Abstract
Estimation of low-density lipoprotein cholesterol (LDL-C) using the Friedewald (FR) formula is often inaccurate when triglycerides are elevated or VLDL particle composition is altered. We hypothesized that LDL-C estimation by the FR formula and other measurement methods might also be inaccurate in individuals treated with a cholesteryl ester transfer protein (CETP) inhibitor. An assay comparison study was conducted using pre and posttreatment serum samples from 280 of the 811 patients treated with the CETP inhibitor anacetrapib in the DEFINE study (determining the ef ficacy and tolerability of CETP in hibition with anac e trapib). After 24 weeks of treatment with anacetrapib, mean LDL-C values by FR formula, Roche direct method (RDM) and Genzyme direct method (GDM) deviated from that measured by the β-quantification (BQ) reference method by –12.2 ± 7.5, –10.2 ± 6.6, –10.8 ± 8.8 mg/dl, respectively. After treatment with anacetrapib, the FR formula and detergent-based direct methods provided lower LDL-C values than those obtained by the BQ reference method. The bias by the FR formula appeared to be due to an overestimation of VLDL-C by the TG/5 component of the formula. Evaluation of the clinical significance of these findings awaits comprehensive lipid and cardiovascular outcome data from ongoing Phase III clinical studies of anacetrapib.
Keywords: Friedewald formula, β quantification, VLDL cholesterol, HDL cholesterol, direct method, low density lipoprotein cholesterol, cholesteryl ester transfer protein
Evaluation of low density lipoprotein cholesterol (LDL-C) in clinical practice has been largely based upon estimation of LDL-C by the Friedewald (FR) formula (1) or by direct measurement of LDL-C using detergent- or antibody-based methods. The FR formula [total cholesterol (TC) – high-density lipoprotein cholesterol (HDL-C) – triglycerides (TG) / 5] uses direct measurements of plasma or serum TC, HDL-C, and TG to estimate LDL-C (1). In the FR formula, VLDL-C is estimated by TG/5 and is based upon the assumption that most of the TG in fasting plasma is in the VLDL fraction and that there is a 5:1 ratio of TG to cholesterol in VLDL particles in the fasting state (2, 3). In clinical settings in which plasma TG is markedly elevated (such as fasting or postprandial chylomicronemia) or in which particle composition of VLDL is altered (such as with fasting TG > 400 mg/dl, and type III hyperlipidemia), the FR formula is known to be inaccurate. In these situations, alternative methods of monitoring LDL-C or levels of atherogenic lipids are recommended (4, 5), such as direct LDL-C measurements by detergent or antibody or monitoring non-HDL-C or apolipoprotein B levels.
Cholesteryl ester transfer protein (CETP) mediates the exchange of cholesteryl esters (CE) and TG between HDL and apoB-containing lipoprotein particles. Anacetrapib (MK-0859) is an orally active, potent, and selective CETP inhibitor in Phase III development (6). Previous studies have shown that anacetrapib treatment increases HDL-C by up to ∼139% and decreases LDL-C by up to ∼40% when calculated by the FR formula (7, 8). These effects on LDL-C and HDL-C were observed when anacetrapib was coadministered with statins for up to 1.5 years of treatment in the DEFINE (determining the ef ficacy and tolerability of CETP in hibition with anac e trapib) trial (clinicaltrials.gov NCT00685776) (9, 10).
Recently, Krauss et al. reported an enrichment of TG and reduction of CE in VLDL, IDL, and the smallest LDL fraction in healthy subjects after 2 weeks of treatment with anacetrapib 150 mg daily (11). In that study, the ratio of TG/CE in VLDL particles was increased approximately 3-fold after treatment with anacetrapib. This increase in the VLDL TG/CE ratio would be expected to impair the accuracy of the TG/5 estimation of VLDL-C used to calculate LDL-C with the FR formula. Furthermore, the change in TG/CE ratio may also affect the sensitivity and specificity of the detergent-based direct methods due to changes in lipoprotein composition. Careful evaluation of seven direct LDL-C quantitation methods has demonstrated that measurement bias is greater when measuring LDL-C in individuals being treated for cardiovascular disease or who had other conditions that might be expected to affect lipoprotein composition compared with measurement of LDL-C from individuals with no known diseases (12).
