORX (Table 1). E2, DHT, and E2+DHT did not increase significantly the total body (350.3 ± 18.12, 405.4 ± 8.2, and 380.2 ± 4.1, respectively) and liver weights (11.2 ± 0.11, 13.4 ± 1.09, and 10.8 ± 0.22, respectively) compared with ND intact control (375.5 ± 21.48 and 10.8 ± 0.69). Treatment effects in intact rats were milder than in ORX HFD control and treated groups (Table 3). Therefore, ORX HFD treatment groups were further compared with only control ORX HFD group, where the changes were more severe.
TABLE 3.
Body, liver, and kidney fat weights; serum biochemical markers; and total cholesterol, triglycerides, HDL-c, LDL-c, and E2 and DHT levels in ORX SD male rats (n = 30 in all groups except n = 10 in the ND group).
| Normal Diet | High-fat Diet |
||||
| Control | Control | E2 | DHT | E2+DHT | |
| Body weight (g) | 391.00 ± 18.69 | 467.92 ± 10.83*, a | 361.68 ± 17.03b | 418.75 ± 12.60c | 385.00 ± 3.08 b |
| Liver weight (g) | 11.74 ± 0.59 | 19.93 ± 2.26*,a | 14.41 ± 0.34b | 16.92 ± 1.13b | 13.34 ± 0.46b |
| Relative kidney fat/body weight | 0.026 ± 0.0051 | 0.021 ± 0.0074a | 0.017 ± 0.0091a | 0.019 ± 0.0081a | 0.016 ± 0.0097a |
| Total cholesterol (mg/dl) | 1.84 ± 0.07 | 4.09 ± 0.27*, a | 3.44 ± 0.27b | 3.05 ± 0.16b | 2.31 ± 0.11c |
| Cholesterol ester (mg/dl) | 1.25 ± 0.21 | 3.21 ± 0.22*, a | 2.44 ± 0.24b | 1.91 ± 0.19b | 1.79 ± 0.24b |
| HDL-c (mg/dl) | 1.32 ± 0.01 | 0.69 ± 0.16*, a | 1.31 ± 0.06b | 0.90 ± 0.07a | 1.37 ± 0.08b |
| LDL-c (mg/dl) | 0.57 ± 0.26 | 1.66 ± 0.07*, a | 1.45 ± 0.56a | 1.25 ± 0.02b | 0.79 ± 0.05c |
| Triglycerides(mg/dl) | 0.49 ± 0.08 | 1.66 ± 0.08*, a | 1.28 ± 0.05b | 1.49 ± 0.13a | 0.87 ± 0.02c |
| ApoB100 (mg/dl) | 0.082 ± 0.02 | 0.101 ± 0.01*, a | 0.084 ± 0.02b | 0.075 ± 0.01b | 0.068 ± 0.01c |
| ALT (U/L) | 30.14 ± 3.88 | 150.10 ± 16.98*, a | 69.48 ± 9.51b | 116.92 ± 13.71a | 53.00 ± 13.67b |
| Serum E2 (ng/dl) | 0.20 ± 0.15 | 0.15 ± 0.11a | 0.43 ± 0.16b | 0.22 ± 0.13a | 0.41 ± 0.13b |
| Serum DHT (ng/ml) | 0.30 ± 0.15 | 0.10 ± 0.13a | 0.28 ± 0.18a | 0.44 ± 0.12b | 0.43 ± 0.14b |
The data groups provided with only different letter superscripts (e.g., a vs. b or a vs. b, c etc.) are statistically significantly different (P < 0.05), where data from E2/DHT/E2+DHT groups should be mainly compared with high-fat control. *P < 0.05, for control HF diet vs. normal diet control groups only
Administration of estrogen/androgen could prevent NAFLD progression in rat liver
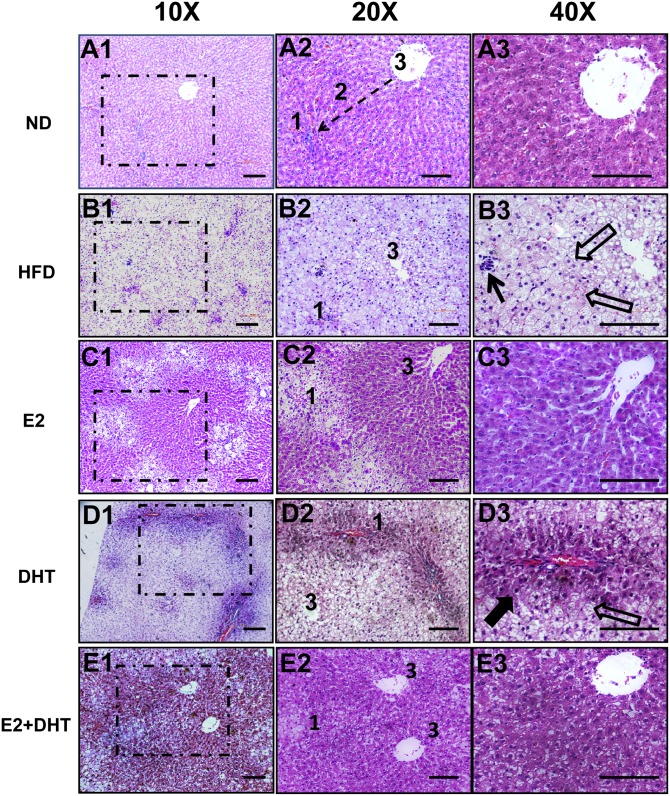
Severe steatosis characterized by macrovacuolar focal necrosis and inflammation was observed in zones 1, 2, and 3 in most (26/30) of the HFD ORX control rats (Fig. 1B1–1B3) (n = 30 per group). ORX rats treated with E2 exhibited reversible phenotype, but macrovacuolar changes still were observed in periportal zone (zone 1) compared with controls, whereas less pronounced microvesicular steatosis occurred in perivenous zone (zone 3) (Fig. 1C1–1C3). In contrast to E2 treatment, DHT (Fig. 1D1–1D3) decreased the level of steatosis in periportal zone (zone 1), but still some macrovacuolar steatosis was observed in the perivenous zone (zone 3) compared with the HFD ORX controls. E2+DHT treatment exhibited the best preventive histopathologic response with apparently normal liver cells in zones 2 and 3, with only some of microvesicular steatosis in zone 1 (Fig. 1E1–E13). Portal inflammation was absent in the E2-, DHT-, and E2+DHT-treated liver samples (Fig. 1C1–E13, 1D1–1D3, and 1E1–1E3). The accumulation of lipids in hepatocytes was further confirmed by ORO staining and morphometric quantification analysis in supplementary Fig. I. The histopathological changes in gonad-intact HFD rats were very similar to the HFD ORX rats after the different treatments; however, the changes were much milder (supplementary Fig. II).
Fig. 1.
