Abstract
The investigation of the human disease sitosterolemia (MIM 210250) has shed light not only on the pathways by which dietary sterols may traffic but also on how the mammalian body rids itself of cholesterol and defends against xenosterols. Two genes, ABCG5 and ABCG8, located at the sitosterolemia locus, each encodes a membrane-bound ABC half-transporter and constitutes a functional unit whose activity has now been shown to account for biliary and intestinal sterol excretion. Knockout mice deficient in Abcg5 or Abcg8 recapitulate many of the phenotypic features of sitosterolemia. During the course of our studies to characterize these knockout mice, we noted that these mice, raised on normal rodent chow, exhibited infertility as well as loss of abdominal fat. We show that, although sitosterolemia does not lead to any structural defects or to any overt endocrine defects, fertility could be restored if xenosterols are specifically blocked from entry and that the loss of fat is also reversed by a variety of maneuvers that limit xenosterol accumulation. These studies show that xenosterols may have a significant biological impact on normal mammalian physiology and that the Abcg5 or Abcg8 knockout mouse model may prove useful in investigating the role of xenosterols on mammalian physiology.
Keywords: Abcg8, Abcg5, phytosterols, adipocytes, infertility, ezetimibe
Cholesterol homeostasis is a tightly regulated process between de novo cholesterol synthesis, exogenous (dietary) cholesterol absorption, and excretion of cholesterol as bile salts or biliary cholesterol (1–3). A normal Western diet contains relatively equal amounts of cholesterol and noncholesterol sterols. These noncholesterol sterols are primarily plant sterols, and the mammalian body prevents the retention of these noncholesterol sterols, only allowing cholesterol to be retained and metabolized by the body. The molecular mechanism(s) by which this occurs was elucidated by studies of the rare genetic disorder sitosterolemia (MIM #210250), which led to the identification of two ABC “half-transporters” that are key to keeping these molecules out of the body (4–8). The genetic defect in Sitosterolemia results in the increased absorption of dietary sterols, including plant sterols, compounded by a profound inability to excrete biliary or intestinal sterols, thus leading to their retention (9–14). Mutations in either ABCG5 (sterolin-1) or ABCG8 (sterolin-2) cause the disease (5, 8, 15). ABCG5 and ABCG8 are found on the apical surface of enterocytes and hepatocytes (16–18), function as obligate heterodimers, and are the long-sought-after sterol exporters responsible for biliary and intestinal excretion (17, 19–24). It is becoming increasingly clear that ABCG5/ABCG8 are responsible for preventing accumulation of a host of dietary noncholesterol sterols (25), and thus, the term “xenosterol” should be used to refer to the class of sterols they prevent from accumulating. Three mouse models for the study of xenosterol/sitosterol trafficking are now available: a murine model simultaneously deficient in both Abcg5/Abcg8 (22, 24), a model lacking only of Abcg8 (20), and one deficient in Abcg5 (21). Despite some differences, all three models recapitulate important sitosterolemic features, such as increased plasma and tissue levels of plant sterols (phytosterols) and massively reduced cholesterol and phytosterol secretion/excretion into bile, but maintained biliary secretion of phospholipids and bile salts.
As part of our characterization on the Abcg8 null mouse, we noted that males showed considerably reduced fertility, but females were almost invariably infertile. Further investigation on the causes of this infertility allowed us to observe that the body fat stores were reproducibly smaller in knockout compared with wild-type mice. Treatment of Abcg8 knockout mice with a pharmacological agent that specifically blocks intestinal sterol uptake reversed the deficit in body fat stores, as well as restored fertility. We report here an investigation into these processes and show that plant sterols are responsible for altering fat storage and fertility in Abcg8 knockout mice.
MATERIAL AND METHODS
Animals and diets
All animals were housed in the facilities for laboratory animals provided by the Animal Research facility (Veterinary Medical Unit) at the Clement J. Zablocki VA Medical Centre, and protocols were approved by the Institutional Animal Care and Use Committee (IACUC). Abcg8−/− (> N10 on a C57Bl/6J background) and their wild-type littermates were used for the majority of the experiments. Cryogenically frozen Abcg5+/− embryos were obtained from a third party with permission of Tularik (South San Francisco, CA), and only one female founder was generated. This female was fertile, a colony backcrossed to C57Bl/6J (N>5) was established, and both Abcg8+/− and Abcg5+/− mice have been deposited with Jackson Laboratories (Bar Harbor, ME) for general distribution. Genotyping for Abcg8 by PCR was performed as previously published (20). All mice were initially maintained on a standard Purina rodent diet 5001 (Purina Mills Inc., St. Louis, MO). Subsequently, we devised a more defined diet described in the supplementary data. In selected studies, rodent chow was supplemented with 0.005% ezetimibe (wt/wt, Zetia, Merck-Schering Plough, Singapore) starting two weeks after birth. We prepared the diet monthly by mixing 200 g powdered Purina rodent diet 5001 with powdered pills containing 10 mg Ezetimibe, stored it at −4°C, and delivered it as powdered diet. In later studies, pelleted-defined diets low in phytosterols were prepared with Ezetimibe (TD 06676) or without Ezetimibe but supplemented with 1% phytosterols (high-sitosterol diet, TD 07306) by a commercial supplier (Harlan Teklad Diets, Madison, WI; see supplementary Table I). The meat diet was commercial minced beef obtained from local stores and supplemented with 1 g of vitamin mixture #40060 (Harlan Teklad) and 1 g of mineral mixture AIN-76 (Harlan Teklad) per every 1 kg raw ground beef, mixed throughout, rolled into small meatballs, and heated on a frying pan until cooked throughout. The meatballs were then stored at −20°C and thawed to room temperature prior to daily use. Because this diet is unconventional, IACUC permission was obtained with the proviso that the studies were to be 8 weeks or less in duration.
To isolate mice transgenic for expression of human ABCG8 driven by the villin promoter, the human cDNA for ABCG8 was cloned into a pBluescriptKS villin promoter construct (gift from Dr. Louvard, Institut Curie, Paris) using Bsi WI and Mlu sites, linearized with Sal I and the ∼11.7 kb fragment (supplementary Fig. I) micro-injected into FVB × C57Bl/6J fertilized eggs. The progeny were screened by PCR, and four founders were identified, of which three showed germ-line transmission. Progeny of all three lines were screened by RT-PCR, and one line, hereafter referred to as villinTgABCG8, was chosen based upon semiquantitative PCR showing robust expression of the transgene mRNA in the intestine (supplementary Fig. I, tracks 6 and 7). It was backcrossed to C57Bl/6J for four generations before crossing with Abcg8 KO lines. Abcg8−/−,villinTgABCG8 lines were fertile on chow diet (see Results) and were maintained as brother-sister matings. Mice that are homozygous villinTgABCG8+/+ did not show any overt phenotypic differences from wild-type mice (data not shown).
Tissue harvesting and histology of perigonadal WAT
Three-month old Abcg8−/− and Abcg8+/+ mice were euthanized, and perigonadal, perirenal white adipose tissue (WAT), subscapular brown adipose tissue, liver, spleen, brain, adrenals, intestinal scrapings, as well as gonads were collected, weighed, and rinsed three times in ice-cold phosphate buffered saline solution and fixed in 10% neutral buffered formalin (Fisher Scientific, Pittsburgh, PA) overnight. Portions were also flash-frozen in liquid nitrogen and stored at −80°C. Fixed tissues were embedded in paraffin, and 5 μm thick slices were obtained. Three slides from each block were stained with a classical hematoxylin and eosin stain (Fisher Scientific, Pittsburgh, PA) and imaged using a Spot digital camera (Diagnostic Instruments, Sterling Heights, MI) connected to an eMac computer (Apple, Cupertino, CA) and mounted on an Olympus CK 40 inverted microscope (Olympus America, Melville, NY). For quantitative analyses, we used ImageJ software version 1.36b (National Institutes of Health, Bethesda, MA, http://rsb.info.nih.gov/ij/); a square with frame of 148 × 148 μm was pasted on each slide, and the area of every cell within or touching the square borders was measured.
Glucose tolerance test
Male mice (n = 4 per group) were housed individually one week before the experiment, fasted for 4 h, and then glucose (2 g/kg body weight) was injected intraperitoneally using a tuberculin syringe with a 27.5 gauge needle. Tail-vein blood glucose was determined at 0, 15, 30, 60, and 120 min after injection using a glucometer (Precision, Abbott, Abbott Park, IL).
Mouse activity
Animals were placed in cages with running wheels, and their total wheel running activity was quantified with ClockLab software (Actimetrics, Wilmette, IL).
RNA isolation and quantitative RT-PCR
Total RNA was isolated from periuterine WAT using Trizol (Invitrogen, Carlsbad, CA). Quantitative RT-PCR was performed as described previously (20, 26). Primers used are shown in supplementary Table II or have been published previously (20, 27, 28). RT-PCR was performed on an Applied Biosystems 7300 Real-Time PCR system (Applied Biosystems, Foster City, CA). All reactions were performed in triplicate. The data were normalized to cyclophilin to compensate for variations in input RNA amounts and analyzed using the comparative threshold cycle method (CT) (29).