In the current study, LDL-C and HDL-C were remeasured by several different assay methods using stored frozen serum samples obtained from 280 individuals in a patient subset at baseline and after 24 weeks of treatment with anacetrapib 100 mg daily in the DEFINE trial. To assess the accuracy of the LDL-C methods, the methods were compared with a modified Centers for Disease Control and Prevention (CDC) LDL-C reference measurement procedure (RMP) that involves ultracentrifugation β-quantification (BQ) (13, 14). The modified BQ procedure involves subtraction of the HDL-C (measured in the supernatant after dextran sulfate precipitation of serum) from the cholesterol measured in the ultracentrifugation infranate (density > 1.006 g/ml) to obtain the LDL-C. We compared the results obtained with BQ with those obtained by three methods commonly used in clinical practice. Values for HDL-C concentration using direct methods were also compared with those obtained after dextran sulfate precipitation of serum.
METHODS
Patient populations
Lipid measurements in the current study were performed using stored frozen serum aliquots from patients who participated in the DEFINE study, a randomized, double-blind, placebo-controlled trial to assess the efficacy and safety profile of anacetrapib in patients with coronary heart disease or at high risk for coronary heart disease (9, 10).Two sets of serum samples were used to perform these measurements. The first set, Study 1, consisted of paired patient samples taken at baseline and Week 24. These samples were selected according to patient baseline LDL-C. Samples from 25 anacetrapib-treated subjects were selected from each of the following three baseline LDL-C intervals of 25 ≤ LDL < 60, 60 ≤ LDL ≤ 90, and > 90 mg/dl (as estimated by the FR formula). Study 1 also included paired samples from 25 additional subjects randomly selected from among patients with LDL-C values < 25 mg/dl as estimated by the FR formula at Week 24 or occurring prior to Week 24, for which the protocol called for discontinuation from the study. The second set of samples, Study 2, consisted of baseline and Week 24 serum samples from two groups of 110 subjects; one randomly selected among patients with baseline LDL-C ≤ 65 mg/dl and the 2nd with baseline LDL-C >65 mg/dl. Across the 2 studies, there were 20 patients in which there was either a missing baseline or on treatment LDL-C value. Since the results of study 1 (n = 100 subjects) and study 2 (n = 220 subjects) were similar to each other, data from both studies were combined for a total of 280 unique patients with complete datasets. In the 20 cases where a patient's serum was analyzed in both studies, only data from Study 2 were used.
Assays
Assays were run by Pharmaceutical Product Development (PPD Global Central Labs, Highland Heights, KY) for study 1 and Pacific Biomarkers (Seattle, WA) for study 2. Serum samples were stored at clinical sites at −20°C or at −70°C for 1–5 months and then at a central facility at −70°C for 18–36 months. Subject sets (baseline and week 24 samples) were analyzed in the same test runs.
Lipid parameters were analyzed on a Roche Modular P automated analyzer. TC was measured by an enzymatic Trinder reaction based on cholesterol esterase and oxidase. TG was measured by an enzymatic method with glycerol blanking to eliminate overestimation of TG concentrations from endogenous or exogenous glycerol. LDL-C was measured by four different methods: i) BQ (15); ii) the Roche direct method (RDM; Roche catalog no. 04714423190, manufactured by Kyowa Medex, Tokyo); iii) the Genzyme direct method (GDM; Genzyme catalog no. 7120, manufactured by Sekisui Medical, Tokyo); and iv) calculated using the FR formula (1). The RDM and GDM were performed according to manufacturers’ instructions.
BQ was performed as follows. Unfractionated sera were centrifuged for 20.5 h at 25,000 rpm (without density adjustment). After centrifugation, the tube was sliced to obtain the top and bottom fractions (density < 1.006 g/ml and density > 1.006 g/ml, respectively). The cholesterol in the top fraction (<1.006 g/ml) reflected the cholesterol content of chylomicrons and VLDL, while the cholesterol in the bottom fraction (>1.006 g/ml) reflected primarily LDL and HDL, with minor contributions from IDL and Lp(a). LDL-C concentration was calculated as the cholesterol level in the total density > 1.006 g/ml fraction less the HDL-C concentration that was measured in the supernatant after dextran sulfate / Mg2+ precipitation of apo-B containing lipoproteins in unfractionated serum. HDL-C was also measured by Roche (Roche catalog no. 04713257190) and Genzyme (Genzyme catalog no. 6121) direct methods as described by the manufacturers. Efficiency of apoB precipitation and subsequent recovery of apoA-I in the supernatant were unaffected by treatment (data not shown).