Histopathology of the liver tissues by hematoxylin and eosin staining from ORX SD male rats (n = 30 per HFD group; n = 10 per ND group). A1–A3: A representative liver specimen from the ORX ND control group. Dotted arrow depicts the liver zonation. Zone 1, cells closest to the portal triad; zone 2, cells in between portal triad and central vein; zone 3, cells near central vein (A2). B1–B3: Liver cells with marked fat accumulation in the ORX HFD control in a representative liver specimen. Closed arrows in B3 depict the inflammation, and the open arrows depict fatty liver cells; 1 and 3 represent zones 1 and 3. C1–C3: A representative liver specimen from the E2-treated group (E2 1.0 mg/kg). Zones 2 and 3 of liver lobules contain normal-appearing liver cells rimmed with marked fatty changes in zone 3. D1–D3: A representative liver specimen from the DHT-treated group (3.0 mg/kg). Zones 1 and 2 of liver lobules contain normal-appearing liver cells rimmed with marked fatty changes in zone 1. Closed arrows in D3 depict the normalized hepatocyte, and the open empty arrows depict the fatty liver cell. E1–E3: Liver from the E2+DHT-treated group (1.0 mg/kg E2+3.0 mg/kg DHT). Zones 2 and 3 of liver lobules contain normal-appearing liver cells rimmed with lesser fatty changes in zone 1. Middle and right panels represent higher magnifications of dotted box areas of left panel. Bars, 50 μm.
Serum E2 and DHT levels
E2, DHT, and E2+DHT treatments increased serum E2 and DHT levels compared with control ORX HFD control groups (Table 3). There was no statistically significant difference between the levels of ND control versus E2 (0.20 + 15 vs. 0.43 + 0.16) or ND control versus DHT-treated (P < 0.08), most likely due to the high individual variance.
Serum parameters and lipid profiles and intrahepatic static levels of palmitic, palmitoleic, and oleic acids
The HFD significantly increased the serum ALT levels compared with the ORX ND control (P < 0.05) (Table 3). There was a significant decrease (P < 0.01) in serum ALT levels in SD rats treated with E2 or E2+DHT compared with the HFD controls (Table 3). Transporting the endogenous cholesterol into liver is the biological function of HDL, and transporting cholesterol into extrahepatic tissues is the biological function of LDL. Thus, hepatic HDL cholesterol (HDL-c) and LDL cholesterol (LDL-c) are important biological parameters of lipid balance. The HFD itself significantly increased (P < 0.01) total hepatic and serum cholesterol, TG, and LDL-c levels and decreased the HDL-c levels compared with the ND in ORX male rats (Table 3). E2 and E2+DHT treatments reduced serum TG and increased HDL-c levels (P < 0.05 compared with the HFD control levels) (Table 3). DHT and E2+DHT significantly reduced serum LDL-c levels (Table 3). Treatment with E2, DHT, and E2+DHT decreased serum total cholesterol and cholesterol ester, respectively (Table 3). ApoB100 was significantly decreased after all treatments (Table 3), highlighting the additive positive treatment effect.
Furthermore, E2, DHT, and E2+DHT treatment significantly decreased the production of palmitic, palmitoleic, and oleic acid by hepatic metabolic analysis for lipogenesis (Table 4). GC measurement of free fatty acids in rat liver could only reflect the static level of changes. Genetic profiling of lipogenesis is shown in Fig. 2.
TABLE 4.
Intrahepatitic static levels of various fatty acids (palmitic, palmitoleic, and oleic acid) from homogenized rat liver tissues with or without different treatments in ORX SD rats (concentration expressed in mg/g tissue)
| Normal Diet | High-fat Diet |
||||
| Control | Control | E2 | DHT | E2+DHT | |
| Palmitic acid | 12.49 ± 0.84 | 35.37 ± 2.74*,a | 6.82 ± 0.64b | 13.1 ± 2.54c | 19.001 ± 3.2c |
| Palmitoleic acid | 0.81 ± 0.16 | 11.41 ± 1.14*,a | 5.85 ± 1.06b | 6.05 ± 1.42b | 5.11 ± 0.75b |
| Oleic acid | 6.45 ± 1.0 | 90.83 ± 6.12*,a | 25.44 ± 4.01b | 62.06 ± 3.7c | 50.67 ± 3.02c |
The data groups provided with only different letter superscripts (e.g., a vs. b or a vs. b, c etc.) are statistically significantly different (P < 0.05), where data from E2/DHT/E2+DHT groups should be mainly compared with high-fat control. *P < 0.05, for control high-fat diet vs. normal diet control groups only.
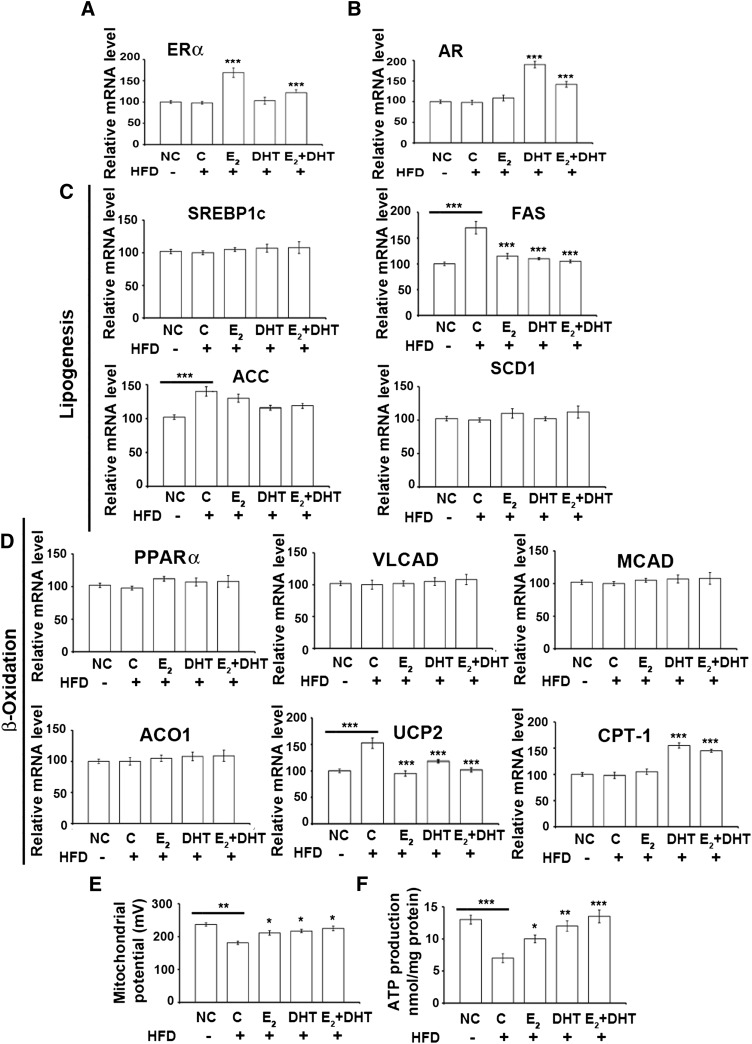
Fig. 2.
Gene expressions of ERα and AR and genes related to de novo synthesis of fatty acids, β-oxidation, and mitochondrial parameters (n = 15 per HFD group; n = 10 per ND group). Gene expression of ERα (A), AR (B), and different components of lipogenesis (C) and β-oxidation (D) at mRNA levels by RT-PCR. E: The mitochondrial membrane potential from the treatments. F: Production of ATP from liver cells. Data from treatment groups are compared with the HFD control group (or HFD compared with ND). *P < 0.05, **P < 0.01, and ***P < 0.001. ACC, acetyl coenzyme A carboxylase; ACO1, acyl-CoA oxidase 1; C-HFD, control high fat diet; CPT1, carnitine palmitotyltransferase1; MCAD, medium-chain acyl-CoA dehydrogenase; NC, normal diet; PPARα, peroxisome proliferator-activated receptor α; SCD1, stearoyl-coenzyme A desaturase 1; SREBP-1c, sterol regulatory element-binding protein-1c; UCP2, uncoupling protein 2; VLCAD, very long-chain acyl-CoA dehydrogenase.