Microarray analyses
Male mice were chosen, since control female mice show estrus cycles, and age-matched wild-type mice, Abcg8−/− on chow or Abcg8−/− on chow supplemented with ezetimibe, were euthanized, testes were harvested, and total RNA was extracted for microarray analyses, which was performed by a commercial vendor (Genus Biosystems, Northbrook, IL). For each genotype, we used two animals, one testes per animal per chip (total 6 chips), using the GE Healthcare/Amersham Biosciences CodeLink UniSet Mouse 20K I Bioarray mouse chips and platform. The results are shown in the supplementary data.
Free fatty acids in mouse plasma
Blood was collected using capillary tubes by orbital puncture from approximately 11-week-old mice (n = 8 for each group) and centrifuged, plasma was collected, and free fatty acids were immediately processed using the NEFA C Assay Kit (Wako Chemicals USA, Richmond, VA) following the manufacturer's protocol.
Plasma hormone measurements
Plasma luteinizing hormone (LH), follicle-stimulating hormone (FSH) and prolactin levels were measured by Dr. A. F. Parlow at the National Hormone and Peptide Program, Harbor-UCLA Medical Center, Torrance, CA. Testosterone and di-hydrotestosterone levels were measured by Dr. F. Pau at the Oregon National Primate Research Center, Beaverton, OR.
Lipolysis in mouse peri-gonadal WAT
Approximately 12-week-old male mice were euthanized (n = 5 for each group), and lipolysis rates were determined as described previously (30).
Hormone-sensitive lipase activity
Hormone-sensitive lipase (Hsl) activity was assayed using a fat-free infranatant of perigonadal WAT homogenate from 12-week-old Abcg8+/+ and Abcg8−/− mice (n = 6 for each group) as described previously (31).
Sterol analyses
Tissue and plasma sterol analyses, using gas chromatography-mass spectroscopy (GC-MS), were performed as reported previously (20), except the column used was Restek and the samples were analyzed on a Thermo-Finnegan FOCUS GC/MS (ThermoFisher).
Statistical analysis
Data are shown as means ± SD. The ANOVA or Student t-test was used to determine the statistical significance of differences between the groups of animals. Significance was set at P < 0.05.
RESULTS
Abcg8- and Abcg5-deficient mice are infertile
In the course of establishing a colony of Abcg8 knockout lines (20), we reported previously that Abcg8−/− mice were viable. However, a breeding colony of homozygous knockout colony proved to be challenging as very few pregnancies resulted, irrespective of whether homozygous males were mated with homozygous females or whether homozygous females were mated with wild-type males. If homozygous males were mated with wild-type females, occasional pregnancies did result in the generation of heterozygous litters, but these were very infrequent. Over the next 5 years of breeding, we noted no more than 10 such litters when homozygous mice were used for breeding (more than 50 breeding pairs housed continuously for more than 12 weeks, see Table 1). Heterozygous mice are indistinguishable from wild-type mice in all of the parameters examined and were used as controls where stated. When we established a colony of Abcg5 knockout mice, we also noted that homozygous mice did not lead to successful pregnancy, with females being almost invariably infertile, and homozygous males occasionally leading to a pregnancy (Table 1). Although a formal study was not carried out, it appeared that any pregnancies, if they occurred, were when the mice were <2 months old. All mice had been maintained on commercial rodent chow. Because the other sitosterolemia mouse model was reported to exhibit normal fertility (22), we were concerned about this discrepancy.
TABLE 1.
Breeding success involving Abcg5 or Abcg8 knockout mice
| Female Genotype | Male Genotype | Number of Crosses | Diet | Number of Deliveries | Average Litter Size | Fertility | |
| Abcg8+/− | × | Abcg8+/− | >20 | Chow | >20 | 7.4 | Fertile |
| Abcg8−/− | × | WT | 14 | Chow | 2 | 8 | Partial |
| WT | × | Abcg8−/− | 12 | Chow | 2 | 6 | Partial |
| Abcg8−/− | × | Abcg8−/− | 16 | Chow | 1 | 4 | Infertile |
| Abcg8+/− | × | Abcg8−/− | 9 | Chow | 2 | 5.5 | Partial |
| Abcg8−/−,VillinTg+ | × | Abcg8−/−,VillinTg+ | >20 | Chow | >20 | 6.7 | Fertile |
| Abcg5−/− | × | Abcg5−/− | 6 | Chow | 0 | N/A | Infertile |
| Abcg5+/− | × | Abcg5−/− | 10 | Chow | 4 | 2.7 | Partial |
| Abcg5+/− | × | Abcg5+/− | 10 | Chow | 10 | 5.8 | Fertile |
| Abcg5−/− | × | Abcg5+/− | 6 | Chow | 0 | N/A | Infertile |
| Abcg8−/− | × | WT | 9 | Meat | 5 | 4.6a | Fertile |
| Abcg8−/− | × | Abcg8−/− | 3 | Meat | 3 | 7.3 | Fertile |
| Abcg8−/− | × | Abcg8−/− | 10 | Chow + ezetimibe | 10 | 7.1 | Fertile |
All mice were weaned at 4 weeks and mated at age 8 weeks. Males and females were placed together for continuous housing, and if there were pregnancies, males were removed until the pups were weaned and females placed back with the males. All wild-type mice were proven to be fertile before mating with Abcg8 knockout mice. Fertility was defined as resulting in pregnancy and pups delivered within 2 months of crossing. Infertility was defined as no pups delivered in 4 months. Partial fertility was defined as 1–2 deliveries in 12 months of continuous breeding. All pregnancies noted in Abcg8−/− mice (irrespective of sex) were when these mice were less than 12 weeks of age. Female mice older than 12 weeks did not get pregnant, including ones that may have delivered once. Male mice older than 12 weeks also did not result in getting wild-type or Abcg8+/− mice pregnant.
Some pups were cannibalized after birth.
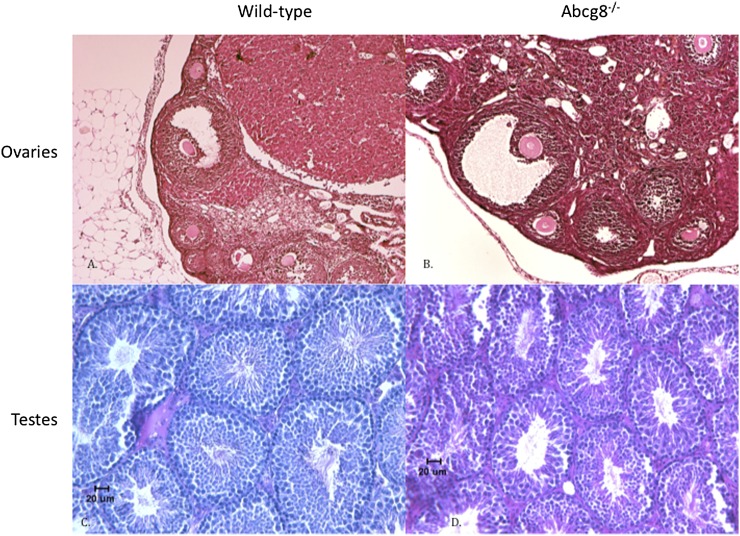
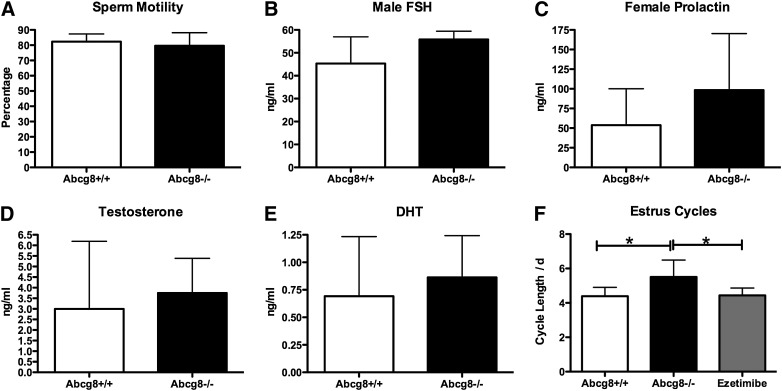
Infertility could result from a structural defect or from a functional defect. Ovaries and testes were examined histologically (Fig. 1). Overall, no structural defects could be identified between gonads from wild-type or Abcg8 KO mice. Examination of the testes from Abcg8 KO mice showed the presence of all stages of sperm development. Sections of ovaries showed the presence of follicles, as well as corpora lutea. For functional analyses, sperm were isolated from testes from wild-type or KO mice and examined for morphology as well as motility (Fig. 2A). No differences were discernible. In vitro fertility testing was not performed. The presence of all forms of sperm during maturation, the preserved sperm motility, etc., suggested that hormonal disruption is also less likely. This was confirmed by hormone measurements of LH/FSH as well as di-hydrotestosterone that showed comparable levels between wild-type and KO mice (Fig. 2B–D). In the case of females, vaginal smears were examined for an estimation of the estrus cycle (Fig. 2F). In general, most Abcg8 females manifested an estrus cycle, and although this was lengthened by at least one day, almost all of the increase was accounted for time spent in postestrus. When more defined diets (as opposed to standard chow) fortified with plant sterols were employed, KO females seemed to be stuck in the postestrus phase (see below). As loss of Abcg8 leads to increased dietary sterol absorption, we reasoned that blocking dietary sterol absorption might lead to amelioration of the lengthening of the estrus cycle. When rodent chow was fortified with ezetimibe, a drug that blocks dietary sterol absorption by >90%, the estrus cycle was shortened to normal (Fig. 2F), and both Abcg8 KO male and female mice were fertile and were able to breed several successive litters. Thus, blocking intestinal sterol entry, cholesterol as well as xenosterols, led to restoration of fertility, suggesting sterol toxicity was leading to infertility in both males and females.