The ratio of TG/C in the VLDL of each patient in study 2 was calculated using VLDL-C and VLDL-TG values obtained by subtracting the cholesterol or TG concentration in the bottom (density > 1.006 g/ml) ultracentrifugal fraction from the cholesterol and TG values in the unfractionated serum.
To calculate LDL-C by the FR formula, TC and TG were measured in unfractionated serum and HDL-C was measured by dextran sulfate / Mg2+ precipitation as described above. LDL-C was calculated as TC – (HDL-C) – (TG/5).
Statistical analyses
The baseline and week 24 on-treatment distributions of LDL-C were summarized for each of the four methods; the resulting six pairwise comparisons between methods were performed to characterize the assay bias relative to the BQ reference. Additionally, the distributions of the absolute and percentage changes in LDL-C from baseline were summarized for each method. Scatterplots and Bland-Altman plots were generated to estimate the bias between each of the methods versus the BQ reference. Pearson correlation coefficients were generated to measure the degree of linear correlation between each of the pairwise comparison of methods.
RESULTS
The primary objective of the current study was to evaluate different methods for measuring the concentration of LDL-C in patients before and after treatment with the CETP inhibitor anacetrapib. The patient population in the current study was a subset of the original 812 patients randomized to 100 mg per day anacetrapib in the DEFINE trial. To evaluate LDL-C measurement methods across a wide range of LDL-C values, the patient subset was selected according to baseline plasma LDL-C values, as calculated by the FR formula using the original plasma lipid measurements performed during the course of the study (10).
The patient population subset was enriched with a greater percentage of patients with lower baseline LDL-C values (see Methods). Mean baseline plasma LDL-C (calculated by the FR formula) in the patient subset was approximately 9% lower than the overall population randomized to anacetrapib (74.1 ± 21.6 versus 81.4 ± 21.2 mg/dl; Table 1). Otherwise, the demographic profile and baseline lipids of the selected patient subset were similar to that of the overall population in the DEFINE trial (Table 1).
TABLE 1.
Patient demographics
| Demographic | LDL Analysis Population | DEFINE Anacetrapib Treatment Group |
| (N = 280) | (N = 811) | |
| Age (years) [mean (SD)] | 62.5 ± 8.8 | 62.5 ± 8.7 |
| <65 | 155 (55.4) | 455 (56.1) |
| ≥65 | 125 (44.6) | 356 (43.9) |
| Gender, N (%) | ||
| Female | 61 (21.8) | 182 (22.4) |
| Male | 219 (78.2) | 629 (77.6) |
| Race, N (%) | ||
| White | 244 (87.1) | 686 (84.6) |
| Asian | 15 (5.4) | 54 (6.7) |
| Other | 13 (4.6) | 49 (6.0) |
| Black or African American | 8 (2.9) | 22 (2.7) |
| Diabetic, N (%) | ||
| Yes | 157 (56.1) | 430 (53.0) |
| No | 123 (43.9) | 381 (47.0) |
| Baseline LDL-C [mean (SD) mg/dl] | 74.1 (21.6) | 81.4 (21.2) |
| Baseline HDL-C [mean (SD) mg/dl] | 40.5 (8.3) | 40.5 (9.3) |
| Baseline TG [median (SD) mg/dl] | 123.5 (57.0) | 127.0 (66.5) |
| Baseline Total-C [mean (SD) mg/dl] | 141.4(25.3) | 150.4 (25.9) |
Stored frozen sera, rather than plasma that was used in the original study, was used to remeasure lipids and lipoproteins and compare assay methods in the current study because of the availability and larger volume of stored frozen serum aliquots. At baseline, serum LDL-C values were similar for all four methods, although the mean, percentiles, and range were slightly lower when LDL-C was calculated by the FR formula (Table 2). At baseline, the mean FR formula, RDM, and GDM LDL-C values deviated from the BQ reference standard by –5.2 ± 7.4, −2.0 ± 6.6, and 0.3 ± 7.6 mg/dl respectively. After 24 weeks of treatment with 100 mg per day anacetrapib, the mean LDL-C concentration calculated by FR formula and the two direct methods were similar to each other. Mean LDL-C by all three methods (FR formula, RDM, and GDM) was lower than the mean LDL-C level by the BQ method with an bias of –12.2 ± 7.5, –10.2 ± 6.6, and –10.8 ± 8.8 mg/dl, respectively (Table 2).
