Abstract
Purpose
Adjuvant! © Online (Adjuvant!) is a user-friendly, web-based tool that provides estimates of adjuvant therapy outcomes for individual patients. While reliable evidence underpins estimates for most patient cohorts, there is a paucity of data on the effect of adding chemotherapy to complete estrogen blockade for premenopausal women with estrogen-receptor positive breast cancer.
Methods
International Breast Cancer Study Group (IBCSG) Trial 11-93 enrolled 174 premenopausal women with estrogen-receptor positive, node-positive breast cancer. Fifty-five percent of patients had 1 positive axillary lymph node and 97% had 3 or fewer positive nodes. Patients were randomized to receive ovarian function suppression plus five years of tamoxifen with or without anthracycline-based chemotherapy. Estimated hazard rates and corresponding 10-year relapse-free survival percents obtained from Trial 11-93 data were compared with those predicted using Adjuvant!.
Results
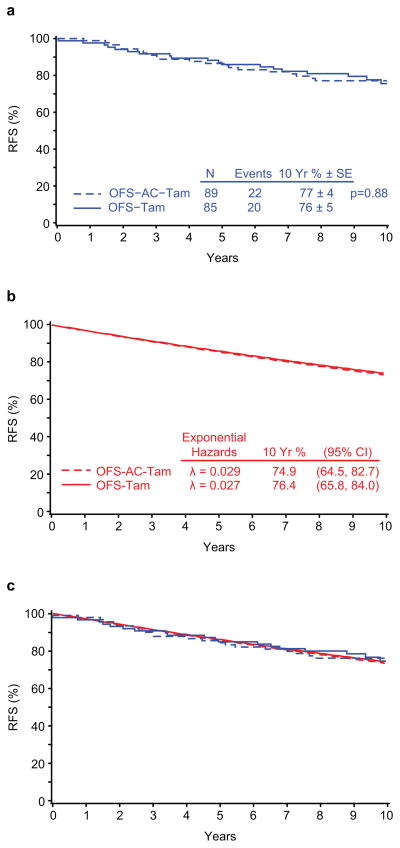
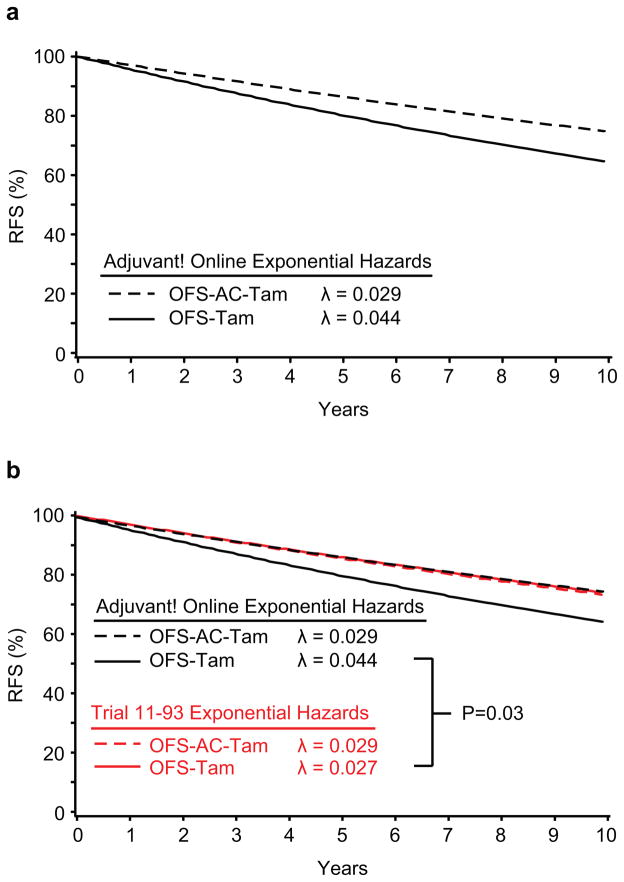
The 10-year relapse-free survival percents predicted from Adjuvant! were 64.4% (95% CI, 61.9% to 67.2%) for endocrine therapy alone and 74.9% (95% CI, 73.1% to 76.8%) for chemoendocrine therapy. By contrast, these estimates in Trial 11-93 were 76.4% (95% CI, 65.8% to 84.0%) for endocrine therapy alone and 74.9% (95% CI, 64.5% to 82.7%) for chemoendocrine therapy. The Adjuvant! estimate for the endocrine alone control group is lower than that observed in Trial 11-93 (p=0.03), while the estimates for the two chemoendocrine therapy groups are similar.