Fig. 1.
Gonadal histology from Abcg8 knockout mice. Abcg8 wild-type or knockout mice were raised on rodent chow. Ovaries (A and B) or testes (C and D) were obtained from adult animals (ages > 12 weeks), fixed, sectioned, and stained with H and E. (A and C) are from wild-type mice, and (B and D) are from Abcg8 knockout mice. No significant differences were discernible between wild-type and knockout mice.
Fig. 2.
Hormonal and infertility profiles in ABCG8 knockout mice. To investigate the infertility of Abcg8 knockout mice, sperm motility (A), male FSH levels (B), female prolactin levels (C), male testosterone (D), male di-hydrotestosterone (E), and female estrus cycles (F) were examined. Except for estrus cycles, no differences were noted between wild-type (open bars) and knockout mice (black bars). Estrus cycles were lengthened by at least one day, and all of the increase was in the postestrus phase (see text). Fortification of the rodent chow diet with the sterol absorption-blocking drug ezetimibe reversed this and restored fertility (see text).
Microarray analyses of testes from Abcg8−/− and Abcg8+/+ mice
To gain mechanistic insights into the loss of fertility, we chose to perform microarray analyses of male testes. Female wild-type mice show normal estrus cycles, and thus, females were not chosen, since the knockout ovaries could be halted at different estrus-cycle phases and this would be difficult to control for. Male sperm development is continuous, and our histological analyses suggested this was not altered in Abcg8 knockout mice. To improve the chances that we might be able to identify pathways that could be mechanistically linked to plant sterol-mediated disruption of fertility, we reasoned that if exclusion of xenosterols restored fertility, we might be able to recognize a gene expression signature that was different between wild-type and Abcg8 knockout mice and that this pattern would be restored toward the wild-type pattern in Abcg8 knockout mice in which plant sterols were blocked. Wild-type mice (n = 2), Abcg8−/− mice fed chow (n = 2), or Abcg8−/− mice fed chow supplemented with ezetimibe to block xenosterol accumulation were used to compare gene expression patterns by microarray analyses of testes (see supplementary data). Heat maps showed that there were few changes in gene expression profiles between any of these groups. Additionally, there were too few expression changes to allow for meaningful pathway analyses (see supplementary data). This finding suggests that the pathophysiological mechanism(s) may involve nontranscriptional pathways.
Xenosterol toxicity, not cholesterol toxicity, leads to infertility
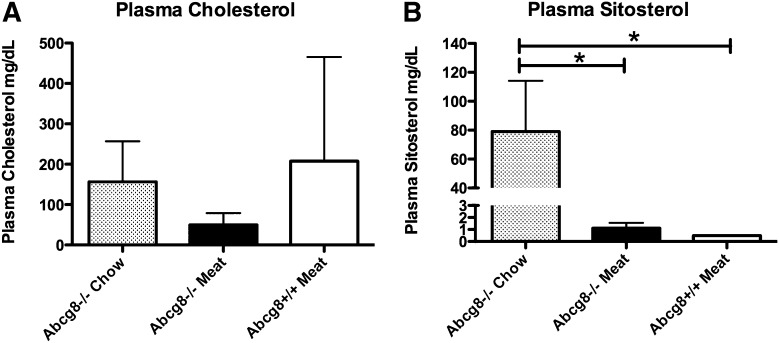
Because the loss of Abcg8 leads to increased accumulation of both cholesterol, as well as xenosterol (and both are prevented from accumulating by ezetimibe), we tested the possibility that the infertility could result as a consequence of cholesterol toxicity and not xenosterol accumulation. It is relatively difficult to remove all sources of xenosterols from a diet suitable for rodent consumption. We therefore reasoned that if a diet were made purely of food source of animal origin supplemented with essential nutrients, xenosterol exposure could be almost eliminated. At weaning, female Abcg8 KO mice were fed a diet consisting of steamed meatballs supplemented with essential nutrients, and after eight weeks, they were mated to check for fertility (see Table 1) and compared with C57Bl/6J mice fed meatballs and Abcg8 mice fed chow. All wild-type (n = 4) as well as Abcg8 female KO mice (n = 6) fed meatballs became pregnant within one week of housing with a stud male and successfully delivered normal litters, whereas none of the Abcg8 female mice fed chow became pregnant, despite continuous housing for more than three weeks with proven stud wild-type males. To confirm that the meatball diet prevented accumulation of xenosterols, plasma was analyzed from the mice (Fig. 3). Although Abcg8 female KO mice raised on chow exhibited significant accumulation of plant sterols, wild-type and Abcg8 KO mice raised on the meat diet had very low levels of plant sterol (Fig. 3B). Thus, despite the accumulation of cholesterol, but not xenosterols, via the meatball diet, fertility was restored. Interestingly, Abcg8 KO mice on the meatball diet showed decreased plasma cholesterol levels (Fig. 3A), although this observation was not pursued further.
Fig. 3.
Effect of meat-only diet in Abcg8 knockout mice. To exclude plant sterols from the diet to see whether fertility could be restored, we weaned female mice and placed these on the meat diet (see Methods). At the end of the experiment (after breeding challenge), plasma was examined by GC for cholesterol (A) and sitosterol (B). On the meat diet, Abcg8 knockout mice (B, middle bars, n = 5) showed significantly lower plant sterol levels compared with knockout mice fed chow (B, n = 4), and although the cholesterol levels were also decreased (A, middle bar), these did not reach statistical significance by ANOVA. Note that the plant sterols attained on the meat diet in the Abcg8 knockout mice were only 1.1 ± 0.4 mg/dl compared with wild-type mice (n = 6) fed the meat diet, 0.5 mg/dl (lower level of detection). On chow, the Abcg8 knockout mice (n = 4) had a sitosterol level of 79 ± 35 mg/dl. All of the Abcg8 knockout mice on the meat diets got pregnant, whereas none of the knockout mice on the chow diet did.
Restoration of intestinal Abcg5/Abcg8 activity restores fertility
To selectively restore sterolin function to the intestine alone, we created a mouse transgenic for human ABCG8, whose expression is driven by the mouse villin promoter villinTgABCG8 (supplementary Fig. I). We reasoned that if Abcg5/Abcg8 act as the first line of defense against xenosterols, restoring this activity in the intestine alone might prove to be insightful. We chose human ABCG8 cDNA, as the mammalian genes are highly homologous and thus expression of human ABCG8 in mouse enterocytes should allow for complementation of sterolin function in Abcg8-deficient mice.