Local ER/AR pathways in the rat liver
E2 increased ERα expression significantly compared with nontreated rats on HFD controls (Figs. 2A and 3A). ERβ signal was undetectable in livers of all groups. DHT significantly increased AR expression (P < 0.01) compared with control ORX HFD mice (Figs. 2B and 3A). Upon E2/DHT treatment, a reciprocal suppression of ERα was found with DHT and of AR with E2 (Figs. 2A, B and 3A).
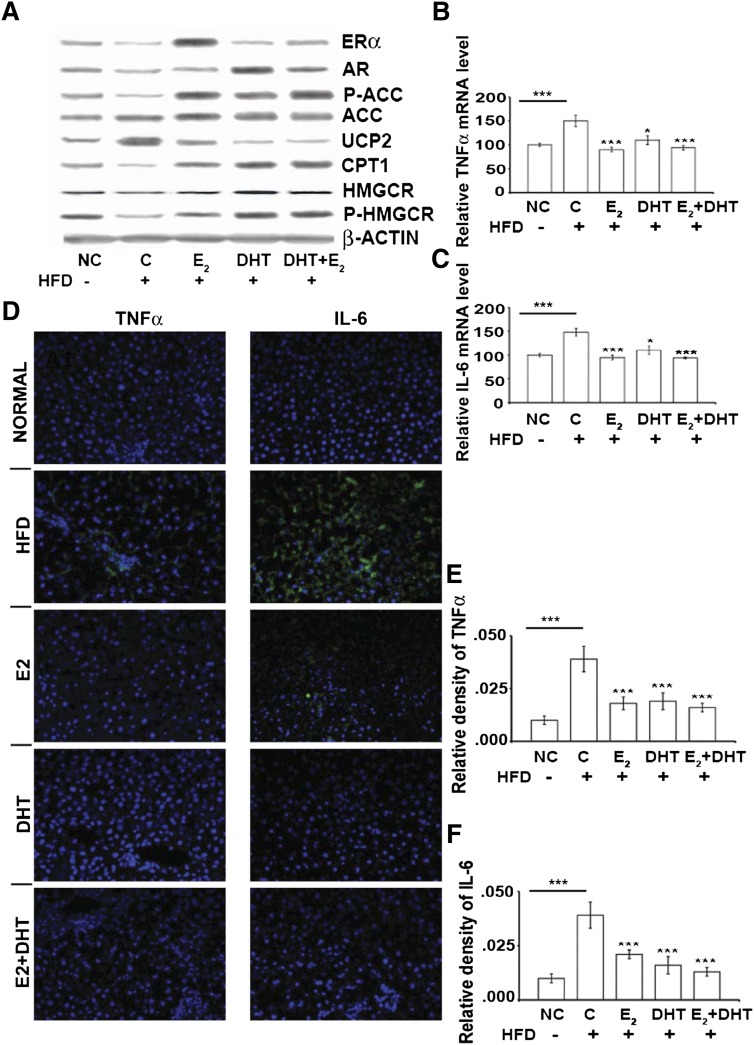
Fig. 3.
Alterations of liver proteins, IL-6, and TNF-α levels after different treatments (n = 15 per HFD group; n = 10 per ND group). A: Protein levels in ORX rat livers after different treatments analyzed by Western blot (β-actin as internal control). mRNA expression of TNF-α (B) and IL-6 (C) by RT-PCR and TNF-α protein (D, left panel) and IL-6 protein (D, right panel) in liver by FITC/DAPI immunofluorescence after different treatments. Green fluorescence is mainly shown in HFD groups (TNF-α, IL-6). Cells were counterstained with DAPI. E and F: Semi-quantification of the positive signals for TNF-α (D, left panel) and IL6 (D, right panel). Data (B, C, E, F) from E2, DHT, and E2+DHT groups are compared with HFD control (or HFD compared with ND). ***P < 0.001. Dose for E2 is 1.0 mg/kg and for DHT is 3.0 mg/kg. C-HFD, control of high fat diet; NC, normal diet.
Estrogen and androgen may regulate the expression of genes involved in de novo synthesis of fatty acids and β-oxidation
Among the key hepatic lipogenic genes, the expression of sterol regulatory element-binding protein-1c, stearoyl-CoA desaturase 1, and fatty acid synthase (FAS) was similar in all treatment groups, except for a significant increase of FAS in the HFD control group compared with ND (Fig. 2C). E2, DHT, or E2+DHT downregulated the FAS compared with HFD. Acetyl-CoA carboxylase (ACC) was significantly increased in the HFD groups compared with ND (Fig. 2C).
We also analyzed mRNA expression of the key genes associated with β-oxidation. The expression levels of peroxisome proliferator-activated receptor α, very long-chain acyl-CoA dehydrogenase, medium-chain acyl-CoA dehydrogenase, and acyl-CoA oxidase 1 did not change in any of the treated groups. However, the expression of uncoupling protein2 (UCP2) was significantly downregulated (P < 0.05) in the E2-, DHT-, and E2+DHT-treated groups compared with HFD controls (Figs. 2D, 3A). Carnitine palmitotyltransferase 1 (CPT1) was increased by DHT and E2+DHT treatment compared with HFD controls (Figs. 2D, 3A). No significant changes were observed in genes involved in TG synthesis (supplementary Fig. III).
Estrogen and androgen may improve MMP and static ATP levels
An increase of MMP (Fig. 2E) and static ATP levels (Fig. 2F) in E2, DHT, and E2+DHT groups compared with the ORX HFD control was found. Both MMP and static ATP significantly decreased with the HFD control groups compared with the ND control group (Fig. 2E, F). Steroid treatments significantly decreased UCP2 and increased MMP and static ATP levels in the ORX rat liver on HFD (Fig. 2E, F).
