Abstract
Diabetes is a major risk factor for cardiovascular disease. To examine how diabetes interacts with a mildly compromised lipid metabolism, we introduced the diabetogenic Ins2C96Y/+ (Akita) mutation into mice expressing human apoE4 (E4) combined with either an overexpressing human LDL receptor gene (hLDLR) or the wild-type mouse gene. The hLDLR allele caused 2-fold reductions in plasma HDL-cholesterol, plasma apoA1, and hepatic triglyceride secretion. Diabetes increased plasma total cholesterol 1.3-fold and increased apoB48 secretion 3-fold, while reducing triglyceride secretion 2-fold. Consequently, diabetic E4 mice with hLDLR secrete increased numbers of small, cholesterol-enriched, apoB48-containing VLDL, although they have near normal plasma cholesterol (<120 mg/dl). Small foam cell lesions were present in the aortic roots of all diabetic E4 mice with hLDLR that we analyzed at six months of age. None were present in nondiabetic mice or in diabetic mice without hLDLR. Aortic expression of genes affecting leukocyte recruitment and adhesion was enhanced by diabetes. ApoA1 levels, but not diabetes, were strongly correlated with the ability of plasma to efflux cholesterol from macrophages. We conclude that the diabetes-induced proinflammatory changes in the vasculature and the hLDLR-mediated cholesterol accumulation in macrophages synergistically trigger atherosclerosis in mice with human apoE4, although neither alone is sufficient.
Keywords: type-1 diabetes, VLDL secretion, HDL-cholesterol, reverse cholesterol transport, Akita mutation, macrophage, leukocyte recruitment, humanized animal models, apolipoproteins, foam cell plaques
More than 10% of the United States adult population is estimated to have diabetes (1), and the leading cause of increased mortality in patients with diabetes is enhanced atherosclerosis (2, 3). A common cluster of harmful changes to lipoprotein metabolism, including increased plasma VLDL and LDL cholesterol, occurs in type-2 diabetic patients and is characterized by the presence of small, dense LDL particles, low HDL, increased triglycerides, and postprandial lipemia (4, 5). Serum lipid and lipoprotein levels in patients who have well-controlled type-1 diabetes are generally not higher than those in people without diabetes. However, hypertriglyceridemia and reduced HDL are common in poorly controlled patients, and plasma apoB100 levels, which indicate the number of LDL particles, correlate with glycemic control (5). This underscores the importance of diabetes-induced changes in lipoprotein composition and distribution as a cardiovascular disease (CVD) risk (4, 5), but how these relatively small changes in lipid metabolism that occur in the general human population lead to increased diabetic complications of atherosclerosis is not completely understood. Current animal models for atherosclerosis have not been able to address this issue satisfactorily, as they rely on gross lipid abnormalities, such as the lack of LDLR or apoE which cause severe hypercholesterolemia. Although modest increases in atherosclerosis have been observed when these hypercholesterolemic mice are made diabetic (6–10), the effect of diabetes on atherosclerosis in mice with more normal, physiological cholesterol levels has not been investigated.
Apolipoprotein E (apoE) is a small circulating protein associated predominantly with VLDL and HDL. It is a primary ligand for the LDLR, a crucial component in the clearance of plasma lipoproteins, a major determinant of plasma cholesterol and CVD risk (8). The APOE gene is polymorphic in the human population, having three common isoforms: apoE2, apoE3, and apoE4. The apoE4 isoform is associated with higher LDL cholesterol and an increased risk of CVD (11). Previously we have reported that mice expressing human apoE4 in place of mouse apoE have normal plasma lipid and lipoprotein profiles when they are on regular chow (12, 13). We have also reported that mice that carry one copy of the hLdlr allele, which encodes human LDLR protein (hLDLR) in place of mouse LDLR and expresses three times normal, have a 40% reduction in plasma cholesterol. The HDL cholesterol of the mice with hLDLR is below 50 mg/dl, and it is close to that normally seen in humans (14). Furthermore, mice expressing both human apoE4 and human LDLR (E4h mice) are highly sensitive to diet-induced dyslipidemia. Although they have low plasma cholesterol on regular chow, they develop hypercholesterolemia and atherosclerosis on a diet adjusted with fat and cholesterol contents mirroring those consumed by humans (14). In the current work, we show that uncontrolled diabetes induces atherosclerosis in these mice on a normal chow diet without the addition of high fat and high cholesterol.
To induce diabetes, we used the Ins2C96Y/+ “Akita” mutation, a genetic model of type-1 diabetes. In Akita mice, a C96Y amino acid substitution in the Ins2 gene leads to improper folding of pro-insulin in the endoplasmic reticulum and causes eventual β-cell death (15). As a consequence, male Akita mice develop hypoinsulinemia and severe hyperglycemia beginning around one month of age. Here, we show that diabetic Akita mice expressing human apoE4 and human LDLR (E4hAkita) develop distinct foam cell lesions despite having plasma total cholesterol within a normal physiological range (∼100 mg/dl). Neither hLDLR allele nor diabetes alone is sufficient, but both are necessary for triggering atherosclerosis.
METHODS
Mice and induction of diabetes
Mice with a replacement of the endogenous Apoe locus with the human APOE*4 (E4) allele and mice carrying the hLdlr allele encoding human LDLR protein were previously described (12–14, 16). Ins2Akita/J mice on a C57BL/6- background were obtained from the Jackson Laboratories (stock #003548). Our experimental mice are all males designated as E4 (Apoe4/4Ldlr+/+Ins2+/+), E4h (Apoe4/4Ldlrh/+Ins2+/+), E4Akita (Apoe4/4Ldlr+/+Ins2C96Y/+), and E4hAkita (Apoe4/4Ldlrh/+Ins2C96Y/+). Mice were fed normal chow diet ad libitum (5.3% fat and 0.02% cholesterol. Prolab IsoPro RMH 3000; Agway Inc.). Animal experiments were conducted in conformity with the Public Health Service policy and approved by the Institutional Animal Care and Use Committees of the University of North Carolina.
Biochemical assays
After a 4 h fast, animals were anesthetized with 2,2,2-tribromoethanol and blood was collected. Cholesterol, phospholipids, glucose, and free fatty acids were measured using commercial kits (Wako, Richmond, VA). Triglycerides were determined using a commercial kit (Stanbio, Boerne, TX). Plasma insulin, interleukin (IL)-6, monocyte chemotactic protein (MCP)-1, and tumor necrosis factor (TNF)-α were assayed using magnetic bead immunoassay kits (Millipore, Billerica, MA) on a MAGPIX platform (Luminex, Austin, TX). Hepatic lipids were extracted by homogenization of liver tissue (∼50 mg) in chloroform:methanol at 2:1 by volume and analyzed as described (17).
Lipoprotein fractionation and VLDL secretion
Lipoprotein distribution and composition was determined with pooled plasma samples (100 µl) fractionated by fast protein liquid chromatography (FPLC) using a Superose 6 HR10/30 column (GE Healthcare, Piscataway, NJ). Pooled plasma (250 µl) was separated by sequential density ultracentrifugation into density fractions ranging from d < 1.006 g/ml (VLDL) to 1.10 < d < 1.21 g/ml (HDL) and subjected to electrophoresis in a 4–20% denaturing SDS-polyacrylamide gel (18). ApoA1 and apoB contents were determined by Western blot in individual plasma or VLDL fraction from pooled (n = 6) plasma samples following ultracentrifugation at d < 1.006 g/ml using 1:2,000 dilution of anti-mouse apoA1 and anti-mouse apoB (Abcam, Cambridge, MA) primary antibodies, respectively, and 1:10,000 dilution of HRP-conjugated anti rabbit IgG as secondary antibody (Cell Signaling, Danvers, MA). For apoE detection, anti-human apoE (EMD Millipore, Darmstadt, Germany) and HRP-conjugated anti-goat IgG as secondary antibody (Sigma-Aldrich, St. Louis, MO) were used. Hepatic secretion of triglyceride (TG) and cholesterol was measured following injection of Tyloxapol (0.7 mg/g body weight, Sigma-Aldrich, St. Louis, MO) via tail vein of mice (n = 5 each genotype) after an overnight fast (17).