TABLE 2.
Serum LDL-C measurements at baseline and after 24 weeks of treatment with anacetrapib
| Method of Analysis | Baseline (n = 280) | 25 Percentile | 75 Percentile | Week 24 (n = 280) | 25 Percentile | 75 Percentile | Change from Baseline | |
| mean (SD) mg/dl | mean (SD) mg/dl | mean (SD) mg/dl | mean (SD) % | |||||
| β-quantification | 76.2 (20.6) | 62.0 | 89.5 | 56.3 (18.7) | 42.0 | 65.5 | −19.9 (21.4) | −23.4 (27.3) |
| Friedewald | 71.0 (21.5) | 55.5 | 85.0 | 44.1 (20.1) | 29.0 | 54.5 | −26.9 (22.4) | −35.6 (30.3) |
| Genzyme | 76.5 (20.3) | 61.0 | 89.5 | 45.5 (17.2) | 33.0 | 54.0 | −31.0 (21.4) | −38.3 (25.1) |
| Roche | 74.3 (21.0) | 59.0 | 88.5 | 46.1 (18.0) | 33.0 | 55.0 | −28.2 (21.1) | −35.7 (26.1) |
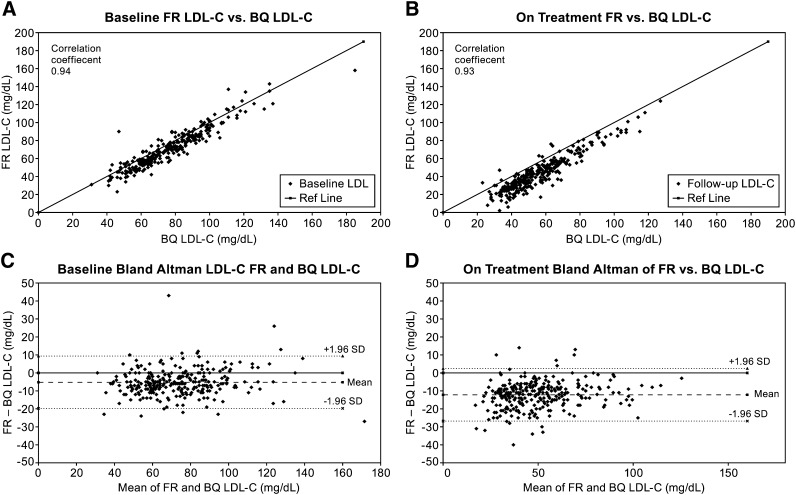
Analyses of individual patient LDL-C data revealed evidence for a fixed bias after treatment. Although there was a strong correlation at both baseline (r = 0.94) and at week 24 (r = 0.93) (Fig. 1A, B) between the FR and BQ methods, the FR formula generated on-treatment LDL-C values that were significantly lower than those measured by BQ (Fig. 1B, D). Similar correlations and differences were obtained when LDL-C levels determined by either of the direct methods were compared with the values obtained by the BQ method (supplementary Figs. I and II). The differences between individual patient FR formula and BQ LDL-C values were also evaluated in patients with and without diabetes and according to on-treatment TG values above and below the median (supplementary Table I and Fig. III). The presence of diabetes or absolute TG level did not appear to alter the mean bias between the methods either at baseline or after treatment with anacetrapib. Additionally, as evidenced by the lack of trends displayed in the Bland-Altman plots, the extent of the bias appeared to be relatively constant across the LDL-C range (Fig. 1C, D, and supplementary Figs. I and II-C, D), with a possibility of greater negative bias at higher TG values after treatment (supplementary Fig. IV-B).