Conclusions
Adjuvant! appears to underestimate the effectiveness of adjuvant endocrine therapy alone for premenopausal women with endocrine responsive breast cancer, thus overestimating the added benefit, if any, from chemotherapy for this patient population.
Keywords: Adjuvant! © Online, Estrogen Receptor, Premenopausal, Chemotherapy, Endocrine Therapy, International Breast Cancer Study Group
INTRODUCTION
Adjuvant! © Online (Adjuvant!) [1, 2] is a web-based tool for estimating risk of relapse and mortality and illustrating the benefits provided by various treatment regimens for newly-diagnosed breast cancer patients. The estimates for risk of death are derived from the Surveillance, Epidemiology and End Results (SEER) data. Adjuvant! calculates estimates for recurrence by adding 14% to the mortality risk “to account for the risk of contralateral breast cancer and local/regional events unlikely to result in breast cancer mortality.” The estimates of treatment benefit are derived from available clinical trial results and data from the 1995 Overview meta-analyses of randomized adjuvant chemotherapy and hormone therapy trials for breast cancer [3], with supplemental information from the 2000 Overview [4]. The background information available on-line for Adjuvant![1] reveals the care used by the developer of this tool to provide the most accurate information on prognosis and treatment benefit for individual patients.
The reliability of the estimates, however, is only as good as the quality and quantity of the data available to inform results for specific patient cohorts and for specific treatment comparisons. Trials completed in the 1970’s showed that the benefits of adjuvant chemotherapy were particularly striking for premenopausal women, and these large effects of chemotherapy for women under 50 years of age were highlighted in overview analyses [3, 4]. In 1993, the International Breast Cancer Study Group (IBCSG) initiated a tailored treatment investigation (IBCSG Trial 11-93) focusing on the specific patient population of premenopausal women with node-positive, endocrine-responsive disease [5,6]. Patients were randomized to receive either adjuvant endocrine therapy alone using a complete estrogen blockade (ovarian function suppression or ablation (OFS) plus five years of tamoxifen (OFS-Tam group)) or the same endocrine therapy plus chemotherapy (four courses of anthracycline plus cyclophosphamide (AC) with the chemotherapy commencing with the OFS and the tamoxifen following the chemotherapy (OFS-AC-Tam group)). The hormonal treatment (OFS + Tam) selected as reference for the trial was – and still remains - considered an ‘optimal endocrine therapy’ in view of its demonstrated superiority to tamoxifen alone in first line therapy in premenopausal women with advanced breast cancer suitable for endocrine treatment [7]. The AC regimen was selected because equivalent efficacy had been demonstrated and it was associated with less toxicity than 6 months of CMF in positive-node breast cancer patients with tamoxifen-nonresponsive tumors [8]. After 5 years, accrual was halted with 174 patients enrolled. Although all patients with node-positive disease were eligible, 55% of patients had 1 positive node, and 97% of patients had 1 to 3 positive nodes. The trial results at 4 years’ median follow up [5] and at 10 years’ median follow up [6] have been published, and show virtually identical outcomes for the patients who were randomized to receive chemotherapy and those who were not, both with respect to disease-free survival and overall survival.
In this paper we compare the recurrence-free survival (RFS) outcomes predicted by Adjuvant! for the 174 patients enrolled in IBCSG Trial 11-93 with the RFS obtained directly from Trial 11-93.
METHODS
Adjuvant! Online
Adjuvant! is a web-based, interactive system that computes RFS and overall survival percentages using the following user-entered patient and disease categories defined in Table 1: age, estrogen-receptor status, tumor grade, tumor size, number of positive nodes, and comorbidity (perfect health, minor problems, average for age, major problems (+10), major problems (+20), major problems (+30)) [1].
Table 1.