Atherosclerosis
At six months of age, mice were euthanized with a lethal dose of 2,2,2-tribromoethanol and perfused at physiological pressure with 4% phosphate buffered paraformaldehyde (pH 7.4). At least 30 serially cut sections of aortic root from each mouse were examined under the light microscope (8 mice per each genotype). Macrophages in the aortic sections were immunostained using anti-mouse monocyte/macrophage antibody (MOMA2) and Cy5 -labeled anti-rat IgG (Abcam, Cambridge, MA).
Lipoprotein uptake by macrophage
Peritoneal macrophages were isolated four days after intraperitoneal injection of 0.5 ml of 4% (w/v) thioglycolate (BD Biosciences). Macrophages were cultured for 48 h in Ham's F-10 medium supplemented with 5% fetal bovine serum, 100 units/ml penicillin, 100 µg/ml streptomycin, 2 mM L-glutamine, and 5 mM (low) or 25 mM (high) glucose. Cells were incubated for 4 h with 1 mg/ml of oxidized human LDL labeled with DiI (1,1’-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine percholorate, Molecular Probes Inc.), and lipoprotein uptake was estimated as described (19). Freshly isolated macrophages were cultured for 2 h to remove nonadhesive cells prior to lipid and gene expression analyses. Lipid contents were determined with Amplex Red Cholesterol Assay Kit (Invitrogen) and Triglyceride Fluorometric Assay Kit (Cayman Chemical).
Cholesterol efflux assay
Peritoneal macrophages isolated from wild-type C57BL/6J mice were cultured in medium containing [3H]choleresterol (1μCi/ml) and 50 μg/ml of acetylated LDL for 16 h. Cells were washed and incubated with 0.8% (v/v) mouse plasma in serum free medium for 2.5 h, and efflux was calculated as counts in medium over counts in medium and cells (19). The ability of individual plasma to promote cholesterol efflux was calculated by subtracting values obtained without plasma.
Gene expression
mRNA was purified from liver, peritoneal macrophage, and thoracic aorta of 4- to 5-month-old mice using an Automated Nucleic Acid Workstation ABI 6700. Real-time PCR was performed in an ABI PRISM 7700 Sequence Detector (Applied Biosystems). β-Actin mRNA was used for normalization. Sequences for primers and probes are available upon request.
Data analysis
Values are reported as mean ± SEM. Two-way ANOVA with diabetes (Akita) and hLDLR genotype as two factors was used for statistical analyses. Tukey-Kramer honestly significant difference was used for posthoc comparisons between the groups.
RESULTS
Akita diabetic mice
By two months of age, E4Akita and E4hAkita mice were fully diabetic as judged by the elevation of their fasting plasma glucose compared with nondiabetic mice. Thus, fasting plasma glucose levels in E4Akita (516 ± 21 mg/dl) and E4hAkita mice (572 ± 26 mg/dl) at four to five months of age were significantly higher than in nondiabetic E4 and E4h mice (157 ± 7 and 149 ± 11 mg/dl, respectively; P < 0.0001 for diabetic effect by two way ANOVA, Fig. 1A). Plasma insulin levels fell to 36 ± 29 and 135 ± 49 pg/ml in diabetic E4Akita and E4hAkita mice, compared with 821 ± 262 and 689 ± 202 pg/ml in nondiabetic E4 and E4h mice, respectively (Fig. 1B). There was no significant effect of LDLR genotype on either fasting plasma glucose or insulin.
Fig. 1.
Plasma glucose, insulin, and lipoproteins. A–D: Plasma glucose (A), insulin (B), triglycerides (C), and cholesterol (D) were measured following a 4 h fast in 4–5 month old nondiabetic and diabetic E4 mice without (white bars) and with hLDL (black bars). The numbers of mice used in each group are shown inside bars. *P < 0.05, **P < 0.001. E: Pooled plasma (100 µl) from nondiabetic E4 and E4h (left panel) and diabetic E4A and E4hA (right panel) mice was separated on a FPLC column, and fractions were analyzed for cholesterol (top) and triglycerides (bottom). Non-HDL to HDL ratio is shown in parentheses. Plasma was pooled from 6–8 mice of each genotype. F, G: ApoB48 (F), apoA1 (G), and apoE (H) in the plasma were measured by Western blot analyzed by densitometry and expressed relative to the mean of E4 plasma as 100. Representative bands from each genotype are shown. I: Apolipoprotein and lipid compositions of VLDL fraction isolated by ultracentrifugation. VLDL fractions from 175 μl plasma of E4 and E4h and from 50 μl plasma of E4A and E4hA were subjected to a SDS gel electrophoresis and stained with Coomassie Brilliant Blue. Pie charts on the right show lipid compositions of E4A-VLDL and E4hA-VLDL.
Mice were maintained on regular chow without any intervention. Despite a 2-fold increase in food intake, diabetic mice had a reduced body weight compared with nondiabetic mice. At six months of age, E4Akita (21.12 ± 4.09 g) and E4hAkita (21.52 ± 1.05 g) mice weighed less than their nondiabetic E4 (30.89 ± 2.2 g) and E4h (28.26 ± 5.0 g) siblings. There was no effect of hLDLR genotype on body weight. Signs of distress in diabetic mice, such as dehydration, hair loss, and significant weight loss, were not present in the experimental mice at six months of age.
Plasma lipids and lipoproteins
Plasma triglyceride levels were not affected by diabetes, but the possession of an hLDLR allele led to a modest but significant reduction of plasma triglycerides (P < 0.004, Fig. 1C). There was no effect of diabetes or LDLR genotype on fasting plasma free fatty acids (data not shown). In contrast, plasma cholesterol levels were significantly increased in diabetic mice compared with nondiabetic mice by about 40 mg/dl (P < 0.0001, Fig. 1D). In nondiabetic mice, plasma total cholesterol was slightly lower (51 ± 4 mg/dl) in the E4h mice than in E4 mice [66 ± 4 mg/dl, not significant (NS)]. However, total plasma cholesterol in the diabetic E4hAkita (102 ± 10 mg/dl) and E4Akita mice (92 ± 6 mg/dl) were indistinguishable (P = 0.09 for interactions between the effects of diabetes and LDLR genotype). Most importantly, the plasma levels of total cholesterol in the diabetic E4hAkita mice remained within the range seen in wild-type mice.
Separating lipoprotein classes by size-exclusion FPLC revealed that nondiabetic E4 mice carry the majority of their cholesterol in the form of HDL and that increasing LDLR levels in E4h mice resulted in a reduction in HDL cholesterol (Fig. 1E, left panel). Diabetes did not change the levels of HDL cholesterol, and HDL in E4hAkita remained lower than in E4Akita mice (Fig. 1E, right panel). VLDL and VLDL remnants were increased in both diabetic groups, and this diabetes-associated increase was magnified in the E4hAkita mice. Thus, although total plasma cholesterol in E4Akita and E4hAkita mice was indistinguishable, there was a dramatic shift in the lipoprotein profile. Cholesterol in the E4Akita mice was primarily in HDL fractions, whereas cholesterol in the E4hAkita mice was primarily in VLDL and VLDL remnant fractions (Fig. 1E, right panel). Consequently, the ratio of non-HDL cholesterol to HDL cholesterol (3.05) in the E4hAkita mice was more than three times higher than in any of the other groups (0.45–0.78, Fig. 1E, in parentheses).
