Abstract
In vitro studies have suggested that HDL and apoB-containing lipoproteins can provide cholesterol for synthesis of glucocorticoids. Here we assessed adrenal glucocorticoid function in LCAT knockout (KO) mice to determine the specific contribution of HDL-cholesteryl esters to adrenal glucocorticoid output in vivo. LCAT KO mice exhibit an 8-fold higher plasma free cholesterol-to-cholesteryl ester ratio (P < 0.001) and complete HDL-cholesteryl ester deficiency. ApoB-containing lipoprotein and associated triglyceride levels are increased in LCAT KO mice as compared with C57BL/6 control mice (44%; P < 0.05). Glucocorticoid-producing adrenocortical cells within the zona fasciculata in LCAT KO mice are devoid of neutral lipids. However, adrenal weights and basal corticosterone levels are not significantly changed in LCAT KO mice. In contrast, adrenals of LCAT KO mice show compensatory up-regulation of genes involved in cholesterol synthesis (HMG-CoA reductase; 516%; P < 0.001) and acquisition (LDL receptor; 385%; P < 0.001) and a marked 40–50% lower glucocorticoid response to adrenocorticotropic hormone exposure, endotoxemia, or fasting (P < 0.001 for all). In conclusion, our studies show that HDL-cholesteryl ester deficiency in LCAT KO mice is associated with a 40–50% lower adrenal glucocorticoid output. These findings further highlight the important novel role for HDL as cholesterol donor for the synthesis of glucocorticoids by the adrenals.
Keywords: lecithin:cholesterol acyltransferase, glucocorticoids, cholesteryl esters
The production and subsequent secretion of glucocorticoids by adrenocortical cells of the zona fasciculata is dependent on the availability of the steroidogenic precursor cholesterol. Unesterified cholesterol is converted to glucocorticoids through a series of side-chain modifications by cytochrome P450 enzymes and hydroxysteroid dehydrogenases (1). The intramitochondrial transfer of unesterified cholesterol by the enzyme steroidogenic acute regulatory protein is considered to be the rate-limiting step in the basal synthesis of glucocorticoids. In vitro studies using isolated adrenocortical cells have suggested that HDL and apoB-containing lipoproteins are able to provide cholesterol as source for the synthesis of glucocorticoids (2–5). We and others have shown that under conditions where glucocorticoids are physiologically relevant (i.e., under stress), the exogenous uptake and intracellular processing of lipoprotein-associated cholesteryl esters becomes of crucial importance to maintain optimal adrenal glucocorticoid function in vivo. Probucol-induced depletion of plasma cholesterol associated with HDL and LDL in C57BL/6 wild-type mice is associated with a lower stress-induced glucocorticoid level (6). In addition, a defect in the hydrolysis of lipoprotein-associated cholesteryl esters in hormone-sensitive lipase knockout (KO) mice is associated with adrenocortical hypofunction (7). Furthermore, apolipoprotein A1 (APOA1) KO mice that virtually lack HDL particles and scavenger receptor BI (SR-BI) KO mice that exhibit an impaired uptake of cholesteryl esters from HDL show a parallel diminished adrenal glucocorticoid function (8–10). Combined, these findings suggest that the uptake of HDL-cholesteryl esters by the adrenals is essential to maintain optimal glucocorticoid production in vivo.
The HDL-associated enzyme lecithin-cholesterol acyltransferase (LCAT) mediates the synthesis of HDL-cholesteryl esters. Human subjects with a deleterious mutation on both alleles of the LCAT gene present with HDL deficiency, whereas heterozygotes typically have HDL cholesterol levels that are half of normal HDL cholesterol (11, 12). Heterozygous and homozygous LCAT KO mice show a similar dose-dependent decrease in plasma HDL levels (13) and thus represent a good mouse model to study the consequences of HDL-cholesteryl ester deficiency on general physiology. To delineate the quantitative contribution of HDL-associated cholesteryl esters to the adrenal glucocorticoid output, here we assessed adrenal glucocorticoid function in LCAT KO mice.
MATERIALS AND METHODS
Animals
LCAT KO mice (14) and C57BL/6 wild-type controls were bred in house and fed a regular chow diet ad libitum. Throughout the experiment both types of mice were housed in the same climate-controlled stable with a 12 h/12 h dark-light cycle and handled identically. Age-matched 10 to 12 week old C57BL/6 mice (n = 10) and LCAT mice (n = 8) were switched to a new cage and fasted overnight (∼18 h) before tail chop blood draws. After an additional 2 weeks, these mice were injected intraperitoneally with 200 μg human ACTH analog (ACTH [1–24]; tetracosactide) followed by tail blood draws at 1, 2, and 3 h after ACTH exposure. Six weeks after the start of experiment, the mice received an intraperitoneal 50 μg/kg sublethal dose of lipopolysaccharide (LPS) (Salmonella minnesota R595) followed by tail blood draws at 1, 2, and 3 h after LPS exposure. Mice were euthanized and tissue was harvested at 4 h after LPS exposure. Before all three types of stress, mice were bled through tail chop to obtain an average basal plasma corticosterone value of each mouse. An additional group of 12 week old LCAT KO (n = 4) and C57BL/6 mice (n = 11) was subjected to overnight fasting (18 h) and subsequently euthanized for tissue harvesting. Animal care and procedures were performed in accordance with the national guidelines for animal experimentation. All protocols were approved by the Ethics Committee for Animal Experiments of Leiden University.
