Abstract
Purpose.
Visual hallucinations (VHs) occur in macular degeneration patients with poor vision but normal cognitive function. The underlying mechanisms are poorly understood. We report the identification of pharmaceutical agents that enhance VH and use these agents to examine the contribution of retinal neurons to this syndrome.
Methods.
We detail clinical observations on VH in five macular degeneration patients treated with proton pump inhibitors having the core structure, 2-pyridyl-methylsulfinyl-benzimidazole. We tested possible retinal mechanisms using paired whole cell recordings to examine effects of these compounds on feedback interactions between horizontal cells and cones in amphibian retina.
Results.
Five patients with advanced wet macular degeneration described patterned VHs that were induced or enhanced by oral proton pump inhibitors. The abnormal images increased with light, disappeared in the dark, and originated in the retina, based on ophthalmodynamometry. Simultaneous paired whole cell recordings from amphibian cones and horizontal cells showed that 2-pyridyl-methylsulfinyl-benzimidazoles blocked the negative shift in voltage dependence and increase in amplitude of the calcium current (ICa) in cones that is induced by changes in horizontal cell membrane potential. These effects disrupt the negative feedback from horizontal cells to cones that is important for the formation of center-surround receptive fields in bipolar and ganglion cells, and thus for normal spatial and chromatic perception.
Conclusions.
Our study suggests that changes in the output of retinal neurons caused by disturbances in outer retinal feedback mechanisms can enhance patterned visual hallucinations.
Introduction
The Charles Bonnet Syndrome (CBS) is named after the Swiss philosopher Charles Bonnet who described visual hallucinations in his blind 89-year-old grandfather.1–3 No psychiatric or cognitive disturbances are present in patients with CBS and, in most cases, there is no gross or microscopic brain pathology.4 The neurophysiological basis of these phenomena has been attributed to the spontaneous discharge of nerve cells from the visual association cortex when blindness leads to reduced sensory input.5,6 This hypothesis is known as the deafferentation theory and has become a widely accepted medical paradigm for explaining the neural basis of CBS.5,7,8 However, this idea has never been validated.
In the current study, we report clinical and experimental observations that suggest altered retinal processing induces or enhances visual hallucinations in macular degeneration patients. We describe a group of five patients that reported the onset or enhancement of patterned visual hallucinations following the use of lansoprazole, omeprazole, and pantoprazole, three proton pump inhibitors (PPIs) sharing the core structure, 2-pyridyl-methylsulfinyl-benzimidazole. Although these drugs do not normally penetrate the blood-brain barrier, patients with exudative macular degeneration have a breakdown in the blood-retinal barrier in the outer retina, a region where proton pumps play important roles in visual processing.
Protons can influence negative feedback interactions between horizontal cells and cone photoreceptors.9–11 This feedback loop is fundamental to the formation of center surround receptive fields in bipolar and ganglion cells.12–14 Center-surround receptive fields improve the detection of edges, a process that is highly conserved from invertebrates to higher vertebrates.15,16 Horizontal cell to cone feedback also appears to be critically important for color perception.14,17–20
We hypothesized that proton pump inhibitors may disrupt normal horizontal cell-photoreceptor cell feedback interactions and thereby alter spatial and chromatic perception. Complementing our clinical observations, we examined the effects of these drugs on horizontal cell to cone feedback interactions directly by recording simultaneously from cones and horizontal cells using an amphibian retina model system.
Methods
We identified five patients with advanced wet macular degeneration and poor vision who reported the onset or enhancement of visual hallucinations with the use of proton pump inhibitors for heartburn management. The diagnosis of wet ARMD was based on the presence of subretinal blood, intraretinal thickening, and exudate. Fluorescein angiography and spectral domain OCT imaging studies demonstrated subretinal neovascularization, retinal edema, and outer retinal disorganization. Each patient was cognitively intact, lived independently, and had no history of dementia, psychosis, or Parkinson's disease. Each patient was aware that the images were not real. IRB approval was obtained from Scripps Memorial Hospital.