Fig. 1.
Analysis of correlations and corresponding individual differences between LDL-C values measured by the Friedewald formula and the β-quantification method at baseline (A, C) and at week 24 (B, D). In (C) and (D), the mean of these differences (bias) is shown as the long dashed line and the upper and lower boundaries are displayed as short dashed lines (±1.96 × SD).
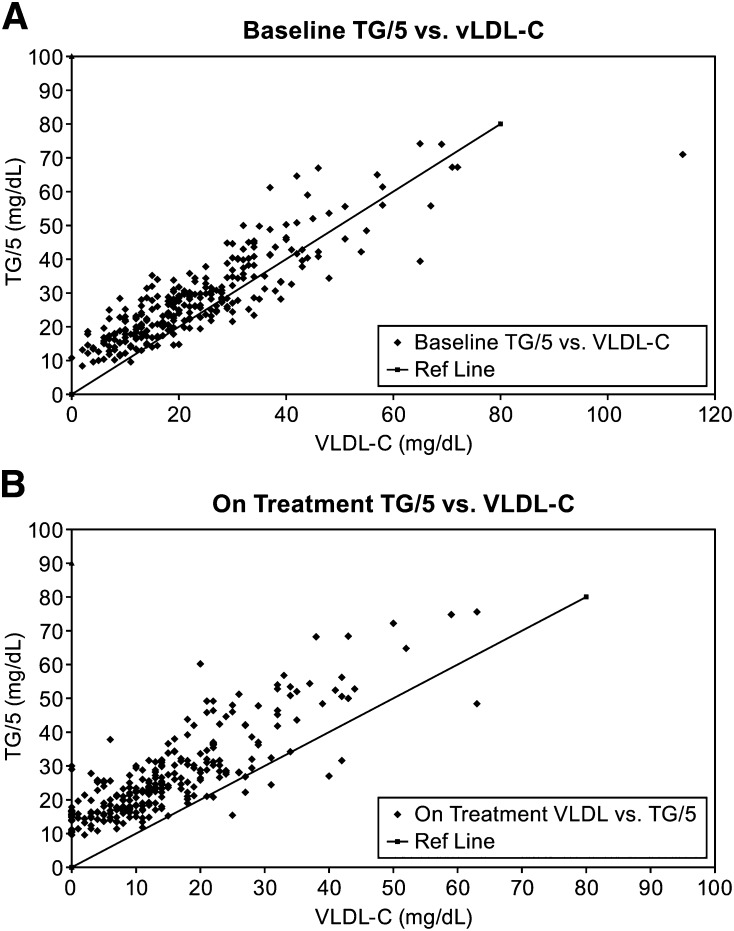
The ratio of TG/C in VLDL was measured before and after treatment with anacetrapib. At baseline, the VLDL TG/C ratio was 4.9 ± 2.1 (n = 201), and it increased to 8.5 ± 6.7 (n = 186) after 24 weeks of treatment with anacetrapib. As TG/5 is used as an estimate of VLDL-C in the FR formula, the correlation and bias between TG/5 and the measured VLDL-C were also analyzed. Although there was a good correlation between TG/5 and VLDL-C at baseline and after anacetrapib treatment (Fig. 2A, B), the mean bias between TG/5 and VLDL-C increased from 5.1 ± 7.3 mg/dl at baseline to 11.9 ± 7.1 mg/dl after treatment.
Fig. 2.
Correlation between VLDL-C estimated by total plasma triglyceride and VLDL-C directly measured by ultracentrifugation at baseline (A) and at week 24 (B).
Baseline and on-treatment HDL-C were measured by RDM, by GDM, and in serum after dextran sulfate precipitation. Using the dextran sulfate as the reference measurement method, mean values for HDL-C concentration at baseline and on treatment were generally similar among the three methods, with the mean GDM having somewhat lower HDL-C values (Table 3).
TABLE 3.