Plasma lipid analyses
Plasma concentrations of free cholesterol, cholesteryl esters, and triglycerides were determined using enzymatic colorimetric assays. The cholesterol distribution over the different lipoproteins in plasma was analyzed by fractionation of 50 μl pooled plasma of each mouse genotype using a Superose 6 column (3.2 × 30 mm; Smart-system, Pharmacia). Free cholesterol and cholesteryl ester content of the effluent was determined using enzymatic colorimetric assays.
Adrenal neutral lipid visualization
Seven micrometer cryosections were prepared on a Leica CM3050-S cryostat. Cryosections were routinely stained with Oil red O for neutral lipid visualization. Nuclei were detected using a hematoxylin stain.
Plasma hormone analysis
Corticosterone levels in plasma were determined using the corticosterone 3H RIA Kit from ICN Biomedicals according to the protocol from the supplier.
Plasma tumor necrosis factor-α analysis
Tumor necrosis factor-α (TNF-α) protein levels were determined in plasma by ELISA (OptEIA kit, BD Biosciences Pharmingen, San Diego, CA) using the standard protocol.
Real-time quantitative PCR
Gene expression analysis was performed essentially as described (15). Equal amounts of RNA were reverse transcribed, and real-time quantitative PCR analysis was executed on the cDNA using an ABI Prism 7500 apparatus (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. Beta-actin and GAPDH were used as housekeeping genes for normalization.
Data analysis
Statistical analysis was performed using Graphpad Instat software (San Diego, CA). Normality of the experimental groups was confirmed using the method of Kolmogorov and Smirnov. The significance of differences was calculated using a two-tailed unpaired t-test or two-way ANOVA where appropriate. Probability values less than 0.05 were considered significant.
RESULTS
LCAT KO mice exhibit isolated HDL-cholesteryl ester deficiency
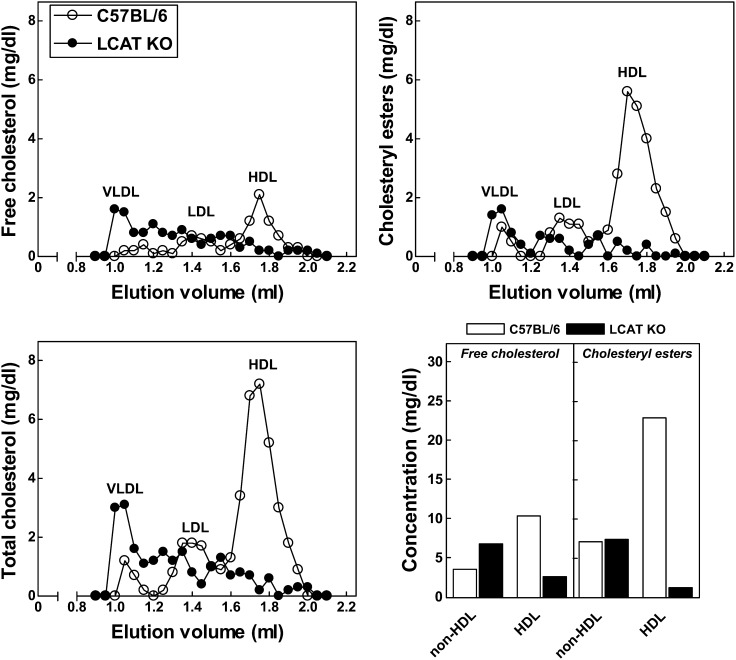
In accordance with a prominent role for LCAT in the esterification of free cholesterol (16), LCAT KO mice exhibited an almost complete absence of cholesteryl esters (−86%; P < 0.001) in plasma with unchanged plasma free cholesterol levels (Table 1). As a result, the free cholesterol to cholesteryl ester ratio was 8-fold higher (P < 0.001) in plasma from LCAT KO mice as compared with C57BL/6 wild-type control mice (Table 1). Fast-protein liquid chromatography lipoprotein analysis revealed that free cholesterol and cholesteryl ester levels in C57BL/6 wild-type mice were primarily associated with the HDL fraction (65% and 78%). As anticipated, virtually no cholesteryl esters were present in the HDL fraction in plasma of LCAT KO mice, whereas the non-HDL cholesteryl ester content was essentially unaffected (Fig. 1). The HDL-associated free cholesterol level was also markedly lower in LCAT KO mice as compared with C57BL/6 mice (Fig. 1). In contrast, the level of free cholesterol associated with apoB-containing lipoproteins was 2.9-fold higher in plasma of LCAT KO mice (Fig. 1). In line with an increased amount of triglyceride-rich apoB-containing VLDL and LDL particles circulating in plasma of LCAT knockout mice, as previously already noted by Sakai et al. (13), we detected a significantly higher level of plasma triglycerides (44%; P < 0.05) in LCAT KO mice (Table 1).
TABLE 1.