Downregulation of lipogenesis by ACC phosphorylation was probably due to estrogen action in rat liver
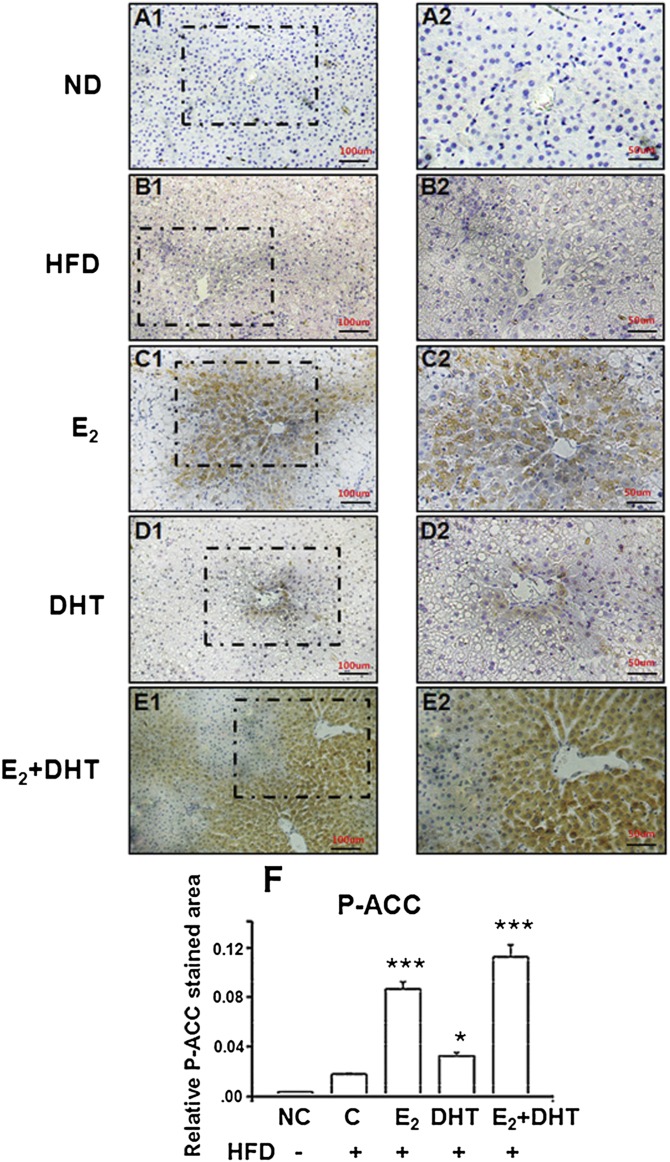
E2, DHT, and E2+DHT treatments significantly increased the phosphorylation of the rate-limiting enzyme in de novo fatty acid synthesis, ACC (P-ACC), compared with HFD controls (Fig. 3A and supplementary Fig. IV), although it seemed that ACC enzyme was not regulated at the level of gene expression by DHT (Figs. 2C and 3A). Abundant P-ACC protein expression was further confirmed by immunohistochemistry and morphometric quantification, showing highest abundance in zone 3 localization (Fig. 4A–F). E2, DHT, and E2+DHT treatments enhanced the protein phosphorylation (i.e., P-ACC/total ACC levels) (supplementary Fig. IV). These results suggested that E2 is involved in the suppression of lipogenesis through P-ACC.
Fig. 4.
A–F: Zone-specific immunohistochemical assay for P-ACC in SD rat liver tissues (n = 30 per HFDt group; n = 10 per ND group). A1 and A2: A representative specimen liver from the ORX ND control group. Dotted arrow depicts the liver zonation. Zone 1, cells closest to the portal triad; zone 2, cells in between portal triad and central vein; zone 3, cells near central vein (A2). B1 and B2: Liver cells with marked fat accumulation in the ORX HFD control. C1 and C2: A representative specimen liver from the E2-treated group. Brownish stained cells observed in cytosol in zones 2 and 3 of liver lobules represent the P-ACC-positive cells. D1 and D2: A representative specimen liver from the DHT-treated group. Only a few brownish cytosol stained cells were observed in zone 3 of liver lobules in the liver samples. E1 and E2: A representative specimen liver from the E2+DHT-treated group. Zones 2 and 3 of liver lobules contain strong brownish cytosol stained cells, whereas no positive signal was observed among hepatocytes near zone 1. Right panels represent higher magnifications of dotted box areas of left panel; 1 and 3 represent zones 1 and 3. F: Morphometric quantification analysis of the P-ACC-stained areas of the liver shown as mean ± SEM. *** P < 0.001 versus C-HFD (control of high-fat diet).
Downregulation of cholesterol biosynthesis by increasing HMGCR phosphorylation was associated most likely with androgen action
Treatment with DHT resulted in a marked decrease in the concentrations of total cholesterol, LDL-c, and ApoB. Marked increase of HMGCR phosphorylation in the DHT and E2+DHT groups (Fig. 3A) and enhanced phosphorylation related to protein levels (i.e., 3-hydroxy-3-methyl-glutaryl-CoA reductase [P-HMGCR]/total HMGCR levels) (supplementary Fig. IV) was observed.
Estrogen and androgens are associated with the prevention of nonalcoholic steatohepatitis progression by amelioration of tumor necrosis factor-α and interleukin-6
Tumor necrosis factor-α (TNF-α) (Fig. 3B) and interleukin-6 (IL-6) (Fig. 3C) were significantly downregulated in all HFD ORX rats treated with E2, DHT, and E2+DHT compared with the HFD control. Conversely, TNF-α and IL-6 (Fig. 3B, C) were upregulated in the ORX HFD group compared with ND (P < 0.05). These alterations (Fig. 3D) were confirmed by immunofluorescence visualization. E2, DHT, and E2+DHT significantly decreased the signals of TNF-α and IL-6 (shows only FITC/DAPI signal) (Fig. 3E, F).
Molecular actions of estrogen and androgen in a rat BRL3A hepatocyte cell line in vitro
With 330 µmol/l palmitic acid and 670 µmol/l OA, the rat BRL3A hepatocyte controls formed abundant reddish ORO-positive cells, whereas the E2 and DHT treatments resulted in scant ORO staining (Fig. 5A). The addition of 1 μM ICI or Flu to the BRL3A cells reflected the red staining comparable to controls (Fig. 5A). The combination of E2+DHT totally blocked the ORO-positive formation (Fig. 5A), and morphometric quantification is shown in Fig. 5B. Furthermore, treatment with E2, DHT, and E2+DHT significantly decreased the production of total cholesterol and cholesterol ester in BRL3A hepatocytes (supplementary Table III). Due to insufficient sensitivity of the assays, no data could be obtained on TG, HDL-c, and LDL-c.
Fig. 5.