Plasma protein levels of apoB100 were very low in all groups. Diabetes significantly increased plasma apoB48 levels (Fig. 1F). There was a decrease in plasma apoA1 in the mice with hLDLR, indicating a reduced number of HDL particles (Fig. 1G). Plasma apoE4 was also low in the mice with hLDLR independent of diabetes (Fig. 1H). SDS gel electrophoresis of plasma VLDL fraction isolated by ultracentrifugation confirmed that hLDLR caused dramatic reduction of apoB100, apoE, and apoC (Fig. 1I). The reduction of apoE and apoC proteins likely accounts for the reduction in the triglyceride content in the remnant particles.
Hepatic lipid content and VLDL secretion
Reduced insulin signaling is associated with increased VLDL secretion, and lipid availability is the primary determinant of hepatic VLDL secretion (20, 21). Hepatic cholesterol stores were similar among all the groups (Fig. 2A). In contrast, diabetes significantly lowered hepatic triglycerides (P = 0.04, Fig. 2B). The diabetes effect was more pronounced in E4Akita mice compared with E4 mice, and the liver triglyceride contents in E4hAkita mice were similar to those in E4h mice.
Fig. 2.
Hepatic lipids and VLDL secretion. A, B: Liver cholesterol (A) and triglycerides (B) in nondiabetic and diabetic E4 (white bars) and E4h mice (black bars). Numbers of mice used in each group are shown inside bars. *P < 0.05. C, D: Hepatic secretion of VLDL triglycerides (C) and cholesterol (D) following injection of the detergent Tyloxapol via the tail vein. Triangles are nondiabetic mice (left panel), and squares are diabetic mice (right panel). Open symbols are mice without hLDLR, and filled symbols are mice with hLDLR. F: ApoB was measured by Western blot in pooled samples (n = 5) of VLDL (d < 1.006 g/ml density fractions) at 2, 60, and 120 min postinjection.
We estimated the rate of hepatic VLDL secretion by injecting mice with Tyloxapol, which inhibits particle uptake and lipolysis, to mice fasted for at least 16 h to minimize intestinal contribution. TG accumulation in the plasma following Tyloxapol injection was similar for the first 30 min postinjection but slowed significantly in mice with hLDLR at 1 and 2 h in both the nondiabetic (Fig. 2C, left panel) and diabetic (Fig. 2C, right panel) state (P < 0.001). Additionally, diabetes had a small but significant TG secretion-lowering effect (P = 0.02). While diabetes resulted in an approximately 40% increase in cholesterol secretion (P < 0.01), LDLR genotype had no significant effect (Fig. 2D). Together, both diabetes and hLDLR significantly altered the ratio of TG/cholesterol in the particles accumulated during 2 h after Tyloxapol injection (24 ± 3, 18 ± 2, 12 ± 2, and 6 ± 1 for E4, E4h, E4Akita, and E4hAkita, respectively; P < 0.0001 for diabetes effect, P = 0.01 for hLDLR effect with no interaction).
To estimate the number of lipoprotein particles being secreted, we measured the amount of apoB in the VLDL (d < 1.006 mg/dl) fraction isolated from pooled plasma at 2, 60, and 120 min posttyloxapol injection. The secretion of total apoB protein was similar between the nondiabetic E4 and E4h mice (Fig. 2E, left panel), although relative ratio of apoB100/apoB48 was higher in E4-VLDL than in E4h-VLDL. Diabetes increased the secretion of total apoB by about 3-fold (P < 0.001), particularly in apoB48 form. Although the amount of total apoB secreted over 2 h was also similar between the E4hAkita and the E4Akita mice, E4hAkita mice secreted primarily apoB48-containing particles (Fig. 2E, right panel). Taken together, these data demonstrate that during times of fasting, the livers of diabetic E4hAkita mice secrete an increased number of proatherogenic VLDL particles that are small, apoB48-containing, triglyceride poor, and cholesterol-rich.
Hepatic expression of the lipid metabolism-related genes
To gain insight into the effects of hypoinsulinemia on lipid uptake and VLDL secretion in the presence or absence of hLDLR, we measured the liver expression of several key genes for lipid metabolism (Table 1). As expected, the hLDLR allele led to a 5- to 6-fold higher LDLR gene expression, but it did not affect the expression of other genes significantly, with the exception of scavenger receptor (SR)-B1, whose expression tended to be higher in E4h mice than in E4 mice. Neither diabetes nor LDLR genotype altered the expression of the genes for apoE, lipoprotein lipase (LPL), or N-deacetylase/N-sulfotransferase (NDST)1, an enzyme responsible for sulfation of heparin sulfate proteoglycans. However, diabetes was associated with a dramatic 6-fold increase in expression of CD36, VLDL receptor (VLDLR), and leptin receptor (LEPR). Diabetes also increased SR-A1 expression almost 2-fold. A 3-fold increase in SR-B1 was observed, but only in mice without hLDLR (E4Akita). Conversely, expression of preprotein convertase serine kexin (PCSK)9, which facilitates degradation of LDLR protein, and expression of fatty acid synthase (FASN) were reduced significantly in diabetic mice. Reduction in PCSK9 and FASN appears to be enhanced in diabetic mice with hLDLR, but the differences between E4Akita and E4hAkita livers were not significant. Together, these changes reflect that the livers in the diabetic mice are preferentially metabolizing lipids.
TABLE 1.