Plasma lipids in wild-type C57BL/6 and LCAT KO mice
| C57BL/6 | LCAT KO | P Value | |
| Free cholesterol (FC; mg/dl) | 21 ± 1 | 20 ± 2 | N.S. |
| Cholesteryl esters (CE; mg/dl) | 50 ± 2 | 7 ± 1 | <0.001 |
| Total cholesterol (TC; mg/dl) | 71 ± 3 | 27 ± 2 | <0.001 |
| FC/CE ratio | 0.41 ± 0.01 | 3.31 ± 0.57 | <0.001 |
| Triglycerides (mg/dl) | 93 ± 4 | 134 ± 18 | <0.05 |
Fig. 1.
Distribution of free cholesterol and cholesteryl esters over HDL and non-HDL VLDL and LDL fractions in pooled plasma of wild-type C57BL/6 and LCAT KO mice.
LCAT KO mice show a diminished adrenal corticosterone output
The basal secretion of glucocorticoids by adrenals in mice is relatively low and is generally assumed to be independent of the acquisition of extracellular cholesterol pools because endogenous de novo production of cholesterol from acetyl-CoA should be sufficient to maintain basal levels. However, although the effect failed to reach statistical significance (P = 0.073), we did observe a marked decrease in basal plasma levels of corticosterone—the primary glucocorticoid circulating in rodents—in response to LCAT deficiency (96 ± 20 ng/ml for LCAT KO mice vs. 148 ± 20 ng/ml for C57BL/6 mice).
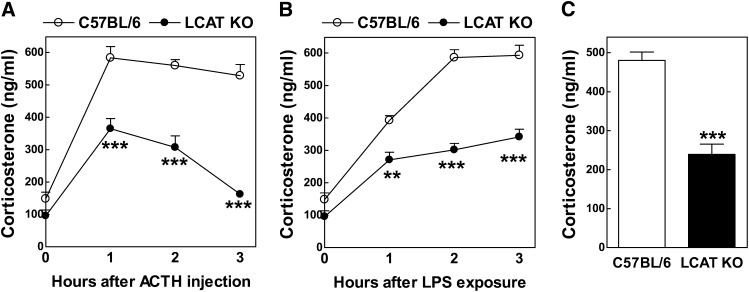
Activation of the hypothalamus-pituitary-adrenal axis results in the secretion of glucocorticoids by the adrenals at levels that effectively activate downstream glucocorticoid receptor signaling, an essential part of the body's response to physiological stressors. As anticipated, adrenocortical cell activation upon the administration of a synthetic mimetic of the pituitary-derived hormone ACTH (tetracosactide; 200 μg intraperitoneally)—a potent activator adrenal steroidogenesis—was associated with an acute rise in plasma corticosterone levels in C57BL/6 wild-type mice. Plasma corticosterone reached a plateau concentration at 1 h after the tetracosactide injection, which remained maximal until 3 h after the administration (Fig. 2A). Tetracosactide exposure also increased plasma corticosterone levels in LCAT KO mice; however, the peak concentration of corticosterone in plasma of LCAT KO mice was 37% lower than the level detected in C57BL/6 mice after 1 h of tetracosactide exposure (365 ± 31 ng/ml vs. 584 ± 34 ng/ml; P < 0.001) (Fig. 2A). In contrast to wild-type mice, the concentration of corticosterone rapidly declined in LCAT KO mice after 1 h and returned to basal levels at 3 h (Fig. 2A). This suggests that the adrenals of LCAT KO mice are only capable of realizing a short attenuated glucocorticoid response upon activation of the hypothalamus-pituitary-adrenal axis.
Fig. 2.
Plasma corticosterone levels in wild-type C57BL/6 (n = 10) and LCAT KO (n = 8) mice. Concentrations in plasma were measured after injection with the human ACTH analog tetracosactide (A), injection with a sublethal dose of LPS (50 μg/kg) (B), or ∼18 h of overnight fasting (C). **P < 0.01 and ***P < 0.001 versus C57BL/6 mice.
Endogenous glucocorticoids protect against sepsis and other inflammation-associated pathologies (17). In line with an essential role for glucocorticoids in the response to infection, exposure to a sublethal dose of endotoxin (lipopolysaccharide [LPS]; 50 μg/kg intraperitoneally) also induced a rapid increase in plasma corticosterone levels in C57BL/6 mice, which reached a plateau of 586 ± 24 ng/ml at 2 h after endotoxin exposure (Fig. 2B). Plasma corticosterone levels did rise in LCAT KO mice upon endotoxin exposure (Fig. 2B). However, LCAT KO mice displayed a more gradual increase up to 3 h after LPS exposure (341 ± 24 ng/ml), which was markedly lower as compared with the maximal level observed in C57BL/6 mice (593 ± 31 ng/ml; P < 0.001) (Fig. 2B). The observed maximum plasma corticosterone levels (∼600 ng/ml for C57BL/6 mice vs. ∼300 ng/ml for LCAT KO mice) and the area-under-the-curve (∼1400 ng/ml/h for C57BL/6 mice vs. ∼800 ng/ml/h for LCAT KO mice) were similar for either genotype after tetracosactide and endotoxin exposure. It is therefore suggested that both treatments induced a maximal acute adrenal steroid output, which is apparently 40–50% lower in LCAT KO mice.