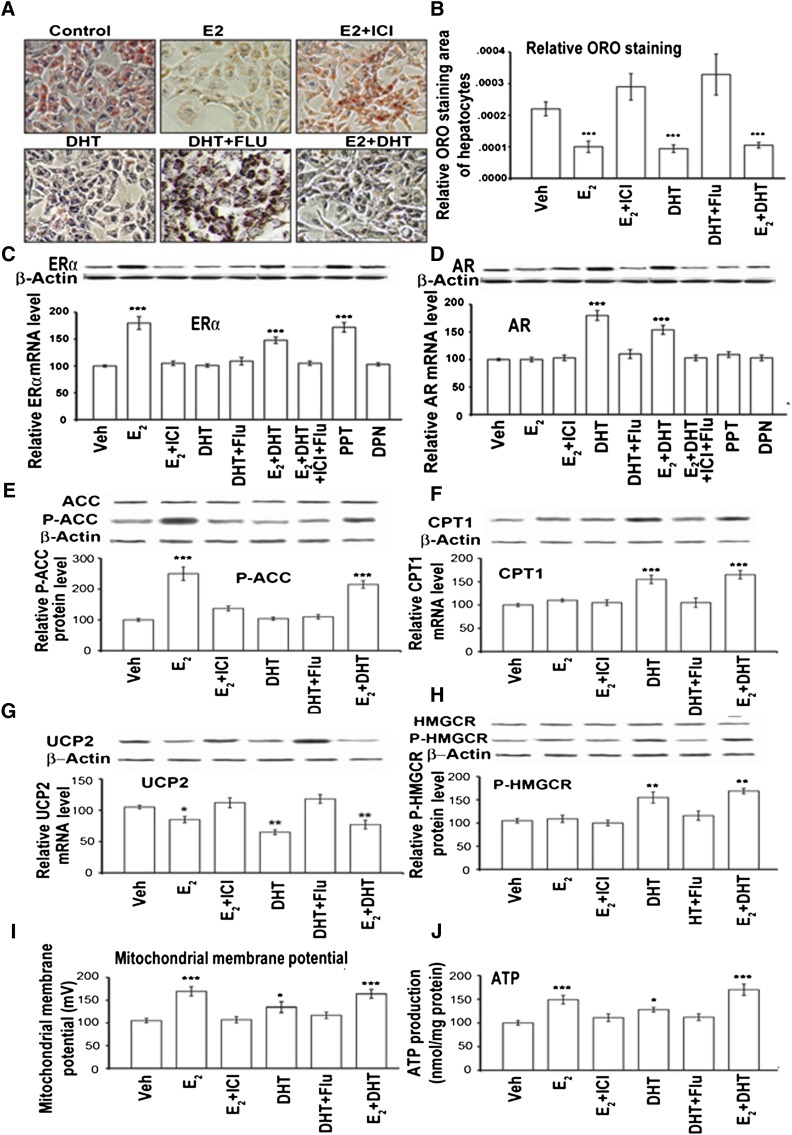
Estrogen and androgen action on liver steatosis by ORO staining on ERα/AR levels and MMP and static ATP level changes in vitro in a rat BRL3A hepatocyte cell line. BRL3A rat hepatocytes pretreated with 330 µmol/l of PA and 670 µmol/l of OA in ORO staining without (control) and with different treatments (E2, E2+ICI, DHT, DHT+Flu, E2+DHT, PPT, and DPN). A: E2 and DHT treatments resulted in less dense ORO staining than control. The addition of 100 nM ICI or Flu reflected the red staining comparable to the control. E2+DHT totally blocked the formation of ORO-positive cells. B: Semiquantification of the ORO-positive signals. Western blot analysis of proteins (C and D, upper panel bands) and semiquantification mRNA (C and D, lower graph panels; RT-PCR to β-actin) and protein levels of ERα (C) and AR (D). Western blot analysis of proteins (E–H, upper panel bands) and semiquantification mRNA (F and G, lower graph panels; RT-PCR to β-actin) and protein levels of P-ACC; below P-ACC protein semiquantification levels of ACC (E), CPT1 (F), UCP2 (G), below P-HMGCR protein semiquantification levels of HMGCR (H) to β-actin in rat BRL3A hepatocytes. Mitochondrial membrane potential (I) and ATP levels (J) in rat BRL3A hepatocytes. Data from treatment groups were compared with vehicle control. The addition of PPT and DPN is at 10 nM. *P < 0.05, **P < 0.01, and ***P < 0.001.
Treatment of the rat BRL3A hepatocytes in vitro for 24 h with E2, E2+DHT, and PPT resulted in upregulation of ERα (Fig. 5C). This ERα upregulation was blocked by ICI (P < 0.05; E2+ICI vs. E2 or PPT), but DPN did not induce any changes (Fig. 5C). Additionally, AR expression was significantly upregulated by DHT and E2+DHT and downregulated by Flu (Fig. 5D).
To verify the E2/ER- and/or DHT/AR-mediated blockage of TG, we analyzed genes associated with de novo fatty acid synthesis and β-oxidation in vitro in BRL3A cells. The expression of sterol regulatory element-binding protein-1c, stearoyl-CoA desaturase 1, and FAS was similar among the treated groups (supplementary Fig. VA). E2 significantly upregulated the P-ACC levels, which were significantly suppressed by ICI. DHT alone did not alter the level of P-ACC in hepatocytes (Fig. 5E).
The expression of peroxisome proliferator-activated receptor α, very long-chain acyl-CoA dehydrogenase, medium-chain acyl-CoA dehydrogenase, and acyl-CoA oxidase 1 was similar among groups (supplemental Fig. VB). DHT significantly increased CPT1, and this DHT-mediated increase could be disrupted by Flu (Fig. 5F). UCP2 expression was significantly downregulated (P < 0.05) by E2 and DHT (Fig. 5G), and this suppression was reversed by ICI and Flu (Fig. 5G). UCP2 protein was nearly absent after the treatment with E2+DHT (Fig. 5G). DHT and E2+DHT significantly increased the P-HMGCR (Fig. 5H), and this increase could be significantly suppressed by Flu. There was an increase in mitochondrial membrane potential after E2, DHT, and E2+DHT compared with control (Fig. 5I). Moreover, E2, DHT, and E2+DHT increased intracellular static ATP, which was significantly suppressed by Flu (Fig. 5J).
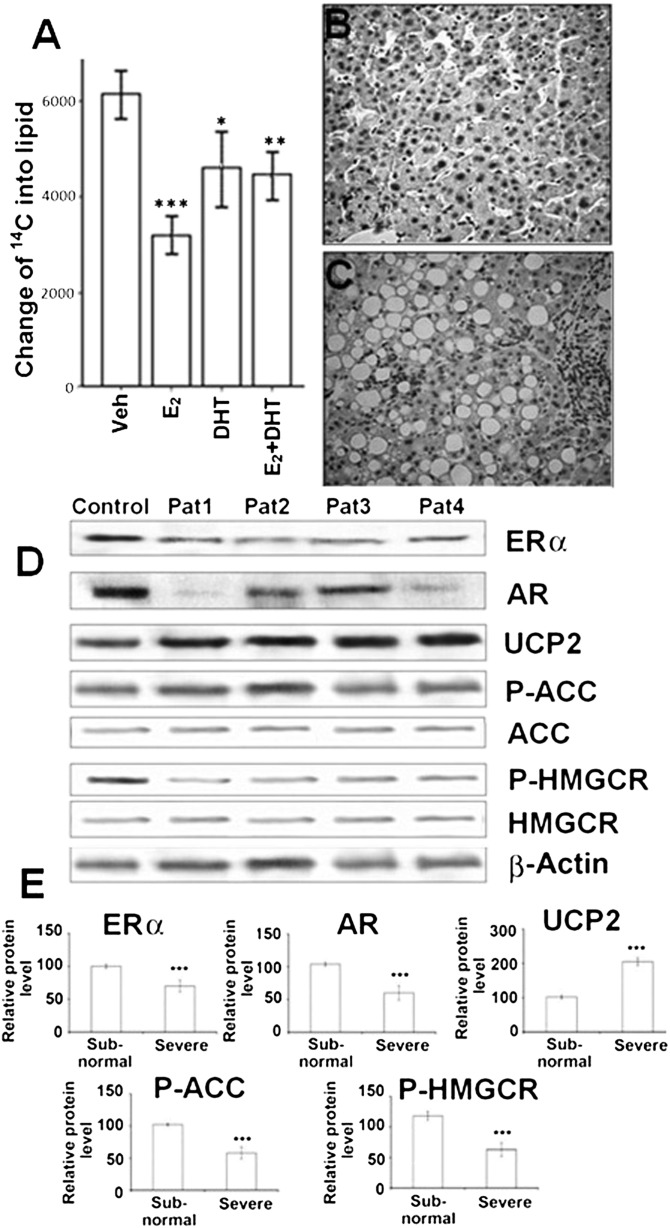
We measured the incorporation rate for de novo lipogenesis by using 14C-labeled acetic acid method in hepatocytes. Treatment with E2, DHT, and E2+DHT significantly decreased the incorporation of 14C from the lipid-containing phase (Fig. 6A). In this de novo lipogenesis by 1-14C-labeling acetic acid procedure, we traced the total radiation that can reflect the lipogenesis. Acetate is the precursor of lipid synthesis, and fatty acid is the substrate of sterols and lipid synthesis. The 14C-acetate labeling procedure done in BRL3A cells reflected lipogenesis. The neutral solvent extraction method used could have collected labeled sterols and other lipids, but fatty acids would likely predominate among radiolabeled lipids.