Patient and disease characteristics according to treatment assignment used by Adjuvant! Online to provide estimates of outcome
| Characteristics | OFS-Tam (n = 85) | OFS-AC-Tam (n = 89) | Total (n = 174) |
|---|---|---|---|
| Age, years | |||
| Median | 46 | 45 | 45 |
| Range | 26 – 54 | 27 – 56 | 26 – 56 |
| Estrogen receptor status, % | |||
| Positive | 99 | 94 | 97 |
| Negative | 1 | 6 | 3 |
| Number of positive nodes, % | |||
| 0 | 0 | 0 | 0 |
| 1 – 3 | 95 | 98 | 97 |
| 4 – 9 | 4 | 2 | 3 |
| Greater than 9 | 1 | 0 | 1 |
| Tumor size, % | |||
| 0 cm | 1 | 0 | 1 |
| 0.1 – 1.0 cm | 7 | 12 | 10 |
| 1.1 – 2.0 cm | 45 | 48 | 47 |
| 2.1 – 3.0 cm | 38 | 28 | 33 |
| 3.1 – 5.0 cm | 7 | 9 | 8 |
| > 5.0 cm | 0 | 0 | 0 |
| Undefined | 2 | 2 | 2 |
| Tumor grade, % | |||
| 1 | 24 | 20 | 22 |
| 2 | 52 | 54 | 53 |
| 3 | 22 | 25 | 24 |
| Undefined | 2 | 1 | 2 |
| Comorbidities, % | |||
| Present | 12 | 11 | 11 |
Abbreviations: OFS, ovarian function suppression; Tam, tamoxifen; AC, doxorubicin (or epirubicin) and cyclophosphamide
A computer program generates estimates for both mortality and relapse according to adjuvant endocrine therapies, chemotherapies, and their combination. The five categories for the endocrine therapies and nine categories of chemotherapies are listed in Table 2 [1]. Adjuvant! projects that ovarian ablation combined with other hormonal therapy such as tamoxifen, has approximately the same effectiveness as tamoxifen.
Table 2.
Treatment groups defined by Adjuvant! Online (from ref. 1)
| Endocrine therapy regimens | Examples |
|---|---|
| • Tamoxifen (Tam) for 5 years | |
| • Aromatase inhibitor (AI) for 5 years | |
| • Tam for 2–3 years followed by AI for 2–3 years | |
| •Ovarian ablation | |
| •Ovarian ablation plus Tam (or another hormonal agent)a | |
|
| |
| Chemotherapy regimens | |
|
| |
| • 6 cycles of cyclophosphamide( C), methotrexate (M), 5-fluorouracil (F) [CMF*6] (Overview 2000) | |
| • Anthracycline-based (Overview 2000) | |
| • 1st generation regimen (G1)b (Shown to be equal to CMF or to be better than no therapy to a degree similar to that expected for CMF.) CA*4, CMF*6, FE(50)C*6 |
Four cycles of C, doxorubicin (A) [CA*4] CMF * 6 F, epirubicin (E)(50), C [FE(50)C*6] |
| • 2nd generation regimens (G2) (Superior to G1, assumes15% – 20% better relative efficacy than CMF.) | CAF *6 FEC*6 FE(100)C*6 FAC*6 CA*4 followed by 4 cycles of taxanes (T) given every 3 weeks [CA*4 then T*4 (q3w)] |
| CA*4 then T*4 (q3w) Anthracycline-based regimen with more than 4 cycles and more than two agents |
|
| • 3rd generation regimens (G3) (15% – 20% better than G2 which are 15% – 20% better than G1–this makes G3 regimens 35% better than CMF) | TAC*6 FE(100)C*3 followed by 3 cycles of (D) dose-dense CA*4 T *4 (all ) |
| • Chemotherapy benefit adjusted by user. | TAC FEC*3+docetaxel (D)*3 CA(Q2W)*4 + Taxol(Q2W)*4 FEC*4+T *8 (Q1W) |
Regimen matching the IBCSG Trial 11-93 prescribed endocrine therapy
Regimen matching the IBCSG Trial 11-93 prescribed chemotherapy
IBCSG Trial 11-93
Trial 11-93 enrolled 174 premenopausal patients suitable for endocrine therapy alone (estrogen receptor (ER) and/or progesterone receptor (PgR) positive) between May 1, 1993 and November 1, 1998. Tumors were classified as ER positive if 1) immunohistochemistry (IHC) quantitative results were available and reported as greater than 25%; 2) IHC quantitative less than 25%, with IHC qualitative reported as ‘positive’ or ‘strongly positive’ 3) IHC quantitative unavailable, but IHC qualitative reported as ‘positive’ or ‘strongly positive’; 4) neither IHC assays available, but biochemical assay equal to 10 fmol/mg cytosol protein or greater.