Because glucocorticoids, through activation of the nuclear glucocorticoid receptor, are important regulators of gluconeogenesis and glucose utilization, overnight fasting is associated with an obligatory stimulation of adrenal glucocorticoid secretion to overcome hypoglycemia (10, 18, 19). In line with this observation, after ∼18 h of fasting we observed 51% lower (P < 0.001) corticosterone levels in LCAT KO mice as compared with C57BL/6 control mice (Fig. 2C). Combined, these findings suggest that HDL deficiency in LCAT KO mice is associated with a diminished adrenal corticosterone output in response to stress.
Adrenals of LCAT KO mice are deprived of neutral lipids despite compensatory up-regulation of genes associated with cholesterol acquisition
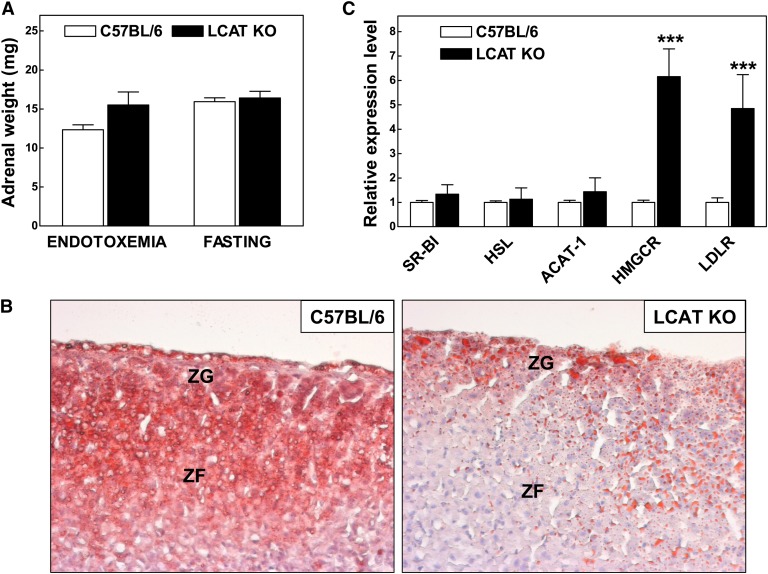
No significant difference in the weight of the adrenals between LCAT KO mice and C57BL/6 mice during endotoxemia (i.e., 4 h after LPS exposure) or under fasting stress conditions was noted (Fig. 3A). Ng et al. (14) have previously described that adrenals from LCAT knockout mice are under normal conditions deprived of cholesteryl esters. In our group of LCAT KO mice, this deficiency of neutral lipids in adrenocortical cells could be verified using Oil red O neutral lipid staining. As evident from Fig. 3B, specifically the glucocorticoid-producing adrenocortical cells within the zona fasciculata in LCAT KO mice lack the intense Oil red O staining as seen in the C57Bl/6 mice.
Fig. 3.
A: Adrenal weights in wild-type C57BL/6 (n = 10) and LCAT KO (n = 8) mice that either suffered from sublethal endotoxemia or were fasted overnight for ∼18 h. B: Representative Oil red O neutral lipid staining of cortical zones in adrenals from C57BL/6 and LCAT KO mice. ZF, zona fasciculata; ZG, zona glomerulosa. C: Relative mRNA expression levels of cholesterol metabolism-associated genes in adrenals of LCAT KO mice (n = 4) as fold compared with those found in C57BL/6 control mice (n = 11). ***P < 0.001 versus C57BL/6 mice.
The unesterified cholesterol pool used for steroidogenesis can be supplied by (1) endogenous synthesis of cholesterol in which HMG-CoA reductase catalyzes the rate-limiting step (2), hydrolysis of stored cholesteryl esters, or (3) uptake of exogenous lipoprotein-associated cholesterol. To identify potential compensatory gene regulation, using quantitative real-time PCR, we measured gene expression levels in adrenals harvested from 18 h-fasted LCAT KO mice and C57BL/6 wild-type control mice (Fig. 3C). A 6-fold stimulation of HMG-CoA reductase (P < 0.001) mRNA expression levels was detected in the adrenals of LCAT KO mice. The relative expression levels of enzymes crucially involved in synthesis (acetyl-CoA acetyltransferase 1) and hydrolysis (hormone-sensitive lipase) of cholesteryl esters within the adrenals were unaffected. Adrenal scavenger receptor BI (SR-BI) expression was unaltered, whereas the LDL receptor expression level was significantly increased (384%; P < 0.001) in LCAT KO mice. It thus seems that adrenals of LCAT KO mice as compared with those of wild-type control mice, probably as a compensatory response, attempt to synthesize more cholesterol and acquire increased amounts of LDL-cholesterol through receptor-mediated uptake.