Fig. 6.
14C acetate incorporation analysis in hepatocytes and the expression of genes in severe and milder/subnormal human male steatotic liver samples. A: BRL 3A hepatocytes treated with E2 and DHT were labeled with 14C acetate, and the levels of 14C incorporation into the lipid fraction are shown relative to untreated controls. B: Milder/subnormal human male steatotic liver sample (n = 5). C: Severe human male steatotic liver sample (n = 5). D: Protein bands of ERα, AR, UCP2, ACC, P-ACC, HMGCR, and P-HMGCR and β-actin (as control) by Western blot analysis. Pat 1–4 represents four individual patients’ liver samples. E: The relative protein levels of ERα, AR, UCP2, P-ACC, and P-HMGCR to β-actin. *P < 0.05, **P < 0.01, ***P < 0.001. Pat, patient.
Gene expression changes in liver steatosis in men are similar to the NAFLD rat model
To demonstrate the clinical relevance of this NAFLD rat model for human patients, we analyzed human liver samples for pathological changes (Fig. 6B, C) and protein expressions of ERα, AR, P-ACC, ACC, UCP2, HMGCR, and P-HMGCR in severe (Fig. 6D; n = 5) and milder/subnormal (Fig. 6D; n = 5) male NAFLD. The expression of ERα and AR were markedly decreased in severe steatotic livers compared with the subnormal control ones (Fig. 6D, E). Decreased P-ACC (Fig. 6D, E), increased UCP2, and decreased P-HMGCR expression could be observed in severe steatotic livers compared with mild/subnormal controls (Fig. 6D, E). There was no significant difference between the subnormal/milder control and severe groups in serum lipid parameters, T and E2, because all the serum parameters from the controls were of abnormal range (supplementary Fig. VI).
DISCUSSION
Numerous clinical and experimental studies have demonstrated that estrogen (19, 34) or androgen (25, 35) elicit favorable effects on reducing body fat mass, thereby improving the liver steatosis. Recently, it has been shown that E2 deficiency accelerated nonalcoholic steatohepatitis (NASH) progression in ovariectomized mice fed a high–fat, high-cholesterol diet and that this effect was improved by estrogen therapy (36). We have demonstrated recently in a pilot study that strong suppression of the actions of endogenous estrogens and/or androgens by Tam, Flu, or Tam/Flu induced NASH in adult intact male SD rats receiving HFD (but never with ND) (24). In the current study, we depleted endogenous steroids by ORX rats and rats supplemented with E2, DHT, or E2+DHT to study the precise differential action of estrogen and androgens on HFD-treated NAFLD.
We demonstrated that the downregulation of lipogenesis with E2 treatment in abundance in zone 3 might be associated with an ERα-mediated increase in ACC phosphorylation. Accordingly, E2 treatment significantly decreased serum TG levels in ORX SD rats and upregulated ERα. We have shown previously that Tam treatment significantly downregulated ERα and increased TG levels in intact SD rat liver. Because we could not detect any ERβ expression (24), this estrogen effect must be mediated by the ERα pathway. This ERα-mediated inhibition of lipogenesis was due to increased P-ACC, which we confirmed by treating hepatocytes with PPT and E2. Accordingly, a recent study has shown that Tam promotes hepatic steatosis by increasing lipogenesis through the reduction of P-ACC (37). DHT treatment significantly increased CPT-1 expression, with the highest abundance in zone 1 through a significant increase of β-oxidation and a decrease of cholesterol biosynthesis by HMGCR phosphorylation via the AR pathway. Our study also revealed enhanced the protective effects of E2 and DHT on liver lipid metabolism. These additive effects of E2 and DHT treatments on lipid homeostasis were demonstrated by normalization of liver histology in zones 1, 2, and 3, as measured by decreased lipogenesis via P-ACC. This was further confirmed by decreased de novo lipogenesis, which in turn decreased serum TG levels and normalized β-oxidation with increased MMP and static ATP in vivo and in vitro. Although our results show that mitochondrial activity is involved in ER/AR of lipid homeostatis, a putative role of oxidative stress triggering inflammation associated with NASH cannot be ruled out and needs to be studied further. We found that cholesterol biosynthesis was decreased as indicated by P-HMGCR (which was further confirmed by the significant decrease in total hepatic and serum cholesterol, LDL-c, and serum ApoB concentrations in E2/DHT groups). A clinical study on transdermal DHT in older men with partial androgen deficiency demonstrated decreased total and LDL-c and unchanged HDL-c and TG (38), which supports our findings that DHT decreases cholesterol biosynthesis to some extent. DHT treatment did not decrease serum E2 levels in that study (38), which may also implicate that the balance of estrogen and androgen levels is crucial for the maintenance of lipid homeostasis in men.
In line with this induced NAFLD rat model, we detected decreased levels of ERα and AR and P-ACC in steatosis patients compared with subnormal/milder controls. Using subnormal/mild liver as control could have caused an underestimation of the alterations in gene expression. Despite these subnormal controls, significantly increased UCP2 and decreased P-HMGCR levels were observed in severe human liver steatosis. These preliminary findings highlight the need for a large-scale clinical investigation on estrogen and androgen actions on human NAFLD.
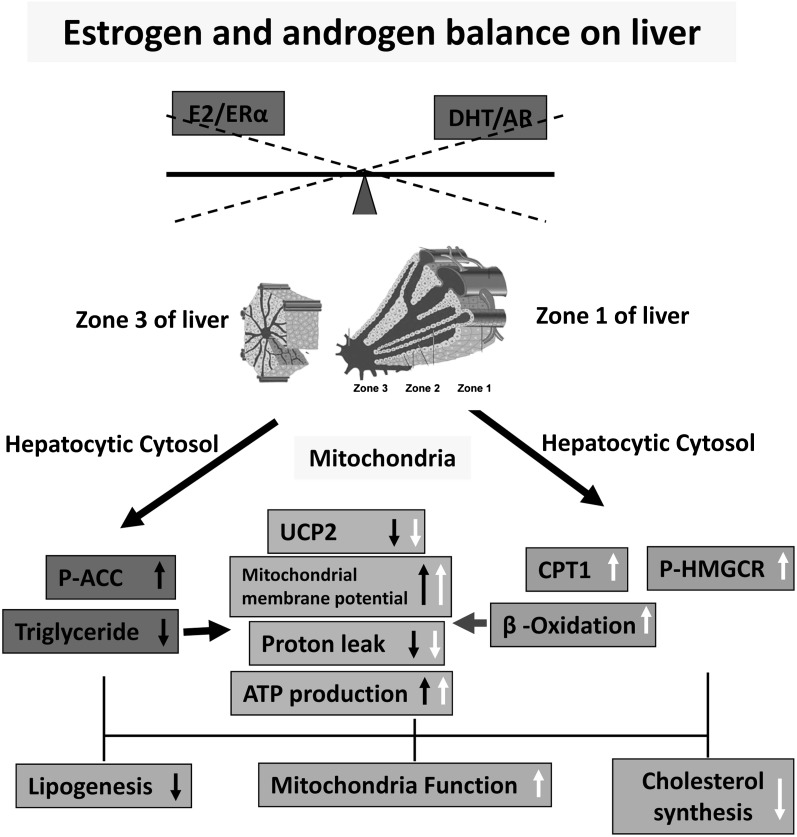
We showed here a possible molecular mechanism explaining the additive positive effects of estrogen and androgen in the prevention of NAFLD (Fig. 7). The estrogen/ERα pathway mainly suppresses hepatic steatosis by decreasing lipogenesis mostly in zone 3 through the induction of P-ACC and, in turn, improving mitochondrial function by inhibiting proton leakage (Fig. 7, closed arrows). The androgen/AR pathway mainly suppresses hepatic steatosis by increasing CPT-1-mediated β-oxidation mainly in zone 1 and decreasing cholesterol biosynthesis (Fig. 7, open arrows). Applications of estrogen and androgen modulate hepatic lipid metabolism by decreasing lipogenesis and cholesterol biosynthesis and by increasing β-oxidation; both pathways subsequently normalize the MMP and ATP production and prevent NASH by down-regulating TNF-α and IL-6. Our findings suggest that the combined administration of selective ER and AR modulators (SERMs and SARMs) could be a potential therapeutic approach for NAFLD.