Patients were randomized to receive either endocrine therapy alone or chemotherapy plus endocrine therapy. Endocrine therapy was expected to provide a complete estrogen blockade from OFS plus five years of tamoxifen (20 mg daily). OFS could be achieved by bilateral surgical oophorectomy via laparotomy or laparoscopy, bilateral ovarian radiation, or GnRH analogue (3.6 mg goserelin every 28 days) continued for 2 years or until the patient was 55 years of age, whichever was longer. OFS followed by ovarian ablation was also acceptable. The group assigned to chemotherapy (AC) received four courses of anthracycline (either doxorubicin 60 mg/m2 or epirubicin 90 mg/m2 i.v. day 1) plus cyclophosphamide (600 mg/m2 i.v. day 1) repeated every 21 days. Patients in the combined arm who opted to receive GnRH analog as the method of OFS were to start AC and GnRH analogue concurrently. If the OFS method was surgery or radiotherapy, then it preceded the chemotherapy, which was followed by tamoxifen to complete 5 years. The planned sample size was 760, later amended to 400 patients, but enrollment was slow and accrual was halted after 5 years with 174 patients entered. The prognostic features were well balanced between the two treatment assignments (Table 1). The current report uses the database published in 2009 [6]. Trial 11-93 was coordinated and funded by the IBCSG. The ethics committees and required health authorities of each participating center approved the study protocol, and all patients gave written informed consent.
Statistical Analyses
Patient and disease characteristics collected during the routine conduct of IBCSG Trial 11-93 were grouped according to the Adjuvant! categories (Table 1). Individual patient characteristics were entered into the interactive screens via the Adjuvant! website [1]. The Adjuvant! website returned the calculated estimates of percent alive and without breast cancer at ten years, and the additional absolute benefit at ten years provided by the Adjuvant! treatment groups that matched the randomized treatment arms of Trial 11-93 – OFS plus tamoxifen and OFS plus tamoxifen plus an anthracycline-based regimen. Each patient’s Adjuvant!-estimated 10-year RFS percent was converted into an annual hazard rate based on an exponential distribution, which assumes a constant rate of failure over time. Then a mean hazard rate with standard error was computed for both the endocrine therapy and the chemoendocrine therapy groups. RFS events were defined as any breast cancer recurrence or contralateral tumor, and second primary neoplasms were ignored.
Comorbidity categories to match those defined by Adjuvant! were difficult to assign for the twenty patients on Trial 11-93 for whom a comorbid illness had been reported: 8 hypertension, 3 arthrosis/arthritis, 4 hypothyroidism, 2 fibromyalgia, and one each of struma nodosa, hypercholesterolemia, and diabetes. No details were collected regarding severity or control of these comorbidities, so a sensitivity analysis was performed by estimating the risk of relapse for each of these patients assigning them to perfect health, average health, and minor problems. Three mean hazards for each of the two regimens were then calculated using the estimates generated from these different comorbidity categories. The overall estimates for the treatment groups were essentially the same, irrespective of how comorbidity was incorporated, so the estimates for this report assigned all patients to the category of perfect health.
The Adjuvant! definition of RFS was used in the analysis of IBCSG Trial 11-93. Assuming exponential distribution for the RFS in IBCSG Trial 11-93, mean hazard rates and their 95 percent confidence intervals were calculated using Trial 11-93 data (Table 3).
Table 3.
Mean annual hazard rates for recurrence and 10-year RFS percents derived from Adjuvant! Online and IBCSG Trial 11-93 analyses assuming exponential RFS distributions
| Adjuvant! Online | IBCSG Trial 11-93 | |
|---|---|---|
| Mean annual hazard rates (95% CI) | ||
| OFS-AC-Tam | 0.029 (0.026, 0.031) | 0.029 (0.019, 0.044) |
| OFS-Tam | 0.044 (0.040, 0.048) | 0.027 (0.017, 0.042) |
| 10-year recurrence-free survival percents (95% CI) | ||
| OFS-AC-Tam | 74.9 (73.1, 76.8) | 74.9 (64.5, 82.7) |
| OFS-Tam | 64.4 (61.9, 67.2) | 76.4 (65.8, 84.0) |
The bootstrap method was used to estimate the standard errors for differences in outcomebetween Trial 11-93 and Adjuvant! and a p-value was calculated.