Liver expression of genes related to lipid/lipoprotein metabolism and inflammation
| Genotype (n) |
P (ANOVA) |
||||||
| Gene | E4 | E4h | E4Akita | E4hAkita | Diabetes | LDLR | Interaction |
| LDLR | 100 ± 17 (7) | 618 ± 113 (6) a | 125 ± 18 (6) b | 523 ± 115 (10) ac | 0.72 | <0.0001 | 0.54 |
| APOE | 100 ± 19 (13) | 94 ± 17 (7) | 106 ± 15 (12) | 123 ± 15 (13) | 0.32 | 0.76 | 0.52 |
| APOB | 100 ± 5 (11) | 115 ± 10 (9) | 121 ± 14 (9) | 112 ± 7 (15) | 0.36 | 0.72 | 0.18 |
| VLDLR | 100 ± 17 (13) | 84 ± 26 (6) | 691 ± 123 (11) ab | 632 ± 125 (13) ab | <0.0001 | 0.71 | 0.83 |
| PCSK9 | 100 ± 15 (11) | 111 ± 24 (8) | 61 ± 16 (9) | 43 ± 8 (15) | 0.001 | 0.82 | 0.35 |
| FOXO1 | 100 ± 20 (12) | 83 ± 16 (8) | 135 ± 29 (9) | 88 ± 014 (15) | 0.37 | 0.15 | 0.52 |
| MTTP | 100 ± 8 (11) | 136 ± 18 (9) | 152 ± 24 (9) | 111 ± 11 (15) | 0.99 | 0.45 | 0.21 |
| NDST1 | 100 ± 12 (13) | 85 ± 17 (9) | 100 ± 14 (11) | 100 ± 13 (13) | 0.75 | 0.77 | 0.41 |
| CD36 | 100 ± 18 (13) | 86 ± 19 (7) | 563 ± 53 (11) ab | 585 ± 70 (13) ab | <0.0001 | 0.65 | 0.49 |
| SR-B1 | 100 ± 9 (8) | 162 ± 30 (6) | 288 ± 56 (6) ab | 186 ± 26 (10) | 0.002 | 0.53 | 0.014 |
| SR-A1 | 100 ± 10 (8) | 99 ± 23 (6) | 164 ± 40 (6) | 163 ± 19 (10) | 0.01 | 0.97 | 0.99 |
| ABCA1 | 100 ± 14 (8) | 115 ± 20 (6) | 76 ± 14 (6) | 100 ± 16 (10) | 0.24 | 0.25 | 0.81 |
| LPL | 100 ± 10 (8) | 98 ± 11 (6) | 109 ± 21 (6) | 119 ± 12 (10) | 0.28 | 0.78 | 0.64 |
| FASN | 100 ± 27 (8) | 116 ± 21(6) | 83 ± 16 (6) | 34 ± 4 (10) ab | 0.02 | 0.24 | 0.14 |
| LEPR | 100 ± 31 (7) | 100 ± 38 (6) | 505 ± 178 (6) ab | 487 ± 85 (9) ab | 0.0005 | 0.92 | 0.92 |
| CTP1a | 100 ± 16 (8) | 88 ± 10 (6) | 120 ± 25 (6) | 128 ± 19 (10) | 0.13 | 0.92 | 0.60 |
| PPARa | 100 ± 16 (8) | 104 ± 18 (6) | 119 ± 28 (6) | 133 ± 23 (10) | 0.30 | 0.69 | 0.83 |
| LOX1 | 100 ± 15 (7) | 95 ± 16 (6) | 70 ± 15 (6) | 77 ± 13 (10) | 0.12 | 0.95 | 0.71 |
| CD68 | 100 ± 26 (12) | 111 ± 24 (10) | 178 ± 58 (10) | 118 ± 34 (13) | 0.25 | 0.51 | 0.34 |
| MCP-1 | 100 ± 30 (12) | 137± (10) | 392 ± 195 (10) | 329 ± 115 (13) | 0.053 | 0.98 | 0.61 |
| IL-6 | 100 ± 20 (12) | 186 ± 41 (10) | 486 ± 186 (10) | 463 ± 182 (13) | 0.02 | 0.82 | 0.69 |
Mice on normal chow were between four and six months of age. Data are mean ± SE of mRNA levels relative to the mean of levels in E4 mice as 100. Significant P values are in bold by ANOVA using diabetes (Akita) and LDLR genotype (h) as two factors.
P < 0.05 by Tukey-Kramer HSD test against E4.
P < 0.05 by Tukey-Kramer HSD test against E4h.
P < 0.05 by Tukey-Kramer HSD test against E4Akita.
ApoB, as well as the insulin-inhibited transcription factor forkhead box protein O1 (FOXO1) and its downstream target microsomal triglyceride transfer protein (MTTP), are key players in the production of VLDL (22). However, expression of the genes for these proteins was not significantly altered either by diabetes or by LDLR genotype. Liver expression of ATP binding cassette transporter (ABC)A1, a key enzyme for HDL production, was not altered.
Diabetes-induced atherosclerosis in E4hAkita mice
We examined the proximal aortas of mice at six months of age for atherosclerotic changes. Under the light microscope, no sign of vascular damage or atherosclerosis was noted in the of nondiabetic E4 or E4h aorta (Fig. 3A). Likewise, none of the aortas dissected from the diabetic E4Akita mice showed any visible signs of atherosclerosis (Fig. 3B). In marked contrast, all of the eight diabetic E4hAkita mice analyzed in this study had atherosclerotic alterations in their aortic roots. The probability of all eight E4hA mice having lesions but none in other groups is <0.0001. Fatty depositions, although small, were clearly present within subendothelial areas of all mice (Fig. 3C, D, black arrows). Foam cells were not only present on the luminal surface but also were present in medial layers in two of the eight mice (Fig. 3E, F, white arrows). These foam cells are of monocyte origin, as they reacted with MOMA2. No advanced plaques with necrotic core or fibrous cap formation were observed. Thus, E4hAkita mice develop atherosclerosis in the absence of severe hyperlipidemia.
Fig. 3.
Atherosclerotic lesions at the aortic root. At six months of age, mice were euthanized and perfused with 4% PFA, and 8 µm sections of the aortic root were cryosectioned. Sections were stained with Sudan IVB to highlight lipid (red) and counterstained with hematoxylin. Representative images of aortic root vessel walls are shown for E4h (A), E4A (B), and E4hA (C–G) mice. None of the examined mice from the E4, E4h, or E4A group but all from the E4hA group showed signs of fatty streaks or macrophage foam cell formation. Black arrows in (C) and (D) indicate fatty intimal depositions. White arrows in (E) and (F) indicate foam cell infiltration in the medial layers. G: Moma2 immunostaining of fatty intimal depositions (red on the bright field) in the neighboring section of (E).
Macrophage cholesterol uptake and reverse cholesterol transport
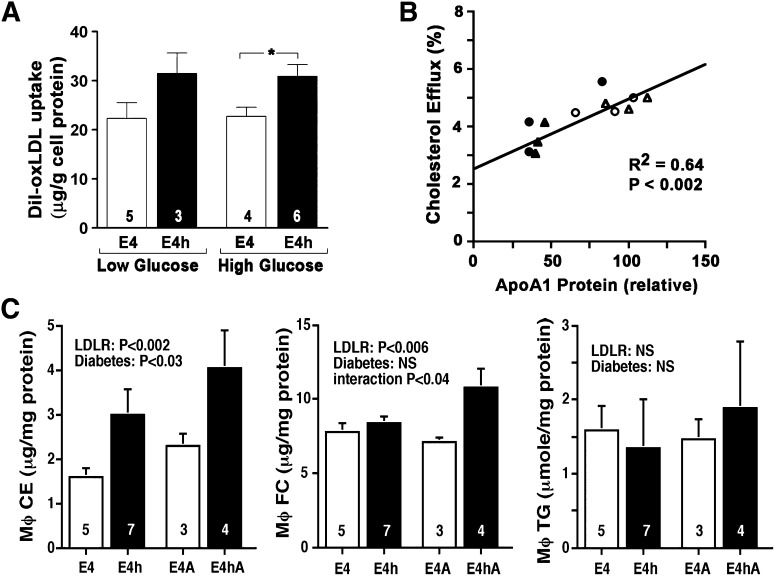
As E4hAkita mice have plasma cholesterol levels below 150 mg/dl, we looked for factors other than plasma lipids that might influence plaque development in these mice. We have previously shown that increases in LDLR expression significantly increase cholesterol delivery into macrophages in culture (19). To examine whether diabetes also affects uptake of modified lipoproteins in macrophages and, thus, foam cell and atherosclerotic plaque formation in a hyperglycemic environment, we measured uptake of DiI-labeled oxidized-LDL in vitro using peritoneal macrophages isolated from nondiabetic E4 and E4h mice. To mimic normal and hyperglycemic environments, macrophages were cultured in medium containing 5 mM glucose or 25 mM glucose for 48 h. As shown in Fig. 4A, uptake of oxidized-LDL was not affected by the glucose levels in the culture medium, but the E4h macrophages took up more oxidized-LDL than E4 macrophages in both conditions (hLDLR effect P < 0.01). Thus, increased LDLR expression enhances lipoprotein uptake in macrophages at low- or high-glucose concentrations.