Fig. 7.
Possible mechanisms of estrogen/androgen action in the prevention of NAFLD/NASH. The estrogen/ERα pathway mainly suppresses hepatic steatosis by decreasing lipogenesis in zone 3 through the induction of P-ACC and improving mitochondrial function by inhibiting proton leakage (closed arrows). The androgen/AR pathway mainly suppresses hepatic steatosis by increasing CPT-1-mediated β-oxidation in zone 1 and decreasing cholesterol synthesis (open arrows). Altogether, estrogen/androgen modulate hepatic lipid metabolism and subsequently normalize the mitochondrial function.
Supplementary Material
Acknowledgments
The authors thank Dr. Stanford Chan from Imperial College London for revising the English language of the revised manuscript.
Footnotes
Abbreviations:
- ALT
- alanine aminotransferase
- AR
- androgen receptor
- ARKO
- androgen receptor knockout mouse
- CPT1
- carnitine palmitotyltransferase1
- DHT
- 5α-dihydrotestosterone
- DPN
- 2,3-bis(4-hydroxyphenyl) propionitrile
- E2
- estradiol
- ER
- estrogen receptor
- αERKO
- knockout mouse for ERα
- FAS
- fatty acid synthase
- HDL-c
- high density lipoprotein cholesterol
- HFD
- high-fat diet
- IR
- insulin resistance
- LDL-c
- low density lipoprotein cholesterol
- MMP
- mitochondrial membrane potential
- NAFLD
- nonalcoholic fatty liver disease
- NASH
- nonalcoholic steatohepatitis
- ORO
- Oil Red O
- ORX
- orchidectomized
- P-ACC
- acetyl coenzyme A carboxylase
- P-HMGCR
- 3-hydroxy-3-methyl-glutaryl-CoA reductase
- PPT
- 4,4,4′-(4-propyl-[1H]pyrazole-1,3,5-triyl) triphenol
- SD
- Sprague-Dawley
- Tam
- tamoxifen
- TG
- triglyceride
- UCP2
- uncoupling protein 2
- VP
- ventral prostate
This work was supported by Ministry of Science and Technology Grants (MOST) 2012AA020601, Beijing Natural Science Foundation 5111002, and MOST 2011CB944103 (X.L.); and National Natural Science Foundation of China 881021003 (G.F.G.).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of four tables and seven figures.
REFERENCES
- 1.Cheung O., Kapoor A., Puri P., Sistrun S., Luketic V. A., Sargeant C. C., Contos M. J., Shiffman M. L., Stravitz R. T., Sterling R. K., et al. 2007. The impact of fat distribution on the severity of nonalcoholic fatty liver disease and metabolic syndrome. Hepatology. 46: 1091–1100 [DOI] [PubMed] [Google Scholar]
- 2.Sanyal A. J. 2002. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 123: 1705–1725 [DOI] [PubMed] [Google Scholar]
- 3.Hewitt K. N., Pratis K., Jones M. E., Simpson E. R. 2004. Estrogen replacement reverses the hepatic steatosis phenotype in the male aromatase knockout mouse. Endocrinology. 145: 1842–1848 [DOI] [PubMed] [Google Scholar]
- 4.Jones M. E., Thorburn A., Britt K. 2000. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc. Natl. Acad. Sci. USA. 97: 12735–12740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murata Y., Ogawa Y., Saibara T., Nishioka A., Fujiwara Y., Fukumoto M., Inomata T., Enzan H., Onishi S., Yoshida S. 2000. Unrecognized hepatic steatosis and non-alcoholic steatohepatitis in adjuvant tamoxifen for breast cancer patients. Oncol. Rep. 7: 1299–1304 [DOI] [PubMed] [Google Scholar]
- 6.Carani C., Qin K., Simoni M., Faustini-Fustini M., Serpente S., Boyd J., Korach K. S., Simpson E. R. 1997. Effect of testosterone and estradiol in a man with aromatase deficiency. N. Engl. J. Med. 337: 91–95 [DOI] [PubMed] [Google Scholar]
- 7.Smith E. P., Boyd J., Frank G. R., Takahashi H., Cohen R. M., Specker B., Williams T. C., Lubahn D. B., Korach K. S. 1994. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N. Engl. J. Med. 331: 1056–1061 [DOI] [PubMed] [Google Scholar]
- 8.Maffei L. 2004. Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: effects of testosterone, alendronate, and estradiol treatment. J. Clin. Endocrinol. Metab. 89: 61–70 [DOI] [PubMed] [Google Scholar]
- 9.Lubahn D. B., Moyer J. S., Golding T. S., Couse J. F., Korach K. S., Smithies O. 1993. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc. Natl. Acad. Sci. USA. 90: 11162–11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Couse J. F., Hewitt S. C., Bunch D. O., Sar M., Walker V. R., Davis B. J., Korach K. S. 1999. Postnatal sex reversal of the ovaries in mice lacking estrogen receptors alpha and beta. Science. 286: 2328–2331 [DOI] [PubMed] [Google Scholar]
- 11.Lemieux C., Phaneuf D., Labrie F., Giguere V., Richard D., Deshaies Y. 2005. Estrogen receptor alpha-mediated adiposity-lowering and hypocholesterolemic actions of the selective estrogen receptor modulator acolbifene. Int J Obes (Lond). 29: 1236–1244 [DOI] [PubMed] [Google Scholar]
- 12.Boyanov M. A., Boneva Z., Christov V. G. 2003. Testosterone supplementation in men with type 2 diabetes, visceral obesity and partial androgen deficiency. Aging Male. 6: 1–7 [PubMed] [Google Scholar]
- 13.Kapoor D., Goodwin E., Channer K. S., Jones T. H. 2006. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur. J. Endocrinol. 154: 899–906 [DOI] [PubMed] [Google Scholar]
- 14.Haider A., Gooren L. J., Padungtod P., Saad F. 2010. Improvement of the metabolic syndrome and of non-alcoholic liver steatosis upon treatment of hypogonadal elderly men with parenteral testosterone undecanoate. Exp. Clin. Endocrinol. Diabetes. 118: 167–171 [DOI] [PubMed] [Google Scholar]
- 15.Fan W., Yanase T., Nomura M., Okabe T., Goto K., Sato T., Kawano H., Kato S., Nawata H. 2005. Androgen receptor null male mice develop late-onset obesity caused by decreased energy expenditure and lipolytic activity but show normal insulin sensitivity with high adiponectin secretion. Diabetes. 54: 1000–1008 [DOI] [PubMed] [Google Scholar]
- 16.Sato T., Matsumoto T., Yamada T., Watanabe T., Kawano H., Kato S. 2003. Late onset of obesity in male androgen receptor-deficient (AR KO) mice. Biochem. Biophys. Res. Commun. 300: 167–171 [DOI] [PubMed] [Google Scholar]