LCAT KO mice display hepatocyte but not leukocyte glucocorticoid insufficiency
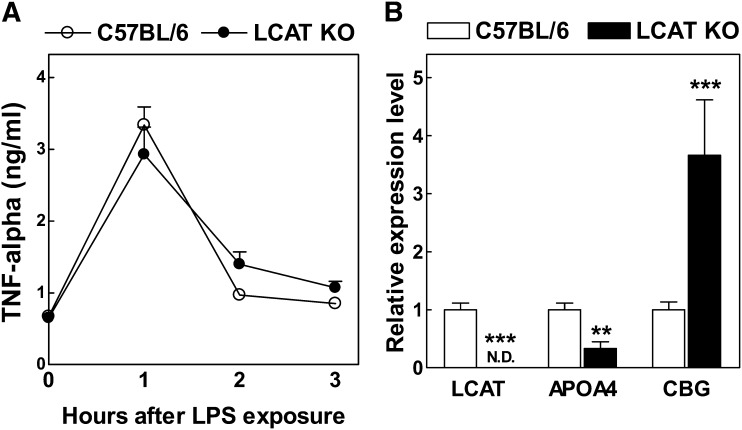
The glucocorticoid receptor is highly expressed in leukocytes where it modulates inflammatory responses (20) and in hepatocytes where it regulates glucose metabolism (21). Because leukocyte glucocorticoid insufficiency is associated with an enhanced endotoxemia-associated cytokine profile (22, 23), we determined the effect of LCAT deficiency on the LPS-induced TNF-α response. Plasma levels of the early response cytokine TNF-α were not significantly different at any time point measured after LPS (50 μg/kg intraperitoneally) exposure (0–3 h) (Fig. 4A). It thus seems that LCAT KO and C57BL/6 wild-type mice reach a similar (optimal) anti-inflammatory glucocorticoid receptor signaling level in leukocytes after LPS exposure.
Fig. 4.
A: Plasma TNF-α levels in wild-type C57BL/6 (n = 10) and LCAT KO (n = 10) mice. Concentrations in plasma were measured before and after injection with a sublethal dose of LPS (50 μg/kg). B: Relative mRNA expression levels of LCAT, APOA4, and CBG in livers of LCAT KO mice (n = 4) as fold compared with those found in C57BL/6 control mice (n = 11). **P < 0.01 and ***P < 0.001 versus C57BL/6 mice.
As anticipated, LCAT mRNA expression was absent in livers of LCAT KO mice (P < 0.001) (Fig. 4B). The relative expression level of the hepatocyte-specific glucocorticoid-induced gene APOA4 encoding apolipoprotein A4 (18), however, was also significantly reduced in livers of overnight-fasted LCAT KO mice (−66%; P < 0.01) (Fig. 4B). Furthermore, LCAT deficiency was associated with a marked 3.7-fold increase (P < 0.001) (Fig. 4B) in the relative hepatic mRNA expression level of the glucocorticoid carrier protein corticosteroid binding globulin, whose gene expression level in hepatocytes is subject to negative feedback control by glucocorticoids (24). Thus, downstream glucocorticoid receptor signaling pathways appear to be relatively deactivated in hepatocytes within the liver of LCAT KO mice. Combined, these findings suggest that LCAT KO mice do not suffer from total body glucocorticoid insufficiency but rather display hepatocyte-specific glucocorticoid insufficiency.
DISCUSSION
Although it is widely acknowledged that both HDL and non-HDL apoB-containing lipoproteins can be used as sources of cholesterol for the production of glucocorticoids by adrenocortical cells, the relative contribution of the separate lipoprotein classes to this process in vivo is not known. In the current study, we assessed glucocorticoid function in LCAT KO mice to investigate the contribution of specifically HDL-associated cholesteryl esters to adrenal steroidogenesis. Despite relatively high circulating non-HDL cholesterol levels and a compensatory up-regulation of genes associated with adrenal cholesterol acquisition, LCAT KO mice that lack HDL-cholesteryl esters are unable to execute a full glucocorticoid response (40–50% lower as compared with C57BL/6 wild-type control mice) to three types of stress (tetracosactide administration, LPS exposure, and overnight fasting). The VLDL cholesteryl ester composition (saturation state) is significantly changed in LCAT KO mice (25), which can theoretically also affect the adrenal glucocorticoid output (26). However, isolation of VLDL fractions from pooled plasma of C57BL/6 and LCAT KO mice (n = 13 each) using ultracentrifugation indicated that both types of mice do not have sufficiently high levels of VLDL to carry out in vitro steroidogenesis studies using H295R adrenocortical cells (data not shown). This suggests that any differences in the VLDL cholesteryl ester composition are unlikely to have a major impact on the glucocorticoid insufficiency phenotype observed in LCAT KO mice. We thus consider our current findings clear proof that HDL-associated cholesteryl esters make an essential contribution to in vivo adrenal glucocorticoid production. Accordingly, data from studies in human LCAT-deficient subjects further support this concept because LCAT deficiency in humans is associated with a lower adrenal steroid output as measured by a decrease in the urinary excretion of 17-ketogenic steroids and 17-hydroxycorticoids (27).