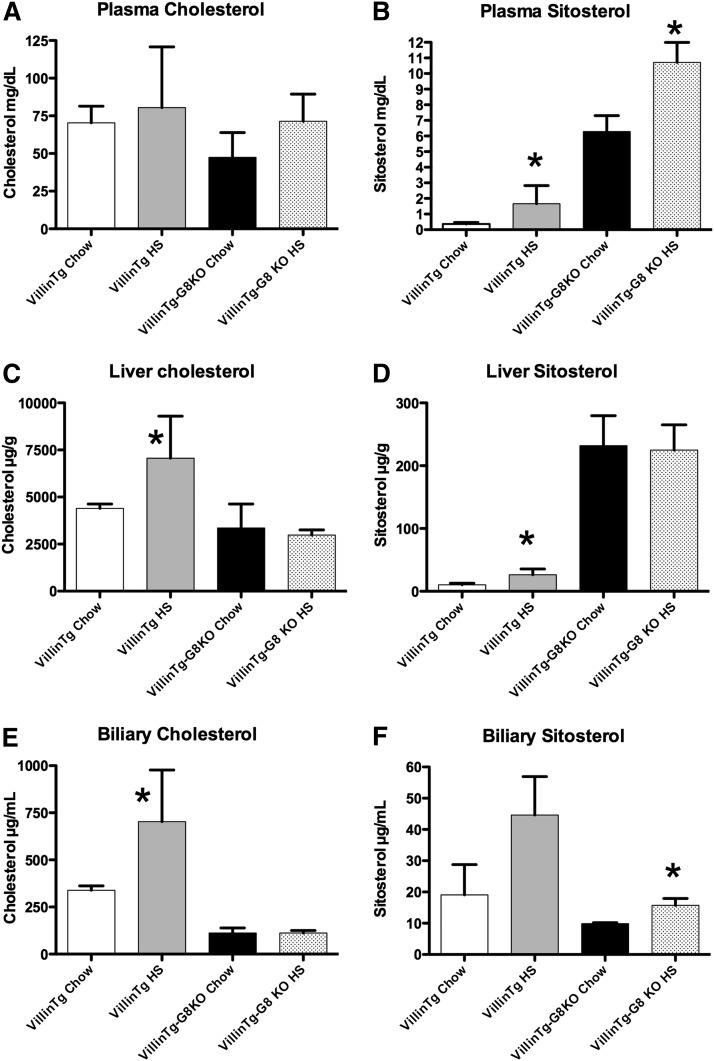
Mice transgenic for human ABCG8 expression via the villin promoter were established. Using RT-PCR, expression of ABCG8 mRNA was confined to intestine; no signal could be obtained in livers from transgenic mice (supplementary Fig. I). To test whether restoration of normal sterolin function in the intestine could rescue the infertility, Abcg8+/− were crossed with villinTgABCG8+ mice, and the heterozygous F1 mice were crossed to generate Abcg8−/−,villinTgABCG8+ mice. On chow diet, both males and females were fully fertile and exhibited no differences in body weight gain or litter sizes. Plasma cholesterol levels were not affected by the transgene (Fig. 4A). However, biochemical analyses of plasma showed that there were significant elevations of plant sterols when mice were fed chow (Fig. 4B) compared with wild-type mice but that the levels of sitosterolemia (∼6 mg/dl) were considerably less than Abcg8−/− mice (typically >20 mg/dl). When we had developed a more defined diet that allowed us to regulate the phytosterol content, placing these mice on a high-sitosterol diet showed that the level of sitosterolemia was considerably ameliorated by the transgenic complementation in the intestine of the Abcg8 KO mice; the sitosterol levels were ∼10 mg/dl compared with ∼70 mg/dl in Abcg8 KO mice on a high-sitosterol diet.
Fig. 4.
Effect of villinTg-ABCG8 gene expression in Abcg8 knockout mice on sterol profiles. Mice expressing human ABCG8, driven by the intestine-specific villin promoter, were made and bred into the Abcg8 knockout line (see Methods) and placed on chow or a high-sitosterol (HS) diet. On chow or HS diet, plasma cholesterol levels (A) were not altered by the presence of transgene (the genotypes are as indicated on the x axis). On a HS diet, plasma sitosterol levels were significantly increased in villin Tg mice (B, second bar) compared with mice raised on chow alone. However, these levels are small (1.7 ± 1.2 mg/dl, n = 4). On a chow diet, villin Tg-Abcg8 knockout mice (B, third bar) showed very mild elevations in plasma sitosterol levels (6.3 ± 1 mg/dl), and more remarkably, when placed upon a HS diet (B, last bar), showed significantly higher, but greatly attenuated levels (10.3 ± 1.2 mg/dl) compared with the chow group. Note that hepatic deficiency of Abcg8 function was not altered by the transgene expression; liver sitosterol levels were high in villinTg-Abcg8 KO mice (D), and biliary excretion of cholesterol and sitosterol remained low (E and F). Although villinTg mice on a HS diet showed increased liver cholesterol levels compared with chow, this observation was not pursued further (C).
Although the presence of the transgene significantly ameliorated the plasma phytosterol levels, liver (Fig. 4C, D) as well as biliary sterol profiles (Fig. 4E, F) showed that plant sterol accumulation was comparable to Abgc8−/− mice and that the biliary defect of sterol secretion remained. This finding further supports the contention that the infertility was secondary to the entry of dietary xenosterols and their accumulation in the body. Importantly, intestine-alone complementation of sterolin function was sufficient, and this finding may have implications for development of therapies for the human disease.
Adipose tissue is reduced in Abcg8−/− mice
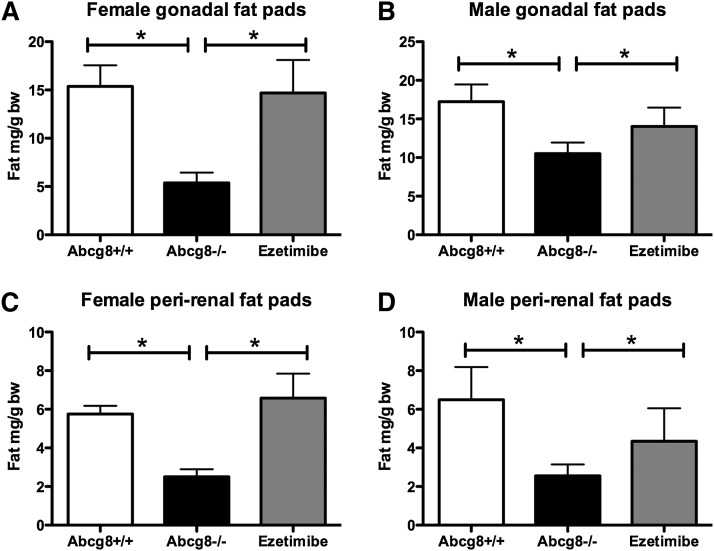
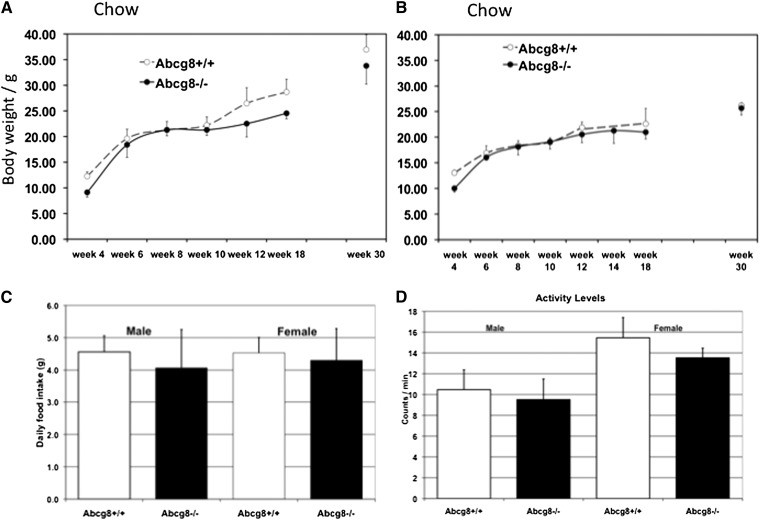
During the course of the studies to harvest organs for analyses, we noted that it was easier to harvest organs from Abcg8 KO mice than it was from control mice. The reason became obvious: there was less periorgan adipose tissue in Abcg8 KO mice fed chow than in wild-type mice, making organ identification and dissection much easier (supplementary Fig. II). Quantitative analyses of various fat depots are shown in Fig. 5. In Abcg8 KO mice fed ezetimibe, the fat pads were comparable to wild-type mice (Fig. 5, compare gray columns with white columns). Interestingly, on chow, serial total body weights did not appear to be different (Fig. 6). Both sexes showed a significantly reduced body weight at age of four weeks, but their weights were comparable to wild-type mice by six weeks age. Abcg8−/− males, but not females, showed a slightly decreased total body weight by week 12 compared with their wild-type counterparts (Fig. 6A), but this was not statistically significant. We saw no difference in the daily dietary intake between Abcg8−/− and Abcg8+/+ mice of both sexes (Fig. 6C). As increased activity could lead to increased energy expenditure and thus reduced fat stores, we performed a simple activity test over one week, using wheel running. No differences between Abcg8−/− and Abcg8+/+ mice were noted (Fig. 6D).
Fig. 5.
Quantitation of regional fat depots in Abcg8 knockout mice. Fat depots as indicated were dissected, weighed, and normalized to body weights in wild-type (open bars, n = 4), knockout (black bars, n = 4), or knockout mice fed chow supplemented with ezetimibe (gray bars, n = 4 for males and n = 4 for females). Mice were ∼16 weeks old at sacrifice and were fed chow. In knockout mice, fat depots were significantly reduced in the gonadal (A and B) as well as perinephric area (C and D), whereas in knockout mice fed chow supplemented with ezetimibe, there were no differences when compared with wild-type mice raised on chow.
Fig. 6.
Growth charts for Abcg8 knockout mice. Serial weights of male (A) and female mice (B) were monitored. On chow, Abcg8 knockout mice (filled circles) do not show any significant differences compared with wild-type mice. Food intake was determined by serial food weighing and no differences (C) (males 4.30 ± 0.98 versus 4.53 ± 0.48 g/day; females 4.07 ± 1.19 versus 4.56 ± 0.50 g/day, respectively) were noted. Activity levels also seemed comparable (D) (males 9.51 ± 1.99 versus 10.47 ± 1.9 counts/min; females 13.53 ± 0.93 versus 15.46 ± 1.93 counts/min, respectively).