Electrophysiology: Whole-Cell Recordings
Normal horizontal cell-photoreceptor cell feedback interactions were measured in retinal slices from the aquatic tiger salamander (Ambystoma tigrinum), a species whose large retinal cells allow one to record simultaneously from both horizontal cells and cones and thereby test the effect of proton pump-mediated feedback interactions directly. Horizontal cell feedback has been shown to operate by similar mechanisms in both amphibian and mammalian retina.21 Experimental procedures were approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Male and female adult aquatic tiger salamanders (Ambystoma tigrinum, 18–25 cm in length; Kons Scientific, Germantown, WI, and Charles Sullivan Co., Nashville, TN) were maintained on a 12-hour day/night cycle. Because maintained illumination can promote disassembly of synaptic ribbons in rods,22–24 animals were sacrificed 1 to 2 hours after the beginning of subjective night. Retinal slices were prepared and whole cell voltage clamp recordings were obtained from synaptically connected cone and horizontal cell pairs as described in detail previously.9,21 Briefly, ICa was measured in the cone by applying a ramp voltage protocol (−90 to +60 mV, 0.5 mV/ms, applied from a steady holding potential of −70 mV). The strength of horizontal cell–cone feedback was altered by changing the membrane potential of the horizontal cell between 0; −40 (approximating the dark resting membrane potential); −70 (approximating the membrane potential in light); and −90 mV. Changing the horizontal cell–membrane potential produced changes in both the amplitude and the voltage dependence of the cone ICa.9 The changes in ICa caused by horizontal-cone negative feedback interactions were compared in the presence and absence of lansoprazole (Prevacid 100 μM; Sigma-Aldrich, St. Louis, MO) and omeprazole (Prilosec 100 μM; Sigma-Aldrich), two compounds that are representative of the structurally-related class of PPIs.
Results
The Table summarizes the clinical features of five patients who developed patterned visual hallucinations after treatment with a proton pump inhibitor for gastroesophageal reflux disease. Concurrently, all patients had wet macular degeneration with active subretinal neovascularization, a condition that causes breakdown in the blood-retinal barrier and leakage of blood products into the subretinal space. Three patients had no prior history of