Our previous data from SR-BI KO mice that lack a functional HDL receptor indicated that disrupted adrenal uptake of cholesteryl esters from HDL is associated with glucocorticoid insufficiency because these mice display (1) a lowered hepatic glucocorticoid signaling and (2) an enhanced susceptibility to endotoxemia (9, 10, 28). In the current study we observed that LCAT KO mice that completely lack HDL-cholesteryl esters also display diminished glucocorticoid signaling in hepatocytes because they exhibit a lowered hepatic APOA4 mRNA expression level and a compensatory up-regulation of liver corticosteroid binding globulin expression. In line with a generally lower hepatic glucocorticoid signaling in LCAT KO mice, Ng et al. (29) have previously detected a lower relative expression level of the glucocorticoid-responsive gene PEPCK and a parallel decrease in plasma glucose levels in response to LCAT deficiency. In contrast to the change in hepatic glucocorticoid action in LCAT KO mice, leukocyte-specific glucocorticoid signaling is apparently normal in these mice because the susceptibility to endotoxemia is unaffected. Such a difference in cell-specific glucocorticoid sensitivity (i.e., high glucocorticoid receptor sensitivity in leukocytes and low glucocorticoid receptor sensitivity in hepatocytes) has also been detected in our previous adrenalectomy and adrenal transplantation studies (30).
Total SR-BI knockout mice thus display a more severe glucocorticoid insufficiency phenotype than that observed in LCAT KO mice. SR-BI is the sole molecule involved in the selective uptake of HDL-associated cholesteryl esters (31). Because both types of mice display hepatocyte-specific glucocorticoid insufficiency, we anticipate that the HDL/SR-BI interaction is essential to generate the bulk of corticosterone needed to effectively activate the glucocorticoid receptor in hepatocytes. However, the difference in leukocyte glucocorticoid insufficiency and endotoxemia susceptibility between the LCAT and SR-BI knockout mice seems to rely on a HDL-cholesteryl ester-independent corticosterone response. SR-BI knockout mice fail to increase their plasma corticosterone levels in response to LPS exposure (9, 28), whereas the present study shows that LCAT KO mice are able to increase, albeit to a lower extent, their plasma glucocorticoid levels in response to endotoxemia. Both our LCAT KO mice and SR-BI KO mice (9) display an increase in the adrenal relative expression level of the LDL receptor, the primary protein involved in the clearance of apoB-containing lipoproteins such as VLDL and LDL. Because previous findings by Kraemer et al. (32) have suggested that the LDL receptor does not supply cholesterol the steroidogenic pathway, a qualitative role for the LDL receptor/apoB-lipoprotein interaction in the synthesis of this distinct corticosterone pool in LCAT KO mice can be excluded. Importantly, SR-BI is also able to clear apoB-containing lipoproteins (33–35). LCAT KO adrenals, in contrast to SR-BI KO adrenals, may thus acquire cholesteryl esters from VDL/LDL particles through receptor-mediated uptake by SR-BI, which is sufficient to maintain plasma glucocorticoid levels that effectively activate the glucocorticoid receptor in leukocytes (but not hepatocytes). Future studies with specific SR-BI mutants that are selective for either only HDL or non-HDL binding and the associated selective uptake, as identified by for example the group of Gu et al. (36), will unequivocally show the possible contribution of the non-HDL/SR-BI interaction to total adrenal steroid production.
In conclusion, our studies show that HDL-cholesteryl ester deficiency in LCAT KO mice is associated with a 40–50% lower adrenal glucocorticoid stress response and, as a result, a hepatocyte-specific glucocorticoid insufficiency phenotype. These findings further highlight the important novel role for HDL as cholesterol donor for the synthesis of glucocorticoids by the adrenals.
Acknowledgments
The authors thank Dr. Jeroen C. Rijk from the RIKILT - Institute of Food Safety of Wageningen UR in The Netherlands for his experimental suggestions.
Footnotes
Abbreviations:
- KO
- knockout
- LPS
- lipopolysaccharide
- SR-BI
- scavenger receptor BI
- TNF-α
- tumor necrosis factor-α
This study was supported by TIPharma (grant T2-110), the Dutch Heart Foundation (grants 2007T056, 2008T070, and 2009B027), and the Landsteiner Foundation for Blood Transfusion Research (grant 0912F).