Sizes, not numbers, of adipocytes are decreased in Abcg8 KO mice, and fat loss is reversed by coadministration of ezetimibe
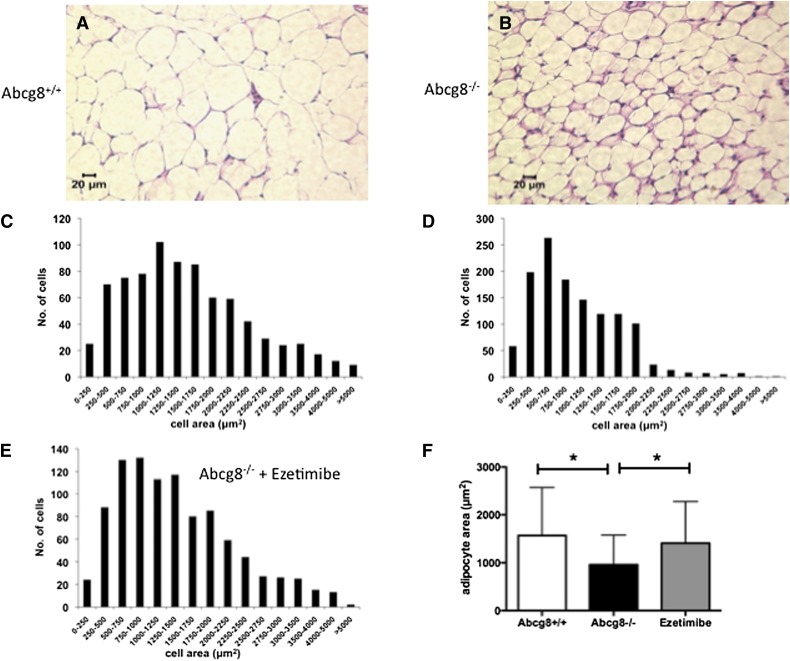
To examine whether the loss of abdominal fat was a result of loss of adipocytes or decrease in triglyceride storage, tissue was fixed and processed for histological staining (Fig. 7A, B). The cell sizes were reduced in Abcg8−/− mice, with a reduced cell area compared with wild-type mice (958 ± 619 versus 1,569 ± 1,004 μm2, respectively; P < 0.05), but this was also restored toward normal in Abcg8−/− mice fed chow fortified with ezetimibe (1,409 ± 872 μm2) (Fig. 7C–E).
Fig. 7.
Adipocyte sizes are reduced in Abcg8 knockout mice and reversed by ezetimibe. (A and B) Representative histological sizes of adipocytes from fat stores from wild-type and knockout mice, respectively. Formal size quantitation is shown below: (C) wild-type, (D) Abcg8 knockout on chow, € Abcg8 knockout on chow supplemented with ezetimibe. The smaller adipocyte cell sizes are restored toward normal in Abcg8 knockout mice when the chow is supplemented with ezetimibe to block dietary sterol entry at the intestinal level (F shows area quantitations).
Were our results unique to Abcg8−/− mice? Were they reproducible in other sitosterolemic mouse models? We analyzed the perigonadal WAT from Abcg5−/− mice and their wild-type littermates maintained on rodent chow. A decrease in perigonadal WAT was notable in Abcg5−/− compared with Abcg5+/−+ mice (males 12.38 ± 3.82 versus 19.95 ± 4.69, females 6.76 ± 1.92 versus 11.53 ± 2.70 mg/g body weight, respectively; P < 0.05). Histologically, reduced adipocyte size distribution was also present in Abcg5−/− peri-gonadal WAT compared with wild-type mice (900 ± 722 versus1,501 ± 900 μm2, respectively, P < 0.05). Sterol analyses of fat stores in male Abcg8 knockout mice on a high- or low-sitosterol diet confirm that plant sterols were dramatically elevated in the high sitosterol-fed mice (supplementary Fig. III).
Glucose tolerance test and fatty acid metabolism in Abcg8−/− mice
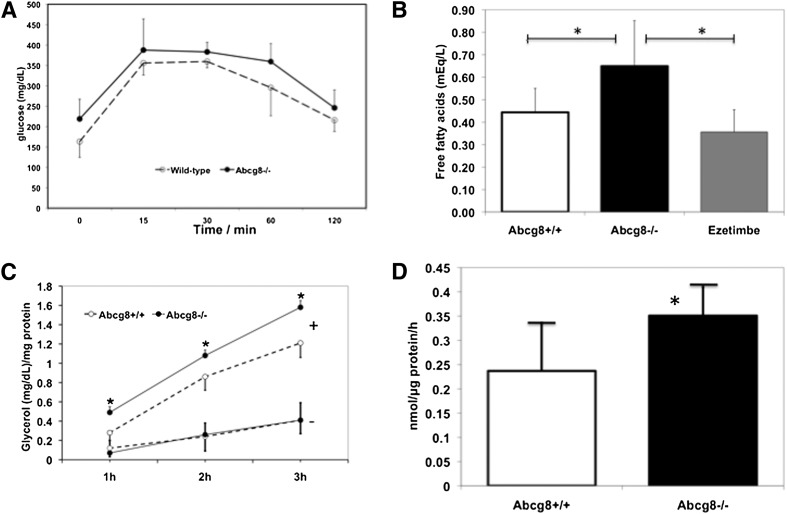
Because of reduced retroperitoneal adipose mass, we performed an intraperitoneal glucose tolerance test. The results of the test in Abcg8−/− and Abcg8+/+ were similar (Fig. 8A). No differences in the glucose excursion curves were noted. Similar data were obtained for Abcg5 knockout mice (supplementary Fig. IV). However, plasma free fatty acid (FFA) levels were increased in Abcg8 KO mice (Abcg8−/− 0.65 ± 0.2 versus Abcg8+/+ 0.44 ± 0.11 mEq/l; P < 0.01) (Fig. 9B). Ezetimibe-supplemented Abcg8 KO mice showed FFA levels comparable to wild-type mice (0.36 ± 0.10 mEq/l), again indicating that blocking dietary sterol absorption is able to reverse this phenotype.
Fig. 8.
Glucose tolerance and adipose tissue fatty acid release in Abcg8 knockout mice. (A) Results of an intraperitoneal glucose tolerance in wild-type (open circles) or knockout mice (n = 4 per group); no differences were observed in the glucose excursions. Plasma baseline free fatty acid levels were significantly increased in the Abcg8 knockout mice (B, middle bar), and these were lowered back to wild-type levels in Abcg8 knockout mice whose diet was supplemented with ezetimibe (B, last bar). To further characterize the increase in fatty acids, adipocytes were isolated and cultured, and the release of fatty acids into media was examined under basal (−) and isoproterenol-stimulated (+) conditions (C). Basal levels of fatty acid release was indistinguishable between wild-type (open circles) and knockout adipocytes (C, filled circles, bottom two lines, marked by “−”). However, upon isoproterenol stimulation, significantly more fatty acid was released by adipocytes from abcg8 knockout mice (C, top two lines marked by “+” n = 5). A classical hormone-sensitive assay was performed on adipocyte homogenates (D), which showed that knockout adipocytes (n = 6) had higher lipolytic activity compared with wild-type adipocytes (n = 5).
Fig. 9.
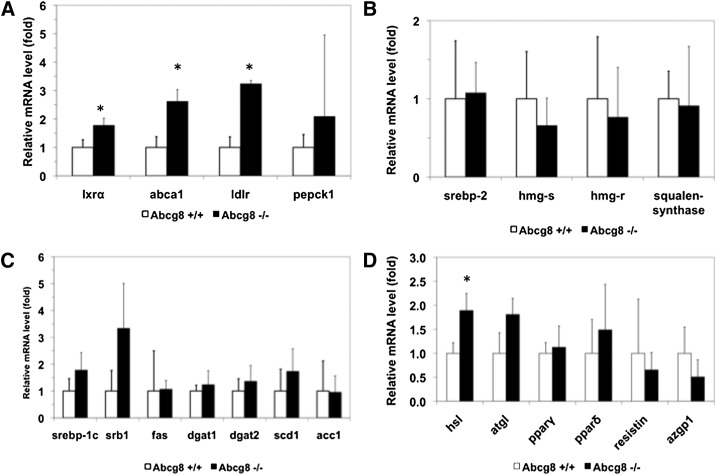
Gene expression profiles of selected mRNAs in adipocytes isolated from Abcg8 knockout mice. Real-time PCR analyses for selected genes were performed from RNA isolated from adipose tissue from wild-type (open bars) or knockout mice (closed bars). Significantly increased gene expression patterns for Lxr, Abca1, Ldl-R (A), and Hsl (D) were noted in fat from knockout mice. Interestingly, sterol and fatty acid syntheses pathways did not seem to be perturbed (B,C).