Serum HDL-C measurements at baseline and after 24 weeks of treatment with anacetrapib
| Method of Analysis | Baseline (n = 280) | 25 Percentile | 75 Percentile | Week 24 (n = 280) | 25 Percentile | 75 Percentile | Change from Baseline |
|
| mean (SD) mg/dl | mean (SD) mg/dl | 25 | 75 | mean (SD) mg/dl | mean (SD) % | |||
| DS Precipitation | 42.4 (8.3) | 36.0 | 48.0 | 93.5 (22.3) | 78.0 | 108.0 | 51.1 (18.7) | 122.9 (45.9) |
| Genzyme Direct | 42.4 (7.9) | 37.0 | 48.0 | 88.3 (20.1) | 74.0 | 102.0 | 45.9 (16.7) | 110.2 (81.3) |
| Roche Direct | 41.9 (8.9) | 35.0 | 48.0 | 94.9 (24.0) | 79.0 | 110.0 | 53.0 (20.2) | 129.8 (51.3) |
DISCUSSION
Current guidelines for the primary and secondary prevention of cardiovascular disease recommend specific LDL-C treatment goals (4, 5, 16). LDL-C estimation in current clinical practice has been based upon either the FR formula calculation of LDL-C or direct LDL-C measurement techniques. It is known that FR formula calculation of LDL-C becomes less reliable in the setting of hypertriglyceridemia; in this setting guidelines recommend alternative methods. The BQ reference method itself is not routinely used in clinical practice because it is a labor-intensive and time-consuming assay (13, 14). The current study demonstrates that, at baseline, there is a small fixed bias (−5 mg/dl) between the serum LDL-C values obtained by the FR formula compared with the BQ reference standard. The fixed bias increases to a mean of −12 mg/dl when LDL-C is measured from samples taken after treatment with the CETP inhibitor anacetrapib. The bias was similar in the presence or absence of a clinical diagnosis of diabetes when TG was above or below the median (supplementary Table I), and the bias was similar across the range of baseline LDL-C. Visual inspection of the relationship between the bias between FR and BQ as a function of TG after treatment may suggest a greater negative bias at higher TG values (supplementary Fig. IV-B); however, the number of patients with moderate to severe hypertriglyceridemia was quite limited in this study because patients were excluded from the DEFINE study for TG > 400 mg/dl. Additional studies will be needed to estimate the assay bias after treatment with anacetrapib in the setting of higher TG and with baseline LDL-C values > 100 mg/dl.
The increase in negative bias for FR formula calculation can be explained by an overestimation of the VLDL-C when assuming that the ratio of TG/C in VLDL is 5. In fact, the ratio in people treated with anacetrapib was greater than 5 (mean of 8.5). The reason for the higher ratio of TG/C in VLDL is that inhibition of CETP decreases both the transfer of TG from VLDL to HDL and of cholesteryl esters from HDL to VLDL. An increase in the ratio of TG/C in the VLDL fraction of patients treated with anacetrapib has also recently been reported in another study (11). The finding that the bias between VLDL-C and the TG/5 component of the FR was of a similar magnitude but directionally opposite to the bias between the FR LDL-C versus BQ method supports the premise that overestimation of VLDL-C is most likely responsible for the underestimation of LDL-C by the FR method in anacetrapib-treated patients. However, the explanation for the same finding when using direct methods for measuring LDL-C is not obvious. It is possible that the specific detergents used in the direct methods have a reduced ability to differentiate cholesterol from the LDL fraction in the presence of an increased TG/C ratio.
Relative to results using the BQ method, the FR equation (and the direct methods) provided lower levels of LDL-C after treatment with anacetrapib. These observations indicate that use of the FR equation to calculate LDL-C will result in a greater apparent LDL-C reduction than when LDL-C is measured by the BQ technique. However, the extent to which the treatment effect was overestimated using the FR equation remains uncertain. Nonrandom selection of the samples, the absence of assay comparison measurements on samples from placebo-treated subjects, and potential preanalytical variability [e.g., matrix differences (4, 17) and effects of long-term frozen storage (18)] may have had an impact on the magnitude of the bias and calculation of the treatment effect in the current study. It is possible that the bias between FR and BQ might also be influenced by the sampling procedures. In a previous 8-week dose-ranging study (7), the mean bias between FR and BQ LDL-C was ∼6 mg/dl after treatment with anacetrapib 150 mg in combination with atorvastatin 20 mg (all assays performed on fresh plasma; unpublished data). Additional studies are clearly needed to evaluate the bias under various clinical settings and sampling paradigms.