REFERENCES
- 1.Sasano H. 1994. Localization of steroidogenic enzymes in adrenal cortex and its disorders. Endocr. J. 41: 471–482 [DOI] [PubMed] [Google Scholar]
- 2.Gwynne J. T., Hess B. 1980. The role of high density lipoproteins in rat adrenal cholesterol metabolism and steroidogenesis. J. Biol. Chem. 255: 10875–10883 [PubMed] [Google Scholar]
- 3.Gwynne J. T., Hess B., Hughes T., Rountree R., Mahaffee D. 1984–1985. The role of serum high density lipoproteins in adrenal steroidogenesis. Endocr. Res. 10: 411–430 [DOI] [PubMed] [Google Scholar]
- 4.Mason J. I., Robidoux W. F. 1979. Steroidogenesis in isolated cells and mitochondria of rat Snell adrenocortical carcinoma 494. Endocrinology. 105: 1230–1236 [DOI] [PubMed] [Google Scholar]
- 5.Higashijima M., Nawata H., Kato K., Ibayashi H. 1987. Studies on lipoprotein and adrenal steroidogenesis: I. Roles of low density lipoprotein- and high density lipoprotein-cholesterol in steroid production in cultured human adrenocortical cells. Endocrinol. Jpn. 34: 635–645 [DOI] [PubMed] [Google Scholar]
- 6.Hoekstra M., Korporaal S. J., Li Z., Zhao Y., Van Eck M., Van Berkel T. J. 2010. Plasma lipoproteins are required for both basal and stress-induced adrenal glucocorticoid synthesis and protection against endotoxemia in mice. Am. J. Physiol. Endocrinol. Metab. 299: E1038–E1043 [DOI] [PubMed] [Google Scholar]
- 7.Li H., Brochu M., Wang S. P., Rochdi L., Côté M., Mitchell G., Gallo-Payet N. 2002. Hormone-sensitive lipase deficiency in mice causes lipid storage in the adrenal cortex and impaired corticosterone response to corticotropin stimulation. Endocrinology. 143: 3333–3340 [DOI] [PubMed] [Google Scholar]
- 8.Plump A. S., Erickson S. K., Weng W., Partin J. S., Breslow J. L., Williams D. L. 1996. Apolipoprotein A-I is required for cholesteryl ester accumulation in steroidogenic cells and for normal adrenal steroid production. J. Clin. Invest. 97: 2660–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoekstra M., Ye D., Hildebrand R. B., Zhao Y., Lammers B., Stitzinger M., Kuiper J., Van Berkel T. J., Van Eck M. 2009. Scavenger receptor class B type I-mediated uptake of serum cholesterol is essential for optimal adrenal glucocorticoid production. J. Lipid Res. 50: 1039–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoekstra M., Meurs I., Koenders M., Out R., Hildebrand R. B., Kruijt J. K., Van Eck M., Van Berkel T. J. 2008. Absence of HDL cholesteryl ester uptake in mice via SR-BI impairs an adequate adrenal glucocorticoid-mediated stress response to fasting. J. Lipid Res. 49: 738–745 [DOI] [PubMed] [Google Scholar]
- 11.Santamarina-Fojo S., Lambert G., Hoeg J. M., Jr Brewer H. B. 2000. Lecithin-cholesterol acyltransferase: role in lipoprotein metabolism, reverse cholesterol transport and atherosclerosis. Curr. Opin. Lipidol. 11: 267–275 [DOI] [PubMed] [Google Scholar]
- 12.Hovingh G. K., Hutten B. A., Holleboom A. G., Petersen W., Rol P., Stalenhoef A., Zwinderman A. H., de Groot E., Kastelein J. J., Kuivenhoven J. A. 2005. Compromised LCAT function is associated with increased atherosclerosis. Circulation. 112: 879–884 [DOI] [PubMed] [Google Scholar]
- 13.Sakai N., Vaisman B. L., Koch C. A., Hoyt R. F., Jr, Meyn S. M., Talley G. D., Paiz J. A., Brewer H. B., Jr, Santamarina-Fojo S. 1997. Targeted disruption of the mouse lecithin:cholesterol acyltransferase (LCAT) gene. Generation of a new animal model for human LCAT deficiency. J. Biol. Chem. 272: 7506–7510 [DOI] [PubMed] [Google Scholar]
- 14.Ng D. S., Francone O. L., Forte T. M., Zhang J., Haghpassand M., Rubin E. M. 1997. Disruption of the murine lecithin:cholesterol acyltransferase gene causes impairment of adrenal lipid delivery and up-regulation of scavenger receptor class B type I. J. Biol. Chem. 272: 15777–15781 [DOI] [PubMed] [Google Scholar]
- 15.Hoekstra M., Kruijt J. K., Van Eck M., Van Berkel T. J. 2003. Specific gene expression of ATP-binding cassette transporters and nuclear hormone receptors in rat liver parenchymal, endothelial, and Kupffer cells. J. Biol. Chem. 278: 25448–25453 [DOI] [PubMed] [Google Scholar]
- 16.Jonas A. 1991. Lecithin-cholesterol acyltransferase in the metabolism of high-density lipoproteins. Biochim. Biophys. Acta. 1084: 205–220 [DOI] [PubMed] [Google Scholar]
- 17.Webster J. I., Sternberg E. M. 2004. Role of the hypothalamic-pituitary-adrenal axis, glucocorticoids and glucocorticoid receptors in toxic sequelae of exposure to bacterial and viral products. J. Endocrinol. 181: 207–221 [DOI] [PubMed] [Google Scholar]
- 18.Hanniman E. A., Lambert G., Inoue Y., Gonzalez F. J., Sinal S. J. 2006. Apolipoprotein A-IV is regulated by nutritional and metabolic stress: involvement of glucocorticoids, HNF-4 alpha, and PGC-1 alpha. J. Lipid Res. 47: 2503–2514 [DOI] [PubMed] [Google Scholar]