Since FFA levels were increased in Abcg8 KO mice, we measured lipolysis in perigonadal WAT. No significant differences in the basal lipolytic rates were noted in periepididymal WAT from Abcg8−/− and Abcg8+/+ mice (Fig. 8C, lower lines). In contrast, when stimulated with isoproterenol, Abcg8−/− WAT showed increased levels of free glycerol (Fig. 8C, upper lines). Lipase activity was also significantly increased in WAT homogenates from Abcg8−/− mice compared with Abcg8+/+ mice (0.36 ± 0.06 versus 0.23 ± 0.9 nmol/µg protein/h; P < 0.05) (Fig. 9D).
Gene expression changes in WAT of Abcg8−/− and Abcg8+/+ mice
To gain mechanistic insights into the loss of lipid storage in the WAT, we measured gene expression changes using quantitative RT-PCR (Fig. 9). Genes involved in triglyceride synthesis (Fig. 9C) showed no differences between the two genotypes, whereas Lxrα-dependent genes were mostly upregulated in Abcg8−/− mice (Fig. 9A). In contrast with previous results in sitosterolemic human and mouse liver as well as mouse adrenal glands (20, 32, 33), the cholesterol synthesis pathway seemed not to be significantly suppressed in Abcg8−/− mice (Fig. 9B). Interestingly, the expression level of Hsl was increased in Abcg8−/− mice (Fig. 9D). A similar tendency, although not significant, was observed for the lipolytic enzyme Atgl (Fig. 9D, P = 0.064).
DISCUSSION
The human disease of sitosterolemia (MIM #210250) is caused by mutations affecting one of two proteins, ABCG5 or ABCG8 (also known as sterolin-1 and sterolin-2, respectively), which comprise a functional heterodimer now known to be responsible for mammalian sterol excretion. In humans, this disease has been reported to lead to increased atherosclerosis, formation of tuberous and tendinous xanthomas, and macrothrombocytopenia, with rare cases of endocrine disruption or cirrhosis of the liver also reported (34). Although an endocrine disruption was reported in one family (35), endocrinopathies and infertility have not been reported. Although sterolins function to secrete/excrete cholesterol into bile or into the lumen of the intestine (and they are expressed only in these tissues), a major key role is in preventing xenosterols from accumulating in the mammalian body. Xenosterols (such as plant sterols that comprise the major food group) are preferentially excreted into bile in humans. It is not clear whether, when sterolin function is impaired, any subsequent pathophysiological issues that arise are because of cholesterol toxicity or xenosterol toxicity (or both). In sitosterolemic individuals, as well as in several in vivo and in vitro studies, phytosterols can affect the activity of several metabolic pathways. In sitosterolemic humans (32) and mice (20), reduced activities of several hepatic enzymes involved in cholesterol synthesis have been shown. Although sitosterol has been shown to have a direct effect on CYP7α activity, it does not affect HMG-CoA reductase, and paradoxically, there is an upregulation of LDL-receptors in tissues from sitosterolemic individuals (32, 36–39). To date, direct biological activity for most of these noncholesterol sterols has been difficult to demonstrate. Sitosterol is the most common noncholesterol sterol humans are exposed to, but this sterol does not seem to have potent biological effects. In contrast, a less abundant plant sterol, stigmasterol, has been shown to activate the transcriptional factor Lxrα and decrease SREBP-2 activity in vitro (33). More recently, it is also been implicated to lead to FXR activation (40). Furthermore, phytosterol mixtures incorporated into macrophage cell membranes affect prostaglandin release, and such mixtures have been shown to affect embryological development and levels of circulating hormones in fish (41–46). Sitosterol has been proposed to affect antioxidant enzymes, to have apoptotic effects on tumor cells, to inhibit hepatic sterol 27-hydroxylase and 5α-reductase, as well as to help improve symptoms of benign prostatic hyperplasia (47–52). Sterols with a double bond at C-22 in the side chain (stigmasterol, brassicasterol, and ergosterol) have been shown to inhibit the activity of sterol Δ24-reductase in vitro (53). Orally administered phytosterols have been suggested to enhance lipolysis and prevent lipid deposition as well as accelerate fatty acid desaturation in isolated rat adipocytes (54, 55). All of these studies support the hypothesis that xenosterols affect mammalian physiology.
In this study of Abcg8 or Abcg5 knockout mice, we report that plant sterols accumulate in these animals fed a chow diet, that these mice exhibit loss of fertility and loss of fat storage, and that all of these effects can be blocked when plant sterols are prevented from accumulating. The evidence for the latter is supported by using a diet that is free of xenosterols (the meat diet), by blocking all dietary sterol entry using the drug ezetimibe, by transgenically restoring normal sterolin function in the intestine using the villin promoter (though the liver continues to be defective), and by devising a purified diet that minimizes the xenosterol content. What is remarkable is the variety of different phenotypes that are manifest when these mice accumulate these xenosterols. The loss of intra-abdominal fat stores is dramatic, and on the more defined diets enriched in plant sterols, fat stores are almost totally absent after eight weeks in female Abcg8 knockout mice (data not shown). This latter point is important, as on chow diet, the animals seemed to have fared well, and this phenotype was not noted until significant time had passed. This may be one reason why studies using Abcg5/Abcg8 knockout had not reported these phenotypes (22). Although initially infertility was not noted to be an issue with Abcg5/Abcg8 knockouts (22), subsequent to their deposition at the Jackson Labs, their website indicates that female fertility of knockouts is impaired (http://jaxmice.jax.org/strain/004670.html). Chow is known to result in uneven and perhaps even poor cholesterol absorption, and the plant sterol content is usually not defined.
In chow-fed knockout mice, fat cells seem to progressively be depleted of triglycerides, with an increase in lipolytic rates, as judged by isolated adipocyte preparations, but with no significant changes in triglyceride or FPLC lipid profiles (20). Reduced food intake did not seem to account for the loss of abdominal fat, although our measures may not have been sensitive enough. However, if accumulation of plant sterols led to reduced food intake, this would also be of interest because it would suggest that accumulation of plant sterols can lead to presumably central nervous system-mediated alteration in food intake or that plant sterols may be sensed as unpalatable by the mice. Clearly, more detailed and targeted studies to explore these concepts are called for. Plant sterols have been shown to accumulate in the CNS (56), though the functional consequence of this has not been established (57).
The cause of infertility by xenosterols remains elusive; there are no structural differences in the ovaries or testes caused by loss of sterolin function or by xenosterol accumulation. There were no major changes in gene expression profiles of whole testes examined by global microarray analyses. It is possible that this approach is too insensitive, that gene expression changes may underlie the pathophysiological mechanism, and that we did not analyze the key cells that are more susceptible to plant sterols. Like the brain, the testes are also thought to have a testes-blood barrier, and this may have accounted for a lack of significant gene expression changes. If so, the reduced fertility is therefore even more difficult to explain. On chow diet, female mice exhibited an estrus cycle, although the postestrus phase was significantly lengthened, supportive of an effect on this pathway. However, it is difficult to conceptualize why male mice also show significantly reduced fertility. Sperm development appeared normal, and isolated sperm motility was also judged normal. One possibility is that the accumulated plant sterols may significantly affect the membranes of both ova and sperm, thus interfering with fertility. Further studies are needed to explore these possibilities. Another possibility is that plant sterols may affect local production of the active sterols, T-MAS and FF-MAS, as well as desmosterol in the respective gonadal tissues (58, 59). These latter sterols are known to affect fertility, and if plant sterols affected their biosynthesis, perhaps infertility can result.
Adrenal glands have been reported to be affected by plant sterol accumulations. Although these animals were not shown to be corticosterone deficient, stimulated corticosterone levels were significantly lower in sterolin-deficient mice (33). We confirmed that the adrenals in Abcg8 KO mice on chow diets are depleted of lipid droplets. However, we did not measure their corticosterone levels. In the study by Yang et al., stigmasterol, but not sitosterol, was implicated as the phytosterol responsible for the effects on the adrenal glands, and this group has reported that rarer plant sterols may be selectively accumulated in sterolin-deficient mice (24). It is likely that the stigmasterol content in the chow diets used may be both highly variable and a factor in published studies. Detailed exploration of this aspect is warranted.
In conclusion, we show that when mice accumulate xenosterols from the diet, a number of different physiological systems are disrupted: the endocrine system is disrupted with infertility in both males and females, and fat metabolism is altered with loss of fat. The use of Abcg8 or Abcg5 knockout mice, together with improved and defined diets, may prove useful in exploring mechanisms whereby xenosterols disrupt mammalian physiology, and we propose that xenosterols also be considered endocrine disruptors.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the advice and assistance provided by Dr. Joseph Besharse, Medical College of Wisconsin, with the activity monitoring; Drs. Demetri Spyropoulos and Eric Klett, Medical University of South Carolina, with making the villin transgenic mice; and Mr. Brian Stogsdill, Ms. Yanhong Cai, Ms. Abigail Maciolek, and Mr. Jesse Buel with sterol determinations, animal husbandry, and technical assistance. The authors are grateful to Dr. Xujing Wang for assistance with re-analyses of the microarray data on the testes. The authors thank Dr. Daisy Sahoo for her critical comments of this manuscript.