RESULTS
IBCSG Trial 11-93 treatment groups achieved similar estimated 10-year RFS assuming an exponential distribution: 76.4% (95% CI, 65.8% to 84.0%) for the OFS-Tam group and 74.9% (95% CI, 64.5% to 82.7%) for the OFS-AC-Tam group. The two regimens also had similar mean hazards (0.027 (95% CI, 0.017 to 0.042) for OFS-Tam; 0.029 (95% CI, 0.019 to 0.044) for OFS-AC-Tam). By contrast, the Adjuvant! estimates suggested a substantial advantage for the addition of the chemotherapy, with differences in the mean hazards (0.044 (95% CI, 0.040 to 0.048) for OFS-Tam; 0.029 (95% CI, 0.026 to 0.031) for OFS-AC-Tam) and in the10-year RFS percents (64.4% (95% CI, 61.9% to 67.2%) and 74.9% (95% CI, 73.1% to 76.8%) without and with chemotherapy) (Table 3). The Adjuvant! estimate for the endocrine alone control group was significantly lower than the control group estimate observed in Trial 11-93 (p=0.03), while the estimates for the two chemoendocrine therapy groups were similar.
Figure 1 presents the Kaplan-Meier curves for RFS calculated from the IBCSG Trial 11-93 data (Fig 1a), the RFS curves estimated assuming an exponential distribution (Fig 1b), and the four curves superimposed (Fig 1c). Figure 2 presents the RFS curves assuming an exponential distribution calculated from the Adjuvant! hazard rate estimates (Fig 2a) and superimposes these RFS curves with those calculated using the Trial 11-93 outcome data (Fig 2b).
Figure 1.
Figure 2.
CONCLUSIONS
Adjuvant! is a user-friendly web-based system that can give health professionals a rapid response for the prognosis for patients in specific subgroups. It is an excellent tool frequently used to assist clinicians in assessing potential benefits of a variety of treatment options. Any such tool, however, is only as good as the evidence on which it is built. One clinical area of controversy for which data are scarce concerns the benefit of chemotherapy when added to the combination of ovarian suppression plus tamoxifen (also referred to as ‘complete estrogen blockade’) for premenopausal patients with estrogen receptor-positive tumors and limited axillary node (1–3+) involvement. In fact, IBCSG Trial 11-93 is the only published randomized trial directly addressing this question. To obtain estimates in this setting, Adjuvant! must rely on data from other populations, such as all trials enrolling premenopausal patients, or trials comparing chemotherapy with no chemotherapy in the absence of hormonal treatment.
Adjuvant! properly describes its limitations [1]. Estimates rely heavily on treatment effects obtained on average from the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) overviews. These may be imprecise because several regimens are often combined in a single analysis, yielding results for a class of treatments and possibly obscuring differences of the effectiveness of a particular regimen. In addition, Adjuvant! cannot provide estimates for therapies that have not been sufficiently evaluated in clinical trials. In the case of this report, due to the paucity of data regarding the benefit of chemotherapy for premenopausal patients with endocrine responsive disease who receive complete estrogen blockade, Adjuvant! must make the assumption that the ‘average’ results are relevant for this patient population. Adjuvant! also assumes that the value of chemotherapy and tamoxifen is independent and roughly additive. Finally, Adjuvant! does not appear to adjust estimates of the effectiveness of chemotherapy regimens based on age or estrogen receptor status. The Adjuvant! online help file states: “In premenopausal women neither ER status nor whether the patient received adjuvant tamoxifen or not did not seem to make a difference in terms of the effectiveness of the adjuvant chemotherapy. In an analysis not presented by the Overview, but derived from the data [sic], it is even clearer that in younger women ER is not an important predictor of the effectiveness of chemotherapy.” [1] While this statement is true with respect to comparisons of chemotherapy plus tamoxifen versus tamoxifen alone, it fails to consider the biologic drivers of the observed results, i.e., the fact that endocrine effects of chemotherapy on the ovarian function (chemocastration) play a role for the population of patients with ER-positive disease.
If OFS is used as an integral part of the endocrine therapy regimen, the ovarian ablative effect of chemotherapy may be superfluous and its cytotoxic effect may not provide much additional benefit for an ER-positive cohort of patients with a limited axillary node (1–3+) involvement. Indeed, long term results of the randomized ZEBRA study demonstrated non-inferiority of goserelin versus CMF (cyclophosphamide, methotrexate and 5-fluorouracil) for adjuvant therapy of premenopausal patients with ER-positive, node-positive early breast cancer [9]. In two subsequent randomized trials of premenopausal patients with ER-positive early breast cancer, complete estrogen blockade combining an LHRH-agonist with tamoxifen was compared to adjuvant chemotherapy [10, 11]. This hormonal treatment was significantly more effective than CMF in the Austrian trial which enrolled patients with stage I and II breast cancer [10], while the French study evaluating patients with one to three positive axillary nodes [11] found this hormonal therapy to be at least equivalent to 6 cycles of FEC50 (fluorouracil, epirubicin and cyclophosphamide).