- 17.Lin H. Y., Yu I. C., Wang R. S., Chen Y. T., Liu N. C., Altuwaijri S., Hsu C. L., Ma W. L., Jokinen J., Sparks J. D., et al. 2008. Increased hepatic steatosis and insulin resistance in mice lacking hepatic androgen receptor. Hepatology. 47: 1924–1935 [DOI] [PubMed] [Google Scholar]
- 18.Alexandersen P., Christiansen C. 2004. The aging male: testosterone deficiency and testosterone replacement. An up-date. Atherosclerosis. 173: 157–169 [DOI] [PubMed] [Google Scholar]
- 19.Simpson E. R., Jones M. E., Clyne C. 2006. Lessons from the ArKO mouse. In Aromatase inhibitors. B. J. A. Furr, editor. Birkhäuser Verlag, Switzerland. 139–155. [Google Scholar]
- 20.Couse J. F., Korach K. S. 1999. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr. Rev. 20: 358–417 [DOI] [PubMed] [Google Scholar]
- 21.Walters K. A., McTavish K. J., Seneviratne M. G., Jimenez M., McMahon A. C., Allan C. M., Salamonsen L. A., Handelsman D. J. 2009. Subfertile female androgen receptor knockout mice exhibit defects in neuroendocrine signaling, intraovarian function, and uterine development but not uterine function. Endocrinology. 150: 3274–3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dieudonne M. N., Pecquery R., Boumediene A., Leneveu M. C., Giudicelli Y. 1998. Androgen receptors in human preadipocytes and adipocytes: regional specificities and regulation by sex steroids. Am. J. Physiol. 274: C1645–C1652 [DOI] [PubMed] [Google Scholar]
- 23.Walters K. A., Simanainen U., Handelsman D. J. 2010. Molecular insights into androgen actions in male and female reproductive function from androgen receptor knockout models. Hum. Reprod. Update. 16: 543–558 [DOI] [PubMed] [Google Scholar]
- 24.Mu Y., She R., Zhang H., Dong B., Huang C., Lin W., Li D., Li X. 2009. Effects of estrogen and androgen deprivation on the progression of non-alcoholic steatohepatitis (NASH) in male Sprague-Dawley rats. Hepatol. Res. 39: 910–920 [DOI] [PubMed] [Google Scholar]
- 25.Moverare-Skrtic S., Venken K., Andersson N., Lindberg M. K., Svensson J., Swanson C., Vanderschueren D., Oscarsson J., Gustafsson J. A., Ohlsson C. 2006. Dihydrotestosterone treatment results in obesity and altered lipid metabolism in orchidectomized mice. Obesity (Silver Spring). 14: 662–672 [DOI] [PubMed] [Google Scholar]
- 26.Foley J. F., Collins J. B., Umbach D. M., Grissom S., Boorman G. A., Heinloth A. N. 2006. Optimal sampling of rat liver tissue for toxicogenomic studies. Toxicol. Pathol. 34: 795–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colnot S., Perret C. 2011. Liver zonation. In Molecular pathology of liver diseases, molecular pathology library 5. Satdarshan P. S. Monga, editor. Springer, New York, Dordrecht, Heidelberg, London. 7–16. [Google Scholar]
- 28.Li X., Nokkala E., Yan W., Streng T., Saarinen N., Warri A., Huhtaniemi I., Santti R., Makela S., Poutanen M. 2001. Altered structure and function of reproductive organs in transgenic male mice overexpressing human aromatase. Endocrinology. 142: 2435–2442 [DOI] [PubMed] [Google Scholar]
- 29.Shen Z. J., Lu Y. L., Chen Z. D., Chen F., Chen Z. 2000. Effects of androgen and ageing on gene expression of vasoactive intestinal polypeptide in rat corpus cavernosum. BJU Int. 86: 133–137 [DOI] [PubMed] [Google Scholar]
- 30.Sambrook J., Russel D. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Woodbury, NY. [Google Scholar]
- 31.Li X., Strauss L., Kaatrasalo A., Mayerhofer A., Huhtaniemi I., Santti R., Makela S., Poutanen M. 2006. Transgenic mice expressing p450 aromatase as a model for male infertility associated with chronic inflammation in the testis. Endocrinology. 147: 1271–1277 [DOI] [PubMed] [Google Scholar]
- 32.Coon H. G., Weiss M. C. 1969. A quantitative comparison of formation of spontaneous and virus-produced viable hybrids. Proc. Natl. Acad. Sci. USA. 62: 852–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yecies J. L., Zhang H. H., Menon S., Liu S., Yecies D., Lipovsky A. I., Gorgun C., Kwiatkowski D. J., Hotamisligil G. S., Lee C. H., et al. 2011. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 14: 21–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohlsson C., Hellberg N., Parini P., Vidal O., Bohlooly M., Rudling M., Lindberg M., Warner M., Angelin B., Gustafsson J. 2000. Obesity and disturbed lipoprotein profile in estrogen receptor-β-deficient male mice. Biochem. Biophys. Res. Commun. 278: 640–645 [DOI] [PubMed] [Google Scholar]
- 35.Vandenput L., Mellstrom D., Lorentzon M., Swanson C., Karlsson M. K., Brandberg J., Lonn L., Orwoll E., Smith U., Labrie F., et al. 2007. Androgens and glucuronidated androgen metabolites are associated with metabolic risk factors in men. J. Clin. Endocrinol. Metab. 92: 4130–4137 [DOI] [PubMed] [Google Scholar]
- 36.Kamada Y., Kiso S., Yoshida Y., Chatani N., Kizu T., Hamano M., Tsubakio M., Takemura T., Ezaki H., Hayashi N., et al. 2011. Estrogen deficiency worsens steatohepatitis in mice fed high-fat and high-cholesterol diet. Am. J. Physiol. Gastrointest. Liver Physiol. 301: G1031–G1043 [DOI] [PubMed] [Google Scholar]
- 37.Cole L. K., Jacobs R. L., Vance D. E. 2010. Tamoxifen induces triacylglycerol accumulation in the mouse liver by activation of fatty acid synthesis. Hepatology. 52: 1258–1265 [DOI] [PubMed] [Google Scholar]
- 38.Ly L. P., Jimenez M., Zhuang T. N., Celermajer D. S., Conway A. J., Handelsman D. J. 2001. A double-blind, placebo-controlled, randomized clinical trial of transdermal dihydrotestosterone gel on muscular strength, mobility, and quality of life in older men with partial androgen deficiency. J. Clin. Endocrinol. Metab. 86: 4078–4088 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.