Footnotes
Abbreviations:
- FSH
- follicle-stimulating hormone
- Hsl
- hormone-sensitive lipase
- KO
- knockout
- LH
- luteinizing hormone
- WAT
- white adipose tissue
This work was supported in part by a Department of Veterans Affairs Biomedical Laboratory R&D Merit Award from the Veterans Health Administration, Office of Research and Development (S.B.P.); the American Heart Association (S.B.P.); the Novartis Foundation (C.S.); the Ruth De Bernardis Foundation Bern/CH (C.S.); and the Balli Foundation Bellinzona/CH (C.S.).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of four figures and three tables.
REFERENCES
- 1.Chiang J. Y. 2002. Bile acid regulation of gene expression: roles of nuclear hormone receptors. Endocr. Rev. 23: 443–463 [DOI] [PubMed] [Google Scholar]
- 2.Kidambi S., Patel S. B. 2008. Cholesterol and non-cholesterol sterol transporters: ABCG5, ABCG8 and NPC1L1: a review. Xenobiotica. 38: 1119–1139 [DOI] [PubMed] [Google Scholar]
- 3.Lu K., Lee M. H., Patel S. B. 2001. Dietary cholesterol absorption; more than just bile. Trends Endocrinol. Metab. 12: 314–320 [DOI] [PubMed] [Google Scholar]
- 4.Lee M-H., Gordon D., Ott J., Lu K., Ose L., Miettinen T., Gylling H., Stalenhoef A. F., Pandya A., Hidaka H., et al. 2001. Fine mapping of a gene responsible for regulating dietary cholesterol absorption; founder effects underlie cases of phytosterolemia in multiple communities. Eur. J. Hum. Genet. 9: 375–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee M-H., Lu K., Hazard S., Yu H., Shulenin S., Hidaka H., Kojima H., Allikmets R., Sakuma N., Pegoraro R., et al. 2001. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat. Genet. 27: 79–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu K., Lee M-H., Hazard S., Brooks-Wilson A., Hidaka H., Kojima H., Ose L., Stanlenhoef A. F. H., Miettinen T., Bjorkhem I., et al. 2001. Two genes that map to the STSL locus cause sitosterolemia: genomic structure and spectrum of mutations involving sterolin-1 and sterolin-2, encoded by ABCG5 and ABCG8 respectively. Am. J. Hum. Genet. 69: 278–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel S. B., Honda A., Salen G. 1998. Sitosterolemia: exclusion of genes involved in reduced cholesterol biosynthesis. J. Lipid Res. 39: 1055–1061 [PubMed] [Google Scholar]
- 8.Patel S. B., Salen G., Hidaka H., Kwiterovich P. O., Stalenhoef A. F., Miettinen T. A., Grundy S. M., Lee M. H., Rubenstein J. S., Polymeropoulos M. H., et al. 1998. Mapping a gene involved in regulating dietary cholesterol absorption. The sitosterolemia locus is found at chromosome 2p21. J. Clin. Invest. 102: 1041–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lutjohann D., Bjorkhem I., Beil U. F., von Bergmann K. 1995. Sterol absorption and sterol balance in phytosterolemia evaluated by deuterium-labeled sterols: effect of sitostanol treatment. J. Lipid Res. 36: 1763–1773 [PubMed] [Google Scholar]
- 10.Miettinen T. A. 1980. Phytosterolaemia, xanthomatosis and premature atherosclerotic arterial disease: a case with high plant sterol absorption, impaired sterol elimination and low cholesterol synthesis. Eur. J. Clin. Invest. 10: 27–35 [DOI] [PubMed] [Google Scholar]
- 11.Salen G., Patel S. B., Batta A. K. 2002. Sitosterolemia. Cardiovasc. Drug Rev. 20: 255–270 [DOI] [PubMed] [Google Scholar]
- 12.Salen G., Shore V., Tint G. S., Forte T., Shefer S., Horak I., Horak E., Dayal B., Nguyen L., Batta A. K., et al. Increased sitosterol absorption, decreased removal, and expanded body pools compensate for reduced cholesterol synthesis in sitosterolemia with xanthomatosis. J. Lipid Res. 1989. 30: 1319–1330 [PubMed] [Google Scholar]
- 13.Salen G., Tint G. S., Shefer S., Shore V., Nguyen L. 1992. Increased sitosterol absorption is offset by rapid elimination to prevent accumulation in heterozygotes with sitosterolemia. Arterioscler. Thromb. 12: 563–568 [DOI] [PubMed] [Google Scholar]
- 14.Shulman R. S., Bhattacharyya A. K., Connor W. E., Fredrickson D. S. 1976. Beta-sitosterolemia and xanthomatosis. N. Engl. J. Med. 294: 482–483 [DOI] [PubMed] [Google Scholar]
- 15.Berge K. E., Tian H., Graf G. A., Yu L., Grishin N. V., Schultz J., Kwiterovich P., Shan B., Barnes R., Hobbs H. H. 2000. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 290: 1771–1775 [DOI] [PubMed] [Google Scholar]
- 16.Bhattacharyya A. K., Connor W. E., Lin D. S., McMurry M. M., Shulman R. S. 1991. Sluggish sitosterol turnover and hepatic failure to excrete sitosterol into bile cause expansion of body pool of sitosterol in patients with sitosterolemia and xanthomatosis. Arterioscler. Thromb. 11: 1287–1294 [DOI] [PubMed] [Google Scholar]
- 17.Graf G. A., Li W. P., Gerard R. D., Gelissen I., White A., Cohen J. C., Hobbs H. H. 2002. Coexpression of ATP-binding cassette proteins ABCG5 and ABCG8 permits their transport to the apical surface. J. Clin. Invest. 110: 659–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Repa J. J., Berge K. E., Pomajzl C., Richardson J. A., Hobbs H., Mangelsdorf D. J. 2002. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J. Biol. Chem. 277: 18793–18800 [DOI] [PubMed] [Google Scholar]
- 19.Graf G. A., Yu L., Li W. P., Gerard R., Tuma P. L., Cohen J. C., Hobbs H. H. 2003. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J. Biol. Chem. 278: 48275–48282 [DOI] [PubMed] [Google Scholar]
- 20.Klett E. L., Lu K., Kosters A., Vink E., Lee M. H., Altenburg M., Shefer S., Batta A. K., Yu H., Chen J., et al. 2004. A mouse model of sitosterolemia: absence of Abcg8/sterolin-2 results in failure to secrete biliary cholesterol. BMC Med. 2: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plosch T., Bloks V. W., Terasawa Y., Berdy S., Siegler K., Van Der Sluijs F., Kema I. P., Groen A. K., Shan B., Kuipers F., et al. 2004. Sitosterolemia in ABC-transporter G5-deficient mice is aggravated on activation of the liver-X receptor. Gastroenterology. 126: 290–300 [DOI] [PubMed] [Google Scholar]
- 22.Yu L., Hammer R. E., Li-Hawkins J., Von Bergmann K., Lutjohann D., Cohen J. C., Hobbs H. H. 2002. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc. Natl. Acad. Sci. USA. 99: 16237–16242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu L., Li-Hawkins J., Hammer R. E., Berge K. E., Horton J. D., Cohen J. C., Hobbs H. H. 2002. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J. Clin. Invest. 110: 671–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu L., von Bergmann K., Lutjohann D., Hobbs H. H., Cohen J. C. 2004. Selective sterol accumulation in ABCG5/ABCG8-deficient mice. J. Lipid Res. 45: 301–307 [DOI] [PubMed] [Google Scholar]
- 25.Gregg R. E., Connor W. E., Lin D. S., Brewer H., Jr 1986. Abnormal metabolism of shellfish sterols in a patient with sitosterolemia and xanthomatosis. J. Clin. Invest. 77: 1864–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J., Batta A., Zheng S., Fitzgibbon W. R., Ullian M. E., Yu H., Tso P., Salen G., Patel S. B. 2005. The missense mutation in Abcg5 gene in spontaneously hypertensive rats (SHR) segregates with phytosterolemia but not hypertension. BMC Genet. 6: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grefhorst A., Elzinga B. M., Voshol P. J., Plosch T., Kok T., Bloks V. W., van der Sluijs F. H., Havekes L. M., Romijn J. A., Verkade H. J., et al. 2002. Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. J. Biol. Chem. 277: 34182–34190 [DOI] [PubMed] [Google Scholar]
- 28.Yang J., Goldstein J. L., Hammer R. E., Moon Y. A., Brown M. S., Horton J. D. 2001. Decreased lipid synthesis in livers of mice with disrupted Site-1 protease gene. Proc. Natl. Acad. Sci. USA. 98: 13607–13612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 25: 402–408 [DOI] [PubMed] [Google Scholar]
- 30.Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J., Heldmaier G., Maier R., Theussl C., Eder S., et al. 2006. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 312: 734–737 [DOI] [PubMed] [Google Scholar]
- 31.Soni K. G., Lehner R., Metalnikov P., O'Donnell P., Semache M., Gao W., Ashman K., Pshezhetsky A. V., Mitchell G. A. 2004. Carboxylesterase 3 (EC 3.1.1.1) is a major adipocyte lipase. J. Biol. Chem. 279: 40683–40689 [DOI] [PubMed] [Google Scholar]
- 32.Honda A., Salen G., Nguyen L. B., Tint G. S., Batta A. K., Shefer S. 1998. Down-regulation of cholesterol biosynthesis in sitosterolemia: diminished activities of acetoacetyl-CoA thiolase, 3-hydroxy-3-methylglutaryl-CoA synthase, reducatse, squalene synthase and 7-dehydrocholesterol delta-7 reductase in liver and mononuclear leucocytes. J. Lipid Res. 39: 44–50 [PubMed] [Google Scholar]
- 33.Yang C., Yu L., Li W., Xu F., Cohen J. C., Hobbs H. H. 2004. Disruption of cholesterol homeostasis by plant sterols. J. Clin. Invest. 114: 813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel S. B., Salen G.2010. Sitosterolemia: xenophobia for the body. In Evidence-based Management of Lipid Disorders. M. N. Vissers, J. J. P. Kastelein, and E. S. Stroes, editors. TFM Publishing, Harley, UK. 217–230.