Premenopausal women with ER-positive disease have proven to be a difficult population in which to evaluate the effectiveness of the addition of chemotherapy. IBCSG Trial 11-93 was closed prematurely due to the inability to recruit sufficient patients and as a result is an underpowered study. A second IBCSG trial for premenopausal patients with estrogen receptor-positive disease evaluating the role of chemotherapy, IBCSG 26-02 PERCHE, was also closed early due to poor accrual despite a worldwide effort (BIG 4-02) [12]. Nevertheless, IBCSG Trial 11-93 remains the only published randomized trial evaluating chemotherapy added to optimal endocrine therapy for premenopausal women with endocrine-responsive disease.
Three main limitations should be considered when interpreting the current results. IBCSG Trial 11-93 recruited only 174 patients (of the accrual goal of 400) and thus provides imprecise estimates, although the difference between the Trial 11-93 and Adjuvant! control groups not receiving chemotherapy was statistically significant (p = 0.03). AC chemotherapy was used in Trial 11-93, and it is possible that more intensive chemotherapy regimens might have produced different results. However, the results from Trial 11-93 and Adjuvant! only differ in the estimated outcome for the non-chemotherapy control groups. Finally, we note that although Trial 11-93 was open for the node-positive population, 55% of patients enrolled had only 1 positive node, and 97% had 1 to 3 positive nodes. Furthermore, only 41 patients (24%) had grade 3 tumors, leaving as an unanswered question the role of chemotherapy for high grade disease. The OFS administered in IBCSG 11-93 by GnRH was prolonged and continued until at least the age of 55 years. Thus, the results shown here are primarily applicable to patients with a low number of positive axillary lymph nodes, lower grade and highly endocrine-responsive tumors, although they might also be extrapolated to node-negative disease.
The MINDACT (Microarray In Node-negative Disease may Avoid ChemoTherapy) and TAILORx (Trial Assigning IndividuaLized Options for Treatment (Rx)) studies both have the goal of evaluating molecular signatures or gene expression profiles for clinical practice [13,14], seeking to identify a subset of patients with operable node-negative breast cancer (TAILORx) or 0–3 positive nodes (MINDACT) who may not need chemotherapy. Neither trial will be able to address the question posed in Trial 11-93 and PERCHE [12]. For example, while the Intergroup TAILORx trial will provide some information regarding the impact of chemotherapy, the endocrine treatment is neither standardized nor declared prior to randomization. It is, therefore, possible that those women not assigned to receive chemotherapy might be given a different endocrine treatment, such as OFS combined with an antihormonal drug, which will make the results difficult to interpret.
Adjuvant! was shown to reliably predict overall survival (OS) and event free survival (EFS) in a large series of 4,083 patients from the British Columbia Breast Cancer Outcomes Unit database [15]. However, when applied to data from 1065 women treated in the United Kingdom, it yielded overly optimistic predictions, which were more pronounced for OS than for EFS, possibly reflecting less availability of new and more effective cancer drugs [16]. The RFS observed in both arms of the IBCSG Trial 11-93 were nearly identical with the RFS predicted by Adjuvant! for the more aggressive treatment strategy (OFS-AC-Tam). The trial results indicate that estimates of chemotherapy benefit underestimate the effectiveness of OFS plus an anti-hormonal compound. Few published trials in this population include OFS, thus Adjuvant! can only evaluate the value of chemotherapy plus tamoxifen compared with that of tamoxifen alone.
A recent presentation by the Austrian Breast Cancer Study Group Trial ABCSG-12 reported excellent 5-year results for both arms of a randomized phase III study of adjuvant endocrine treatment that included both OFS and tamoxifen or an aromatase inhibitor [17]. It is now understood that chemotherapy-induced OFS plays a role for ER-positive disease. Despite the low power of IBCSG Trial 11-93, it is likely that this randomized clinical trial tailored to a specific population and using a total estrogen blockade control arm provides a better estimate of chemotherapy benefit than Adjuvant! in this specific population. While there seems to be no major benefit of systematically adding adjuvant chemotherapy to OFS plus tamoxifen for all hormone receptor positive premenopausal breast cancer patients, there are possibly subgroups in which such a combination may be advantageous. Prospective clinical trials focusing on molecularly defined subgroups (like luminal B or ER+/HER2+ cancers) should evaluate additive or synergistic hormonal and chemotherapy combinations.