In summary, the current study indicates that the LDL-C calculated by the FR formula or measured by direct detergent-based assays underestimates LDL-C after treatment with the CETP inhibitor anacetrapib when compared with LDL-C values obtained by the reference BQ method. This difference results in an overestimation of the percentage change from baseline value; however, the magnitude of the overestimation of the treatment effect is uncertain. Further studies are needed to prospectively evaluate the effects of anacetrapib on LDL-C using the BQ reference method. It is also logical to include measures of apoB and non-HDL-C, neither of which is subject to the degree of variability seen in LDL-C measurement (whether by BQ or other methods) and both of which have been shown to be more predictive of cardiovascular risk than is LDL-C (19, 20). More importantly, the clinical impact of the effects of anacetrapib on multiple lipid parameters is currently being studied in REVEAL (clinicaltrials.gov #NCT01252953), a cardiovascular outcome study of 30,000 individuals with preexisting cardiovascular disease.
Supplementary Material
Acknowledgments
The authors thank Ms. Patrice H. Gibbons and Dr. Edward A. O'Neill (Merck Sharp & Dohme Corp.) for their assistance with the manuscript.
Footnotes
Abbreviations:
- BQ
- β-quantification
- C
- cholesterol
- CE
- cholesteryl ester
- CEPT
- cholesteryl ester transfer protein
- DEFINE
- determining the efficacy and tolerability of CETP inhibition with anacetrapib
- FR
- Friedewald
- GDM
- Genzyme direct method
- LDL-C
- low-density lipoprotein cholesterol
- RDM
- Roche direct method
- TC
- total cholesterol
- TG
- triglyceride
This study was funded by Merck & Co., Inc., Whitehouse Station, NJ.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of four figures and one table.
REFERENCES
- 1.Friedewald W. T., Levy R. I., Fredrickson D. S. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18: 499–502 [PubMed] [Google Scholar]
- 2.Fredrickson D. S., Levy R. I., Lees R. S. 1967 doi: 10.1056/NEJM196701122760206. Fat transport in lipoproteins--an integrated approach to mechanisms and disorders. N. Engl. J. Med. 276: 34-44, 94–103, 148–156, 215–225, 273–281. [DOI] [PubMed] [Google Scholar]
- 3.Hatch F. T. 1968. Practical methods for plasma lipoprotein analysis. Adv. Lipid Res. 6: 1–68 [PubMed] [Google Scholar]
- 4.National Heart Lung and Blood Institute. 2002. Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Accessed September 12, 1012, at: http://www.nhlbi.nih.gov/guidelines/cholesterol/index.htm.
- 5.Reiner Z., Catapano A. L., De Backer G., Graham I., Taskinen M. R., Wiklund O., Agewall S., Alegria E., Chapman M. J., Durrington P., et al. 2011. ESC/EAS guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur. Heart J. 32: 1769–1818 [DOI] [PubMed] [Google Scholar]
- 6.Gutstein D. E., Krishna R., Johns D., Surks H. K., Dansky H. M., Shah S., Mitchel Y. B., Arena J., Wagner J. A. 2012. Anacetrapib, a novel CETP inhibitor: pursuing a new approach to cardiovascular risk reduction. Clin. Pharmacol. Ther. 91: 109–122 [DOI] [PubMed] [Google Scholar]
- 7.Bloomfield D., Carlson G. L., Sapre A., Tribble D., McKenney J. M., Littlejohn T. W., III, Sisk C. M., Mitchel Y., Pasternak R. C. 2009. Efficacy and safety of the cholesteryl ester transfer protein inhibitor anacetrapib as monotherapy and coadministered with atorvastatin in dyslipidemic patients. Am. Heart J. 157: 352–360 [DOI] [PubMed] [Google Scholar]