- 19.Chida D., Nakagawa S., Nagai S., Sagara H., Katsumata H., Imaki T., Suzuki H., Mitani F., Ogishima T., Shimizu C., et al. 2007. Melanocortin 2 receptor is required for adrenal gland development, steroidogenesis, and neonatal gluconeogenesis. Proc. Natl. Acad. Sci. USA. 104: 18205–18210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberman A. C., Druker J., Garcia F. A., Holsboer F., Arzt E. 2009. Intracellular molecular signaling. Basis for specificity to glucocorticoid anti-inflammatory actions. Ann. N. Y. Acad. Sci. 1153: 6–13 [DOI] [PubMed] [Google Scholar]
- 21.Cole T. J., Myles K., Purton J. F., Brereton P. S., Solomon N. M., Godfrey D. I., Funder J. W. 2001. GRKO mice express an aberrant dexamethasone-binding glucocorticoid receptor, but are profoundly glucocorticoid resistant. Mol. Cell. Endocrinol. 173: 193–202 [DOI] [PubMed] [Google Scholar]
- 22.Butler L. D., Layman N. K., Riedl P. E., Cain R. L., Shellhaas J., Evans G. F., Zuckerman S. H. 1989. Neuroendocrine regulation of in vivo cytokine production and effects: I. In vivo regulatory networks involving the neuroendocrine system, interleukin-1 and tumor necrosis factor-alpha. J. Neuroimmunol. 24: 143–153 [DOI] [PubMed] [Google Scholar]
- 23.Bertini R., Bianchi M., Ghezzi P. 1988. Adrenalectomy sensitizes mice to the lethal effects of interleukin 1 and tumor necrosis factor. J. Exp. Med. 167: 1708–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole T. J., Harris H. J., Hoong I., Solomon N., Smith R., Krozowski Z., Fullerton M. J. 1999. The glucocorticoid receptor is essential for maintaining basal and dexamethasone-induced repression of the murine corticosteroid-binding globulin gene. Mol. Cell. Endocrinol. 154: 29–36 [DOI] [PubMed] [Google Scholar]
- 25.Parks J. S., Li H., Gebre A. K., Smith T. L., Maeda N. 1995. Effect of apolipoprotein A-I deficiency on lecithin:cholesterol acyltransferase activation in mouse plasma. J. Lipid Res. 36: 349–355 [PubMed] [Google Scholar]
- 26.Green S. R., Pittman R. C. 1991. Comparative acyl specificities for transfer and selective uptake of high density lipoprotein cholesteryl esters. J. Lipid Res. 32: 457–467 [PubMed] [Google Scholar]
- 27.Bochem A. E., Holleboom A. G., Vergeer M., Brandt A. M., Sweep F. C., Kuivenhoven J. A., Hovingh G. K., Stroes E. S.2012. Lower adrenal steroid production in individuals with low HDL cholesterol due to mutations in ABCA1 and LCAT. Abstract 290 in XVI International Symposium on Atherosclerosis. Sydney, Australia, March 25–29, 2012.
- 28.Cai L., Ji A., de Beer F. C., Tannock L. R., van der Westhuyzen D. R. 2008. SR-BI protects against endotoxemia in mice through its roles in glucocorticoid production and hepatic clearance. J. Clin. Invest. 118: 364–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng D. S., Xie C., Maguire G. F., Zhu X., Ugwu F., Lam E., Connelly P. W. 2004. Hypertriglyceridemia in lecithin-cholesterol acyltransferase-deficient mice is associated with hepatic overproduction of triglycerides, increased lipogenesis, and improved glucose tolerance. J. Biol. Chem. 279: 7636–7642 [DOI] [PubMed] [Google Scholar]
- 30.van der Sluis R. J., van Puijvelde G. H., Van Berkel T. J., Hoekstra M. 2012. Adrenalectomy stimulates the formation of initial atherosclerotic lesions: reversal by adrenal transplantation. Atherosclerosis. 221: 76–83 [DOI] [PubMed] [Google Scholar]
- 31.Out R., Hoekstra M., Spijkers J. A., Kruijt J. K., van Eck M., Bos I. S., Twisk J., Van Berkel T. J. 2004. Scavenger receptor class B type I is solely responsible for the selective uptake of cholesteryl esters from HDL by the liver and the adrenals in mice. J. Lipid Res. 45: 2088–2095 [DOI] [PubMed] [Google Scholar]
- 32.Kraemer F. B., Shen W. J., Patel S., Osuga J., Ishibashi S., Azhar S. 2007. The LDL receptor is not necessary for acute adrenal steroidogenesis in mouse adrenocortical cells. Am. J. Physiol. Endocrinol. Metab. 292: E408–E412 [DOI] [PubMed] [Google Scholar]
- 33.Rhainds D., Brodeur M., Lapointe J., Charpentier D., Falstrault L., Brissette L. 2003. The role of human and mouse hepatic scavenger receptor class B type I (SR-BI) in the selective uptake of low-density lipoprotein-cholesteryl esters. Biochemistry. 42: 7527–7538 [DOI] [PubMed] [Google Scholar]
- 34.Swarnakar S., Temel R. E., Connelly M. A., Azhar S., Williams D. L. 1999. Scavenger receptor class B, type I, mediates selective uptake of low density lipoprotein cholesteryl ester. J. Biol. Chem. 274: 29733–29739 [DOI] [PubMed] [Google Scholar]
- 35.Van Eck M., Hoekstra M., Out R., Bos I. S., Kruijt J. K., Hildebrand R. B., Van Berkel T. J. 2008. Scavenger receptor BI facilitates the metabolism of VLDL lipoproteins in vivo. J. Lipid Res. 49: 136–146 [DOI] [PubMed] [Google Scholar]
- 36.Gu X., Lawrence R., Krieger M. 2000. Dissociation of the high density lipoprotein and low density lipoprotein binding activities of murine scavenger receptor class B type I (mSR-BI) using retrovirus library-based activity dissection. J. Biol. Chem. 275: 9120–9130 [DOI] [PubMed] [Google Scholar]