- 35.Mushtaq T., Wales J. K., Wright N. P. 2007. Adrenal insufficiency in phytosterolaemia. Eur. J. Endocrinol. 157(Suppl. 1): S61–S65 [DOI] [PubMed] [Google Scholar]
- 36.Nguyen L. B., Salen G., Shefer S., Bullock J., Chen T., Tint G. S., Chowdhary I. R., Lerner S. 1994. Deficient ileal 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in sitosterolemia: sitosterol is not a feedback inhibitor of intestinal cholesterol biosynthesis. Metabolism. 43: 855–859 [DOI] [PubMed] [Google Scholar]
- 37.Nguyen L. B., Salen G., Shefer S., Tint G. S., Shore V., Ness G. C. 1990. Decreased cholesterol biosynthesis in sitosterolemia with xanthomatosis: diminished mononuclear leukocyte 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and enzyme protein associated with increased low-density lipoprotein receptor function. Metabolism. 39: 436–443 [DOI] [PubMed] [Google Scholar]
- 38.Nguyen L. B., Shefer S., Salen G., Ness G. C., Tint G. S., Zaki F. G., Rani I. 1990. A molecular defect in hepatic cholesterol biosynthesis in sitosterolemia with xanthomatosis. J. Clin. Invest. 86: 923–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shefer S., Salen G., Bullock J., Nguyen L. B., Ness G. C., Vhao Z., Belamarich P. F., Chowdhary I., Lerner S., Batta A. K., et al. 1994. The effect of increased hepatic sitosterol on the regulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase and cholesterol 7 alpha-hydroxylase in the rat and sitosterolemic homozygotes. Hepatology. 20: 213–219 [DOI] [PubMed] [Google Scholar]
- 40.Carter B. A., Taylor O. A., Prendergast D. R., Zimmerman T. L., Von Furstenberg R., Moore D. D., Karpen S. J. 2007. Stigmasterol, a soy lipid-derived phytosterol, is an antagonist of the bile acid nuclear receptor FXR. Pediatr. Res. 62: 301–306 [DOI] [PubMed] [Google Scholar]
- 41.Gilman C. I., Leusch F. D., Breckenridge W. C., MacLatchy D. L. 2003. Effects of a phytosterol mixture on male fish plasma lipoprotein fractions and testis P450scc activity. Gen. Comp. Endocrinol. 130: 172–184 [DOI] [PubMed] [Google Scholar]
- 42.Leusch F. D., MacLatchy D. L. 2003. In vivo implants of beta-sitosterol cause reductions of reactive cholesterol pools in mitochondria isolated from gonads of male goldfish (Carassius auratus). Gen. Comp. Endocrinol. 134: 255–263 [DOI] [PubMed] [Google Scholar]
- 43.MacLatchy D. L., Van Der Kraak G. J. 1995. The phytoestrogen beta-sitosterol alters the reproductive endocrine status of goldfish. Toxicol. Appl. Pharmacol. 134: 305–312 [DOI] [PubMed] [Google Scholar]
- 44.Mattsson K., Tana J., Engstrom C., Hemming J., Lehtinen K. J. 2001. Effects of wood-related sterols on the offspring of the viviparous blenny, Zoarces viviparus L. Ecotoxicol. Environ. Saf. 49: 122–130 [DOI] [PubMed] [Google Scholar]
- 45.Nakari T., Erkomaa K. 2003. Effects of phytosterols on zebrafish reproduction in multigeneration test. Environ. Pollut. 123: 267–273 [DOI] [PubMed] [Google Scholar]
- 46.Sharpe R. L., Woodhouse A., Moon T. W., Trudeau V. L., Maclatchy D. L. 2007. Beta-sitosterol and 17beta-estradiol alter gonadal steroidogenic acute regulatory protein (StAR) expression in goldfish, Carassius auratus. Gen. Comp. Endocrinol. 15: 34–41 [DOI] [PubMed] [Google Scholar]
- 47.Awad A. B., Downie A. C., Fink C. S. 2000. Inhibition of growth and stimulation of apoptosis by beta-sitosterol treatment of MDA-MB-231 human breast cancer cells in culture. Int. J. Mol. Med. 5: 541–545 [DOI] [PubMed] [Google Scholar]
- 48.Berges R. R., Windeler J., Trampisch H. J., Senge T. 1995. Randomised, placebo-controlled, double-blind clinical trial of beta-sitosterol in patients with benign prostatic hyperplasia. Beta-sitosterol Study Group. Lancet. 345: 1529–1532 [DOI] [PubMed] [Google Scholar]
- 49.Cabeza M., Bratoeff E., Heuze I., Ramirez E., Sanchez M., Flores E. 2003. Effect of beta-sitosterol as inhibitor of 5 alpha-reductase in hamster prostate. Proc. West. Pharmacol. Soc. 46: 153–155 [PubMed] [Google Scholar]
- 50.Nguyen L. B., Shefer S., Salen G., Tint S. G., Batta A. K. 1998. Competitive inhibition of hepatic sterol 27-hydroxylase by sitosterol: decreased activity in sitosterolemia. Proc. Assoc. Am. Physicians. 110: 32–39 [PubMed] [Google Scholar]
- 51.Prager N., Bickett K., French N., Marcovici G. 2002. A randomized, double-blind, placebo-controlled trial to determine the effectiveness of botanically derived inhibitors of 5-alpha-reductase in the treatment of androgenetic alopecia. J. Altern. Complement. Med. 8: 143–152 [DOI] [PubMed] [Google Scholar]
- 52.Vivancos M., Moreno J. J. 2005. Beta-sitosterol modulates antioxidant enzyme response in RAW 264.7 macrophages. Free Radic. Biol. Med. 39: 91–97 [DOI] [PubMed] [Google Scholar]
- 53.Fernandez C., Suarez Y., Ferruelo A. J., Gomez-Coronado D., Lasuncion M. A. 2002. Inhibition of cholesterol biosynthesis by Delta22-unsaturated phytosterols via competitive inhibition of sterol Delta24-reductase in mammalian cells. Biochem. J. 366: 109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katamoto H., Kurihara S., Shimada Y. 1990. Effects of isoprothiolane and phytosterol on lipogenesis and lipolysis in adipocytes from rats of dietary fat necrosis. Nippon Juigaku Zasshi. 52: 1189–1197 [DOI] [PubMed] [Google Scholar]
- 55.Katamoto H., Yoneda N., Shimada Y. 1991. Effects of isoprothiolane and phytosterol on adipocyte metabolism and fatty acid composition of serum and tissue lipids in rats. J. Vet. Med. Sci. 53: 905–910 [DOI] [PubMed] [Google Scholar]
- 56.Vanmierlo T., Weingartner O., van der Pol S., Husche C., Kerksiek A., Friedrichs S., Sijbrands E., Steinbusch H., Grimm M., Hartmann T., et al. 2012. Dietary intake of plant sterols stably increases plant sterol levels in the murine brain. J. Lipid Res. 53: 726–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vanmierlo T., Rutten K., van Vark-van der Zee L. C., Friedrichs S., Bloks V. W., Blokland A., Ramaekers F. C., Sijbrands E., Steinbusch H., Prickaerts J., et al. 2011. Cerebral accumulation of dietary derivable plant sterols does not interfere with memory and anxiety related behavior in Abcg5-/- mice. Plant Foods Hum. Nutr. 66: 149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Byskov A. G., Andersen C. Y., Leonardsen L., Baltsen M. 1999. Meiosis activating sterols (MAS) and fertility in mammals and man. J. Exp. Zool. 285: 237–242 [PubMed] [Google Scholar]
- 59.Rozman D. 2000. Lanosterol 14alpha-demethylase (CYP51)--a cholesterol biosynthetic enzyme involved in production of meiosis activating sterols in oocytes and testis–a minireview. Pflugers Arch. 439 (3 Suppl.): R56–R57 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.