- 8.Krishna R., Anderson M. S., Bergman A. J., Jin B., Fallon M., Cote J., Rosko K., Chavez-Eng C., Lutz R., Bloomfield D. M., et al. 2007. Effect of the cholesteryl ester transfer protein inhibitor, anacetrapib, on lipoproteins in patients with dyslipidaemia and on 24-h ambulatory blood pressure in healthy individuals: two double-blind, randomised placebo-controlled phase I studies. Lancet. 370: 1907–1914 [DOI] [PubMed] [Google Scholar]
- 9.Cannon C. P., Dansky H. M., Davidson M., Gotto A. M., Jr, Brinton E. A., Gould A. L., Stepanavage M., Liu S. X., Shah S., Rubino J., et al. 2009. Design of the DEFINE trial: determining the EFficacy and tolerability of CETP INhibition with AnacEtrapib. Am. Heart J. 158: 513–519 [DOI] [PubMed] [Google Scholar]
- 10.Cannon C. P., Shah S., Dansky H. M., Davidson M., Brinton E. A., Gotto A. M., Stepanavage M., Liu S. X., Gibbons P., Ashraf T. B., et al. 2010. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N. Engl. J. Med. 363: 2406–2415 [DOI] [PubMed] [Google Scholar]
- 11.Krauss R. M., Wojnooski K., Orr J., Geaney J. C., Pinto C. A., Liu Y., Wagner J. A., Luk J. M., Johnson-Levonas A. O., Anderson M. S., et al. 2012. Changes in lipoprotein subfraction concentration and composition in healthy individuals treated with the cholesteryl ester transfer protein inhibitor anacetrapib. J. Lipid Res. 53: 540–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller W. G., Myers G. L., Sakurabayashi I., Bachmann L. M., Caudill S. P., Dziekonski A., Edwards S., Kimberly M. M., Korzun W. J., Leary E. T., et al. 2010. Seven direct methods for measuring HDL and LDL cholesterol compared with ultracentrifugation reference measurement procedures. Clin. Chem. 56: 977–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bachorik P. S., Ross J. W. 1995. National Cholesterol Education Program recommendations for measurement of low-density lipoprotein cholesterol: executive summary. The National Cholesterol Education Program Working Group on Lipoprotein Measurement. Clin. Chem. 41: 1414–1420 [PubMed] [Google Scholar]
- 14.Lipid Research Clinics Program, National Heart and Lung Institute. 1974. Manual of Laboratory Operations: Lipid and Lipoprotein Analysis. National Institutes of Health, Washington, D.C.
- 15.Cole T. G., Ferguson C. A., Gibson D. W., Nowatzke W. L. 2001. Optimization of beta-quantification methods for high-throughput applications. Clin. Chem. 47: 712–721 [PubMed] [Google Scholar]
- 16.Grundy S. M., Cleeman J. I., Merz C. N., Brewer H. B., Jr, Clark L. T., Hunninghake D. B., Pasternak R. C., Smith S. C., Jr, Stone N. J. 2004. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 110: 227–239 [DOI] [PubMed] [Google Scholar]
- 17.Warnick G. R., Kimberly M. M., Waymack P. P., Leary E. T., Myers G. L. 2008. Standardization of measurements for cholesterol, triglycerides, and major lipoproteins. Lab. Med. 39: 481–489 [Google Scholar]
- 18.Shih W. J., Bachorik P. S., Haga J. A., Myers G. L., Stein E. A. 2000. Estimating the long-term effects of storage at −70 degrees C on cholesterol, triglyceride, and HDL-cholesterol measurements in stored sera. Clin. Chem. 46: 351–364 [PubMed] [Google Scholar]
- 19.Benn M., Nordestgaard B. G., Jensen G. B., Tybjaerg-Hansen A. 2007. Improving prediction of ischemic cardiovascular disease in the general population using apolipoprotein B: the Copenhagen City Heart Study. Arterioscler. Thromb. Vasc. Biol. 27: 661–670 [DOI] [PubMed] [Google Scholar]
- 20.Di Angelantonio E., Sarwar N., Perry P., Kaptoge S., Ray K. K., Thompson A., Wood A. M., Lewington S., Sattar N., Packard C. J., et al. 2009. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 302: 1993–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.