Fig. 4.
Macrophage and vascular functions. A: Macrophage LDL uptake. Primary macrophages isolated from E4 mice (white bars) or E4h mice (black bars) were cultured in 5 mM (low) or 25 mM (high) glucose medium for 48 h. Macrophages were cultured with 1 µg/ml DiI-labeled human LDL (left side) or 1 µg/ml DiI-labeled oxidized human LDL (right side) for 4 h, and cellular florescence was measured to estimate LDL uptake. B: Cholesterol efflux to whole plasma correlates with plasma apoA1. Peritoneal macrophages from wild-type mice were labeled with [3H]cholesterol and incubated for 2.5 h with whole plasma (0.8%). Cholesterol efflux to individual plasma is expressed as proportion of labeled cholesterol moved from cells and plotted against their apoA1 levels estimated by Western blot. Open triangle, E4 mice; open circle, E4h mice; filled triangle, E4A mice; filled circle, E4hA mice. C: Cholesterol ester (CE), free cholesterol (FC), and triglyceride content of peritoneal macrophage. Statistics were with ANOVA, with hLDLR and diabetes as two factors.
Plasma HDL is able to mediate reverse cholesterol transport. We therefore measured the capacity of plasma from individual mice to efflux cholesterol using cholesterol-loaded peritoneal macrophages isolated from wild-type C57BL6/J mice in culture. The cholesterol efflux was strongly correlated with the apoA1 concentration of the plasma sample, which we estimated using Western blots (R2 = 0.64; P < 0.002, Fig. 4B). Efflux to plasma from diabetic mice (circles) was not different from that of nondiabetic mice (triangles). In contrast, efflux to plasma from mice with hLDLR (filled symbols) was approximately 75% that from mice without (open symbols), although the effect of hLDLR did not reach significance (P = 0.08) because one E4hAkita plasma had high efflux as well as high apoA1 level.
Consistent with these data, cholesterol ester (CE) content in the peritoneal macrophages isolated from E4h and E4hA mice was significantly higher than that in E4 and E4A mice (P < 0.002 for hLDLR effect, Fig. 4C). E4hA but not E4h macrophages had also increased free cholesterol (FC). Diabetes also had affected the cellular CE content (P < 0.03) but not FC content. TG content was not affected by either diabetes or hLDLR. Macrophage expression of genes for CD36, ABCA1, and ABCG1 that may influence cholesterol transport was not affected by either diabetes or hLDLR (data not shown).
Taken together, these data show that apoE4 mice with increased LDLR expression have enhanced lipid accumulation in macrophages. However, diabetes appears to have only a small effect on this function.
Vascular inflammation and diabetes-induced atherosclerosis
The general inflammatory state of mice as estimated by plasma markers of inflammation at six months of age was unremarkable, as concentrations of circulating TNF-α were below the detectable range (< 9 pg/ml) for all mice (data not shown). However, some mice showed elevated IL-6 and/or MCP-1 concentrations in the plasma, but no genotype association was detected. Liver expression of genes for IL-6 and MCP-1 tended to be elevated in the diabetic mice without the increase in macrophage number as judged by the expression of CD68 (Table 1).
To determine the contribution of diabetes and hLDLR to vascular function, we next examined the expression of several atherosclerosis-modifying genes in thoracic aortas, with an assumption that changes in thoracic aortas would be reflective of those in aortic roots (Fig. 5). Although no atherosclerosis was expected in thoracic aortas of 4- to 5-month-old chow-fed mice, macrophages in diabetic aorta appeared to be increased, as CD68 expression in diabetic mice was higher than in E4 control aorta. Furthermore, the expression of Spp1 (secreted phosphoprotein 1) and of Mmp-12 (metalloproteinase 12), both primarily expressed in foam cells in early atherosclerosis (23, 24), was elevated in aortas with hLDLR. A small but significant increase in mRNA for the vascular cell adhesion molecule (VCAM)-1 was also present in mice with hLDLR (P < 0.02), with no significant effects of diabetes. In contrast, diabetes significantly increased mRNA for intracellular cell adhesion molecule (ICAM)-1 (P < 0.003), MCP-1 (P < 0.0005), and endothelial selectin (SELE; P = 0.0004), with no significant effects of hLDLR. There were no differences in the expression of endothelial nitric oxide synthase, glutathione peroxidase, or endothelin receptor 1a (not shown).
Fig. 5.
Aortic gene expression of genes related to macrophage (A) and endothelial cells (B). Thoracic aortas were dissected from 4- to 5-month-old mice, and mRNA was isolated for gene expression analyses. Data are expressed as fold difference in log scale relative to the mean expression in E4 mice. The numbers of mice used are in parenthesis. Statistical significance of diabetic effects and hLDLR effects are by two-way ANOVA.
These data indicate that both diabetes and hLDLR increase the vascular inflammatory environment, diabetes mainly through the expression of factors known to affect leukocyte recruitment and LDLR mainly through accumulation of lipids in vascular macrophages.
DISCUSSION
The E4hAkita mouse provides a novel model of diabetic atherosclerosis in the absence of severe hyperlipidemia, one in which the diabetes-induced proinflammatory changes in the vasculature and the hLDLR-mediated accumulation of cholesterol in macrophages synergistically trigger foam cell lesions, although neither alone is sufficient. There are several major features that distinguish this model from other mouse models of atherosclerosis. First, none of the genetic components important for lipoprotein metabolism are missing in the E4hAkita mice. Instead, two of the most relevant genes (Apoe and Ldlr) code for human proteins. Second, a genetic model of type-1 diabetes (the Akita mutation) avoids the toxic effects of diabetes inducers, such as streptozotocin or alloxan. Third, no diet manipulation, such as high cholesterol, high fat, or high cholate, is necessary to induce atherosclerosis. Finally and most importantly, diabetes is necessary for plaques to develop.
Mice simultaneously expressing human apoE4 and a high level of hLDLR have normal non-HDL cholesterol on chow diet, but they are sensitive to high-fat/high-cholesterol-induced dyslipidemia and atherosclerosis (14). The clearance rate of postprandial remnants is reduced in these mice, in part because LDLR preferentially sequesters apoE4 on the surface of hepatocytes and limits apoE4 transfer onto lipoproteins, an essential step for apoE-mediated lipoprotein clearance (14, 25). Except for an expected 5-fold higher expression of LDLR, hLDLR does not appear to alter the hepatic expression of genes related to lipoprotein uptake and lipid metabolism. However, hLDLR alters the composition of VLDL secreted from the liver, as indicated in a 2-fold reduction in the rate of hepatic TG secretion, without alterations in the secretion of total cholesterol and apoB amounts. Because each VLDL particle contains a single molecule of apoB protein, this implies that the VLDL particles secreted by E4 mice with hLDLR are the same in number but have less TG and are therefore smaller than those secreted by E4 mice without hLDLR. hLDLR also decreased the apoB100/apoB48 ratio, which is consistent with the report by Twisk et al. who demonstrated that LDLR regulates hepatic apoB secretion and that overexpression of LDLR leads to the degradation of apoB100 but not of apoB48 (21). Importantly, as clearance of apoB48-containing particles from circulation is mediated by apoE, reduced availability of apoE4 leads to accumulation of remnants in plasma.