There is the caveat presented by Adjuvant!: “The estimates given by the program are evidence based, but not the only possible interpretation of the existing evidence. You, the user, should review the evidence, and if you feel other estimates are more appropriate, you should enter your own estimates. It is possible with the program to make estimates of benefit in patient populations where a given adjuvant therapy has not yet been tested or recommended.” [1] Adjuvant! is a user-friendly tool for informing patients and their doctors regarding prognosis and average treatment benefits, however estimates of chemotherapy benefit for premenopausal patients with endocrine-responsive disease and few positive nodes are likely to be misleading.
Acknowledgments
The International Breast Cancer Study Group (IBCSG) funded this research. The IBCSG is funded by the Swedish Cancer Society, The Cancer Council Australia, Australian New Zealand Breast Cancer Trials Group (NHMRC grant number 940892), the Frontier Science and Technology Research Foundation, the Swiss Group for Clinical Cancer Research (SAKK), Cancer Research Switzerland/Oncosuisse, the Foundation for Clinical Research of Eastern Switzerland (OSKK), and the United States National Cancer Institute (CA-75362). We thank Love Nickerson for collecting patient information from Adjuvant! Online.
Contributor Information
Prof. Robert J. Paridaens, Email: robert.paridaens@uz.kuleuven.ac.be, Department of Medical Oncology, University Hospital Gasthuisberg, Catholic University of Leuven, Belgium.
Shari Gelber, Email: shari@jimmy.harvard.edu, IBCSG Statistical Center, Dana-Farber Cancer Institute and Frontier Science and Technology Research Foundation, Boston, MA.
Bernard F. Cole, Email: ccole@cems.uvm.edu, IBCSG Statistical Center, Boston MA and Department of Mathematics and Statistics, College of Engineering and Mathematical Sciences, University of Vermont, Burlington, VT.
Prof Richard D. Gelber, Email: gelber@jimmy.harvard.edu, IBCSG Statistical Center, Dana-Farber Cancer Institute, Harvard School of Public Health and Frontier Science and Technology Research Foundation, Boston, MA.
Prof. Beat Thürlimann, Email: beat.thuerlimann@kssg.ch, Breast Center, Kantonsspital, St. Gallen and Swiss Group for Clinical Cancer Research (SAKK), Switzerland.
Karen N. Price, Email: price@jimmy.harvard.edu, IBCSG Statistical Center and Frontier Science and Technology Research Foundation, Boston, MA.
Stig B. Holmberg, Email: stig.holmberge@vgregion.se, Department of Surgery, Sahlgrenska University Hospital, Gothenburg, Sweden.Diana Crivellari, MD.
Diana Crivellari, Email: dcrivellari@cro.it, Centro di Riferimento Oncologico, Aviano, Italy.
Prof. Alan S. Coates, Email: alan.coates@ibcsg.org, International Breast Cancer Study Group and University of Sydney, Sydney, Australia.
Prof. Aron Goldhirsch, Email: aron.goldhirsch@ibcsg.org, European Institute of Oncology, Milan, Italy, and Oncology Institute of Southern Switzerland, Bellinzona, Switzerland.
References
- 1.Adjuvant! for Breast Cancer (Version 8.0) Adjuvant! Inc; [Accessed 9 July 2009]. http://www.adjuvantonline.com. [Google Scholar]
- 2.Ravdin PM, Siminoff LA, Davis GJ, Mercer MB, Hewlett J, Gerson N, Parker HL. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001;19:980–991. doi: 10.1200/JCO.2001.19.4.980. [DOI] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Polychemotherapy for early breast cancer: an overview of the randomised trials. Lancet. 1998;352:930–952. [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 5.International Breast Cancer Study Group. Randomized controlled trial of ovarian function suppression plus tamoxifen versus the same endocrine therapy plus chemotherapy: Is chemotherapy necessary for premenopausal women with node-positive, endocrine responsive breast cancer? First results of International Breast Cancer Study Group Trial 11-93. The Breast. 2001;10(Suppl 3):130–138. [Google Scholar]
- 6.Thürlimann B, Price KN, Gelber RD, Holmberg SB, Crivellari D, Colleoni M, et al. Is chemotherapy necessary for premenopausal women with lower-risk node-positive, endocrine responsive breast cancer? 10-year update of International Breast Cancer Study Group Trial 11-93. Breast Cancer Res Treat. 2009;113:137–144. doi: 10.1007/s10549-008-9912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klijn JGM, Blamey RW, Boccardo F, Tominaga T, Duchateau L, Sylvester R for the Combined Hormone Agents Trialists’ Group and the European Organization for Research and Treatment of Cancer. Combined Tamoxifen and luteinizing hormone-releasing hormone (LHRH) agonist versus LHRH agonist alone in premenopausal advanced breast cancer: a meta-analysis of four randomized trials. J Clin Oncol. 2001;19:343–353. doi: 10.1200/JCO.2001.19.2.343. [DOI] [PubMed] [Google Scholar]
- 8.Fisher B, Brown AM, Dimitrov NV, Poisson R, Redmond C, Margolese RG, Bowman D, Wolmark N, Wickerham DL, Kardinal CG, et al. Two months of doxorubicin-cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil in positive-node breast cancer patients with tamoxifen-nonresponsive tumors: results from the National Surgical Adjuvant Breast and Bowel Project B-15. J Clin Oncol. 1990;8:1483–96. doi: 10.1200/JCO.1990.8.9.1483. [DOI] [PubMed] [Google Scholar]
- 9.Kaufmann M, Jonat W, Blamey R, Cuzick J, Namer M, Fogelman I, de Haes JC, Schumacher M, Sauerbrei W on behalf of the Zoladex Early Breast Cancer Research Association (ZEBRA) Trialists’ Group. Survival analyses from the ZEBRA study: goserelin (Zoladex ™) versus CMF in premenopausal women with node-positive breast cancer. Eur J Cancer. 2003;39:1711–1717. doi: 10.1016/s0959-8049(03)00392-7. [DOI] [PubMed] [Google Scholar]
- 10.Jakesz R, Hausmaninger H, Kubista E, Gnant M, Menzel C, Bauernhofer T, Seifert M, Haider K, Mlineritsch B, Steindorfer P, Kwasny W, Fridrik M, Steger G, Wette V, Samonigg H. Randomized adjuvant trial of Tamoxifen and Goserelin versus Cyclophosphamide, Methotrexate and Fluorouracil: evidence for the superiority of treatment with endocrine blockade in premenopausal patients with hormone-responsive breast cancer – Austrian Breast and Colorectal Cancer Study Group Trial 5. J Clin Oncol. 2002;20:4621–4627. doi: 10.1200/JCO.2002.09.112. [DOI] [PubMed] [Google Scholar]
- 11.Roché H, Kerbrat P, Bonneterre J, Fargeot P, Fumoleau P, Monnier P, Clavére P, Goudier M-J, Chollet P, Guastalla J-P, Serin D. Complete hormonal blockade versus epirubicin-based chemotherapy in premenopausal, one to three node-positive, and hormone-receptor positive, early breast cancer patients: 7-year follow-up results of French Adjuvant Study Group 06 randomised trial. Annals Oncol. 2006;17:1221–1227. doi: 10.1093/annonc/mdl107. [DOI] [PubMed] [Google Scholar]
- 12.Regan MM, Pagani O, Walley B, Torrisi R, Perez EA, Francis P, et al. Premenopausal endocrine-responsive early breast cancer: who receives chemotherapy? Ann Oncol. 2008;19:1231–1241. doi: 10.1093/annonc/mdn037. [DOI] [PubMed] [Google Scholar]
- 13.MINDACT (Microarray In Node negative Disease may Avoid ChemoTherapy) trial. Breast International Group; 2009. [Accessed 9 July 2009]. http://www.breastinternationalgroup.org/TransBIG/Mindact.aspx. [Google Scholar]
- 14.The TAILORx Breast Cancer Trial. National Cancer Institute. US National Institutes of Health; [Accessed 9 July 2009]. http://www.cancer.gov/clinicaltrials/digestpage/TAILORx. [Google Scholar]
- 15.Olivotto I, Bajdik C, Ravdin P, Speers C, Coldman A, Norris B, Davis G, Chia S, Gelmon K. Population-based validation of the prognostic model ADJUVANT! for early breast cancer. J Clin Oncol. 2005;23:2716–2725. doi: 10.1200/JCO.2005.06.178. [DOI] [PubMed] [Google Scholar]
- 16.Campbell HE, Taylor MA, Harris AL, Gray AM. An investigation into the performance of the Adjuvant! Online prognostic programme in early breast cancer for a cohort of patients in the United Kingdom. British J Cancer. 2009;101:1074–1084. doi: 10.1038/sj.bjc.6605283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Pöstlberger S, Menzel C, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;60:679–91. doi: 10.1056/NEJMoa0806285. Erratum in: N Engl J Med 360:2379. [DOI] [PubMed] [Google Scholar]