Uncontrolled type1 diabetes increases food intake in mice and stresses the remnant clearance in E4 mice, although total cholesterol levels still remain within the normal physiological range of mice (∼100 mg/dl). Insulin has a well-established role as an inhibitor of hepatic VLDL secretion (5, 20, 22). It also promotes degradation of apoB protein (20). Consistent with these observations, we find that diabetes significantly increases the secretion of apoB48, indicating that the number of VLDL particles secreted is increased. Diabetes also caused a 2-fold decrease in the rate of TG secretion, which could be due to reduced hepatic TG stores consequent to the enhanced use of lipids as a source of energy. The decrease in FASN and increase in CD36 and VLDLR expression, which we found in the liver of diabetic mice, supports this explanation. Importantly, the combination of hypoinsulinemia with hLDLR in the E4hAkita liver causes a marked increase in the secretion of small cholesterol-rich, TG-poor, apoB48-containing particles. The efficiency of remnant clearance increases in a particle size-dependent manner (26). Consequently, the small size of the apoB48-containing VLDL particles in the E4hAkita mice impedes their apoE4-dependent clearance and causes an accumulation of VLDL remnants in plasma (Fig. 6).
Fig. 6.
Alterations of vascular environment by diabetes and by hLDLR in mice expressing human apoE4. Arrows moving from left to right indicate the effects of an increase in LDLR expression as a result of the hLdlr allele. Arrows moving from top to bottom indicate the effects of diabetes due to Ins2Akita/+. Nondiabetic E4 mice in top left carry most of plasma cholesterol in HDL (blue circles). Increased LDLR reduces HDL cholesterol in E4h (top right). Diabetes increases plasma glucose (black dots) and VLDL secretion (E4Akita, bottom left) and in E4hAkita mice (bottom right). Interactions between hLDLR and apoE4 compromise the clearance of remnants in E4hAkita mice, leading to an accumulation of VLDL remnants (red circles). In the vasculature, hLDLR increases VCAM-1 gene expression, whereas diabetes increases expression of ICAM-1, SELE, and MCP-1 (upward arrows). Additionally, hLDLR enhances cholesterol uptake while reduces efflux in apoE4-expressing macrophages, leading to foam cell formation only in E4hAkita mice.
HDL-cholesterol levels in the plasma of mice with hLDLR are 60% normal, regardless of diabetes. This is consistent with a previous report that low plasma HDL level occurs in transgenic mice overexpressing human LDLR, which was postulated to be due to increased whole-particle uptake of apoE-containing HDL by LDLR (27). In addition, reduced reverse cholesterol transport and increased liver SR-B1 expression may also contribute to this reduction. Although this degree of HDL reduction is likely to be harmless by itself, when it is combined with a diabetes-induced increase in VLDL remnants, the non-HDL/HDL cholesterol ratio in the E4hAkita mice dramatically increases to 3.05. This is close to that observed in humans (∼3) and very different from the 0.45 observed in wild-type or E4 mice. HDL has a very strong protective effect on CVD risk in humans, and it can significantly decrease the risk even in the presence of high LDL (28). Importantly, the fact that the diabetic E4Akita mice do not develop atherosclerosis although their total plasma cholesterol levels are as high as those of E4hAkita mice, underscores the importance of the types of circulating lipoproteins. However, having an elevated non-HDL/HDL cholesterol ratio by itself is unlikely to be sufficient to account for the diabetes-induced atherosclerosis in the E4hAkita mice. In this regard, Goldberg et al. reported that atherosclerosis was not increased in streptozotocin-induced diabetic LDLR null mice with apoA1-deficiency (29) or in mice expressing human apoB and CETP with LPL deficiency (30), even though these mice had elevated non-HDL/HDL cholesterol ratios. Thus, some factors in addition to the plasma lipoprotein changes are likely to contribute to the atherosclerosis of E4hAkita mice.
CVD risk in patients with diabetes is likely to be increased via several interconnected factors, including inflammation, endothelial dysfunction, and a prothrombotic state that result from a disturbed glucose metabolism (31–33). Our experiments show that diabetes increases MCP-1, ICAM-1, and SELE gene expression 2- to 3-fold in the aorta. These proteins mediate leukocyte recruitment, rolling, and adhesion to the blood vessels, which contribute to the plaque initiation. However, hLDLR did not affect the expression of these genes. Instead, hLDLR, but not diabetes, caused a small 1.5-fold increase of VCAM-1 gene expression in the aorta. VCAM-1 plays a crucial role in monocyte adhesion to the endothelium and in initiation of atherosclerosis in both LDLR-null and apoE-null mice (34, 35). The increased VCAM-1 expression in aortas carrying hLDLR may therefore be a factor that contributes to the atherosclerosis of the E4 diabetic mice. As VCAM-1 gene expression is also increased in E4h mice with low VLDL, this is not likely to be secondary to the relative increase of remnant particles in the E4hAkita mice. On the other hand, because plasma HDL levels are significantly low in hLDLR mice regardless of diabetes, aortic VCAM-1 expression could be related to a reduced cholesterol efflux. Further studies are necessary to elucidate the causative relationships between the hLDLR and VCAM-1 gene expression.
Although macrophage dysfunction is associated with diabetes (36, 37), we find that oxidized LDL uptake in cultured macrophages is increased when they have the hLDLR allele, which is more strongly expressed than the mouse allele (19). ApoE4-expressing macrophages efflux less cholesterol than apoE2- or apoE3-expressing macrophages in culture (19, 38–40), and increased LDLR expression reduces cholesterol efflux from macrophages in an apoE4-dependent manner (19, 38). Additionally, Cash et al. (41) recently reported that macrophages with apoE4, when activated by oxidized LDL, exhibit higher cell stress than those with the other apoE isoforms, mainly through potentiation of endoplasmic reticulum stress signaling. Thus, the combination of high expression of LDLR with human apoE4 induces atherogenic changes in macrophages. In addition, the low plasma levels of apoA1 further reduce the removal of cholesterol from macrophages in mice with hLDLR. Although the resulting macrophage dysfunction is not sufficient by itself to cause foam cell formation, it is a significant factor when combined with the enhanced leukocyte recruitment induced by diabetes (Fig. 6).
The relative contribution of hyperglycemia and dyslipidemia to the accelerated atherosclerosis that presents in diabetes is not well defined. Previously, Renard et al. (8) have shown, in a virus-induced insulinitis model, that diabetes causes accelerated lesion initiation without apparent changes in the lipid/lipoprotein profiles in LDLR-null mice fed a cholesterol-free diet. As diabetic mice heterozygous for LDLR-deficiency have normal plasma cholesterol and do not develop atherosclerosis even when fed a high-cholesterol diet, the authors suggested that moderately elevated basal cholesterol levels (300 mg/dl) of the LDLR-null mice are required for diabetes to induce arterial inflammation and lesion initiation. In our apoE4 mice, in contrast, diabetes caused clear changes in lipoprotein metabolism, including the secretion of a larger number of smaller VLDL particles, although homeostatic adjustments maintained their plasma cholesterol levels within a normal physiological range (<120 mg/dl). We also found that diabetes causes proinflammatory changes in the vasculature of apoE4 mice, although this change alone was not sufficient to initiate atherosclerosis. While the two experiments differ in how type-1 diabetes is induced, the sex of the mice and the mode of lipid metabolism perturbation both clearly demonstrate that diabetes increases atherogenesis with relatively modest changes in lipoprotein metabolism.
Translation of mouse models of atherosclerosis to humans is not straightforward because there are species differences in their lipoprotein metabolism. For example, E4hAkita mice secrete predominantly apoB48-containing lipoproteins, remnants of which are cleared by apoE-mediated mechanisms. This contrasts with humans in which plasma non-HDL lipoproteins contain mainly apoB100 because humans lack hepatic apoB editing (42). Small remnant lipoprotein particles with apoB48, unlike comparable particles with apoB100, cannot be cleared by the LDLR unless they also have apoE. The disturbances in lipoprotein metabolism caused by changes in LDLR levels may therefore be exaggerated in mice by the accumulation of small apoB48 remnants. Nevertheless, recent work by Versmissen et al. indicates that an analogous interaction between the level of LDLR expression and apoE4 isoform may be an important contributing factor in humans (43). Despite the differences between mouse and human lipoprotein metabolism, changes in the lipoprotein profiles, macrophage physiology, and vascular inflammation caused by diabetes and by increased LDLR expression in E4hAkita mice are likely relevant to atherogenesis in humans. Finally, translating our results from a severely hypoinsulinemic model untreated with insulin to human type-1 diabetes treated with insulin or to hyperinsulinemic type-2 diabetes prevalent in humans should be done with caution. Further studies are necessary to determine whether mice expressing human apoE4 and human LDLR are also susceptible to atherosclerosis induced by insulin resistance and type-2 diabetes.
Acknowledgments
The authors thank Marcus McNair, Jennifer Wilder, Sylvia Hiller, and Shinja Kim for technical assistance.
Footnotes
Abbreviations:
- CE
- cholesterol ester
- CVD
- cardiovascular disease
- E4
- Apoe4/4Ldlr+/+Ins2+/+
- E4h
- Apoe4/4Ldlrh/+Ins2+/+
- E4Akita (E4A)
- Apoe4/4Ldlr+/+Ins2C96Y/+
- E4hAkita (E4hA)
- Apoe4/4Ldlrh/+Ins2C96Y/+
- FASN
- fatty acid synthase
- FC
- free cholesterol
- FOXO1
- forkhead box protein O1
- hLDLR
- human LDL receptor
- ICAM-1
- intracellular cell adhesion molecule 1
- IL-6
- interleukin 6
- LDLR
- LDL receptor
- LEPR
- leptin receptor
- LPL
- lipoprotein lipase
- MCP-1
- monocyte chemotactic protein 1
- MMP-12
- metalloproteinase 12
- MTTP
- microsomal triglyceride transfer protein
- NDST1
- N-deacetylase/N-sulfotransferase 1
- NS
- not significant
- PCSK9
- preprotein convertase serine kexin-9
- SELE
- endothelial selectin
- SPP1
- secreted phosphoprotein 1
- SR
- scavenger receptor
- TG
- triglyceride
- TNF-α
- tumor necrosis factor alpha
- VCAM-1
- vascular cell adhesion molecule 1
- VLDLR
- VLDL receptor
This work was supported by National Institutes of Health Grants HL-42630 and HL-087946. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. L.A.J. was supported by American Heart Association Pre-Doctoral Fellowship 10PRE3880032.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2011. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. US Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 2.Kannel W. B., McGee D. L. 1979. Diabetes and cardiovascular disease: the Framingham Study. JAMA. 241: 2035–2038 [DOI] [PubMed] [Google Scholar]
- 3.Grundy S. M., Benjamin I. J., Burke G. L., Chait A., Eckel R. H., Howard B. V., Mitch W., Smith S. C., Jr., Sowers J. R. 1999. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 100: 1134–1146 [DOI] [PubMed] [Google Scholar]
- 4.Goldberg I. J. 2001. Clinical review 124: Diabetic dyslipidemia: causes and consequences. J. Clin. Endocrinol. Metab. 86: 965–971 [DOI] [PubMed] [Google Scholar]
- 5.Jenkins A. J., Lyons T. J., Zheng D., Otvos J. D., Lackland D. T., McGee D., Garvey W. T., Klein R. L. ; DCC/EDIC Research Group 2003. Serum lipoproteins in the diabetes control and complications trial/epidemiology of diabetes intervention and complications cohort: associations with gender and glycemia. Diabetes Care. 26: 810–818 [DOI] [PubMed] [Google Scholar]
- 6.Johnson L. A., Maeda N. 2010. Macrovascular complications of diabetes in atherosclerosis prone mice. Expert Rev. Endocrin. & Metab. 5: 89–98 [DOI] [PubMed] [Google Scholar]
- 7.Hsueh W., Abel E. D., Breslow J. L., Maeda N., Davis R. C., Fisher E. A., Dansky H., McClain D. A., McIndoe R., Wassef M. K., et al. 2007. Recipes for creating animal models of diabetic cardiovascular disease. Circ. Res. 100: 1415–1427 [DOI] [PubMed] [Google Scholar]
- 8.Renard C. B., Kramer F., Johansson F., Lamharzi N., Tannock L. R., von Herrath M. G., Chait A., Bornfeldt K. E. 2004. Diabetes and diabetes-associated lipid abnormalities have distinct effects on initiation and progression of atherosclerotic lesions. J. Clin. Invest. 114: 659–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J., Takeuchi T., Tanaka S., Kubo S. K., Kayo T., Lu D., Takata K., Koizumi A., Izumi T. 1999. A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J. Clin. Invest. 103: 27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou C., Pridgen B., King N., Xu J., Breslow J. L. 2011. Hyperglycemic Ins2AkitaLdlr−/− mice show severely elevated lipid levels and increased therosclerosis: a model of type 1 diabetic macrovascular disease. J. Lipid Res. 52: 1483–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahley R. W., Rall S. C. 2000. Apolipoprotein E: far more than a lipid transport protein. Annu. Rev. Genomics Hum. Genet. 1: 507–537 [DOI] [PubMed] [Google Scholar]
- 12.Sullivan P. M., Mezdour H., Aratani Y., Knouff C., Najib J., Reddick R. L., Quarfordt S. H., Maeda N. 1997. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J. Biol. Chem. 272: 17972–17980 [DOI] [PubMed] [Google Scholar]
- 13.Knouff C., Hinsdale M. E., Mezdour H., Altenburg M. K., Watanabe M., Quarfordt S. H., Sullivan P. M., Maeda N. 1999. Apo E structure determines VLDL clearance and atherosclerosis risk in mice. J. Clin. Invest. 103: 1579–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malloy S. I., Altenburg M. K., Knouff C., Lanningham-Foster L., Parks J. S., Maeda N. 2004. Harmful effects of increased LDLR expression in mice with human APOE*4 but not APOE*3. Arterioscler. Thromb. Vasc. Biol. 24: 91–97 [DOI] [PubMed] [Google Scholar]
- 15.Jun J. Y., Ma Z., Segar L. 2011. Spontaneously diabetic Ins2+/Akita:apoE-deficient mice exhibit exaggerated hypercholesterolemia and atherosclerosis. Am. J. Physiol. Endocrinol. Metab. 301: E145–E154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knouff C., Malloy S., Wilder J., Altenburg M. K., Maeda N. 2001. Doubling expression of the low density lipoprotein receptor by truncation of the 3′-untranslated region sequence ameliorates type iii hyperlipoproteinemia in mice expressing the human apoe2 isoform. J. Biol. Chem. 276: 3856–3862 [DOI] [PubMed] [Google Scholar]
- 17.Johnson L. A., Altenburg M. K., Walzem R. L., Scanga L. T., Maeda N. 2008. Absence of hyperlipidemia in LDL receptor-deficient mice having apolipoprotein B100 without the putative receptor-binding sequences. Arterioscler. Thromb. Vasc. Biol. 28: 1745–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Silva H. V., Mas-Oliva J., Taylor J. M., Mahley R. W. 1994. Identification of apolipoprotein B-100 low density lipoproteins, apolipoprotein B-48 remnants, and apolipoprotein E-rich high density lipoproteins in the mouse. J. Lipid Res. 35: 1297–1310 [PubMed] [Google Scholar]
- 19.Altenburg M., Johnson L., Wilder J., Maeda N. 2007. Apolipoprotein E4 in macrophages enhances atherogenesis in a low density lipoprotein receptor-dependent manner. J. Biol. Chem. 282: 7817–7824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sparks J. D., Sparks C. E. 1994. Insulin regulation of triacylglycerol-rich lipoprotein synthesis and secretion. Biochim. Biophys. Acta. 1215: 9–32 [DOI] [PubMed] [Google Scholar]
- 21.Twisk J., Gillian-Daniel D. L., Tebon A., Wang L., Barrett P. H., Attie A. D. 2000. The role of the LDL receptor in apolipoprotein B secretion. J. Clin. Invest. 105: 521–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamagate A., Dong H. H. 2008. FoxO1 integrates insulin signaling to VLDL production. Cell Cycle. 7: 3162–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirota S., Imakita M., Kohri K., Ito A., Morii E., Adachi S., Kim H. M., Kitamura Y., Yutani C., Nomura S. 1993. Expression of osteopontin messenger RNA by macrophages in atherosclerotic plaques. A possible association with calcification. Am. J. Pathol. 143: 1003–1008 [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumoto S., Kobayashi T., Katoh M., Saito S., Ikeda Y., Kobori M., Masuho Y., Watanabe T. 1998. Expression and localization of matrix metalloproteinase-12 in the aorta of cholesterol-fed rabbits: relationship to lesion development. Am. J. Pathol. 153: 109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altenburg M., Arbones-Mainar J., Johnson L., Wilder J., Maeda N. 2008. Human LDL receptor enhances sequestration of ApoE4 and VLDL remnants on the surface of hepatocytes but not their internalization in mice. Arterioscler. Thromb. Vasc. Biol. 28: 1104–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stalenhoef A. F., Malloy M. J., Kane J. P., Havel R. J. 1984. Metabolism of apolipoproteins B-48 and B-100 of triglyceride-rich lipoproteins in normal and lipoprotein lipase-deficient humans. Proc. Natl. Acad. Sci. USA. 81: 1839–1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yokode M., Hammer R. E., Ishibashi S., Brown M. S., Goldstein J. L. 1990. Diet-induced hypercholesterolemia in mice: prevention by overexpression of LDL receptors. Science. 250: 1273–1275 [DOI] [PubMed] [Google Scholar]
- 28.Cannon C. P. 2007. High-density lipoprotein cholesterol and residual cardiometabolic risk in metabolic syndrome. Clin. Cornerstone. 8(Suppl. 6): S14–S23 [DOI] [PubMed] [Google Scholar]
- 29.Goldberg I. J., Isaacs A., Sehayek E., Breslow J. L., Huang L. S. 2004. Effects of streptozotocin-induced diabetes in apolipoprotein AI deficient mice. Atherosclerosis. 172: 47–53 [DOI] [PubMed] [Google Scholar]
- 30.Kako Y., Massé M., Huang L. S., Tall A. R., Goldberg I. J. 2002. Lipoprotein lipase deficiency and CETP in streptozotocin-treated apoB-expressing mice. J. Lipid Res. 43: 872–877 [PubMed] [Google Scholar]
- 31.Fonseca V., Desouza C., Asnani S., Jialal I. 2004. Nontraditional risk factors for cardiovascular disease in diabetes. Endocr. Rev. 25: 153–175 [DOI] [PubMed] [Google Scholar]
- 32.Chait A., Bornfeldt K. E. 2009. Diabetes and atherosclerosis: is there a role for hyperglycemia? J. Lipid Res. 50: S335–S339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Funk S. D., Yurdagul A., Jr, Orr A. W. 2012. Hyperglycemia and endothelial dysfunction in atherosclerosis: lessons from type 1 diabetes. Int. J. Vasc. Med. 2012: 569654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dansky H. M., Barlow C. B., Lominska C., Sikes J. L., Kao C., Weinsaft J., Cybulsky M. I., Smith J. D. 2001. Adhesion of monocytes to arterial endothelium and initiation of atherosclerosis are critically dependent on vascular cell adhesion molecule-1 gene dosage. Arterioscler. Thromb. Vasc. Biol. 21: 1662–1667 [DOI] [PubMed] [Google Scholar]
- 35.Cybulsky M. I., Iiyama K., Li H., Zhu S., Chen M., Iiyama M., Davis V., Gutierrez-Ramos J. C., Connelly P. W., Milstone D. S. 2001. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J. Clin. Invest. 107: 1255–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang C., Kanter J. E., Bornfeldt K. E., Leboeuf R. C., Oram J. F. 2010. Diabetes reduces the cholesterol exporter ABCA1 in mouse macrophages and kidneys. J. Lipid Res. 51: 1719–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parathath S., Grauer L., Huang L. S., Sanson M., Distel E., Goldberg I. J., Fisher E. A. 2011. Diabetes adversely affects macrophages during atherosclerotic plaque regression in mice. Diabetes. 60: 1759–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucic D., Huang Z. H., Gu D. S., Altenburg M. K., Maeda N., Mazzone T. 2007. Regulation of macrophage apoE secretion and sterol efflux by the LDL receptor. J. Lipid Res. 48: 366–372 [DOI] [PubMed] [Google Scholar]
- 39.Cullen P., Cignarella A., Brennhausen B., Mohr S., Assmann G., von Eckardstein A. 1998. Phenotype-dependent differences in apolipoprotein E metabolism and in cholesterol homeostasis in human monocyte-derived macrophages. J. Clin. Invest. 101: 1670–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hara M., Matsushima T., Satoh H., Iso-o N., Noto H., Togo M., Kimura S., Hashimoto Y., Tsukamoto K. 2003. Isoform-dependent cholesterol efflux from macrophages by apolipoprotein E is modulated by cell surface proteoglycans. Arterioscler. Thromb. Vasc. Biol. 23: 269–274 [DOI] [PubMed] [Google Scholar]
- 41.Cash J. G., Kuhel D. G., Basford J. E., Jaeschke A., Chatterjee T. K., Weintraub N. L., Hui D. Y. 2012. Apolipoprotein E4 impairs macrophage efferocytosis and potentiates apoptosis by accelerating endoplasmic reticulum stress. J. Biol. Chem. 287: 27876–27884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakamuta M., Oka K., Krushkal J., Kobayashi K., Yamamoto M., Li W. H., Chan L. 1995. Alternative mRNA splicing and differential promoter utilization determine tissue-specific expression of the apolipoprotein B mRNA-editing protein (Apobec1) gene in mice. Structure and evolution of Apobec1 and related nucleoside/nucleotide deaminases. J. Biol. Chem. 270: 13042–13056 [DOI] [PubMed] [Google Scholar]
- 43.Versmissen J., Oosterveer D. M., Hoekstra M., Out R., Berbée J. F., Blommesteijn-Touw A. C., van Vark-van der Zee L., Vongpromek R., Vanmierlo T., Defesche J. C., et al. 2011. Apolipoprotein isoform E4 does not increase coronary heart disease risk in carriers of low-density lipoprotein receptor mutations. Circ. Cardiovasc. Genet. 4: 655–660 [DOI] [PubMed] [Google Scholar]