hallucinations. Two patients had a prior history of patterned hallucinations that increased with the use of omeprazole. All hallucinations were induced by bright light and disappeared after 20 to 30 minutes of darkness, indicating a dependence on the initiation of visual transduction and visual processing pathways. Retinal function was required since the hallucinations ceased after applying external pressure to the globe to raise intraocular pressure and reduce retinal circulation, a technique known as ophthalmodynamometry. The hallucinations returned consistently when external pressure was released. With timely discontinuation of the proton pump inhibitors, the hallucinations stopped or returned to baseline.
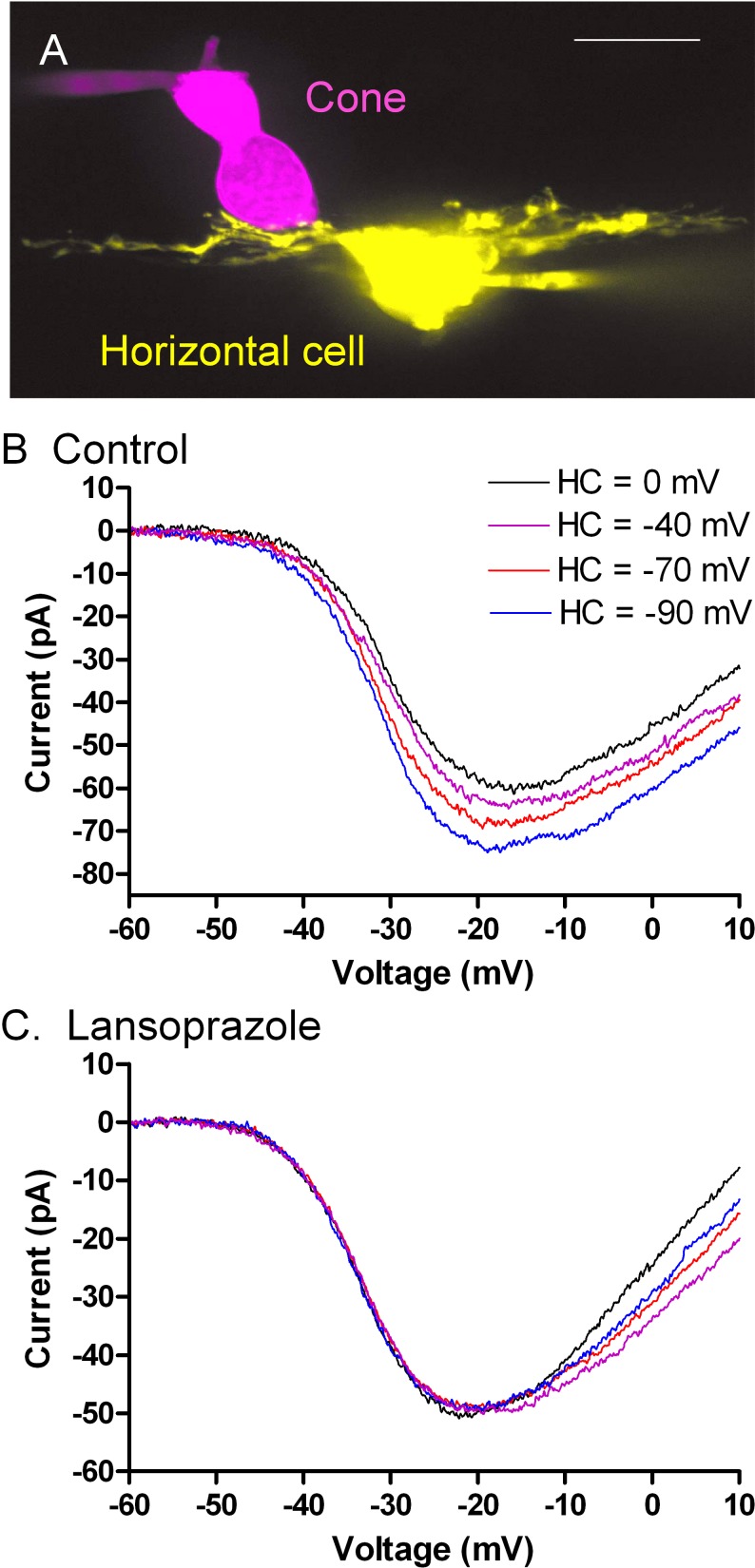
Electrophysiological studies using simultaneous paired whole cell recordings from cone photoreceptor cells and horizontal cells in the amphibian retina were used to evaluate the effects of lansoprazole (Sigma-Aldrich) and omeprazole (Sigma-Aldrich) on negative feedback from horizontal cells to cones. Figure 1A shows an example of a cone and postsynaptic horizontal cell used for simultaneous whole cell recording. Figure 1B shows the current/voltage relationship for cone ICa recorded while voltage clamping the postsynaptic horizontal cell at different membrane potentials. To mimic light-evoked hyperpolarization, we directly hyperpolarized the voltage-clamped horizontal cell, which caused the cone ICa to activate at more negative potentials and slightly increased ICa amplitude. We compared the cone ICa recorded when the horizontal cell membrane potential was clamped at −40 mV (approximating the resting potential in darkness; purple trace) with the cone ICa recorded when the horizontal cell membrane potential was −90 mV (approximating the potential in bright light; blue trace). From this comparison, one can see that hyperpolarization of the horizontal cell caused a negative shift in voltage dependence of cone ICa (leftward shift) and increased its peak amplitude. These effects increase the amplitude of ICa at the cone's normal resting potential of ca. −35 mV and this increase in ICa helps to restore synaptic output from the cone during a light flash. However, in the presence of lansoprazole (100 μM, Sigma-Aldrich), the cone ICa did not respond to changes in the horizontal cell membrane potential, indicating that the normal feedback response was lost (Fig. 1C).
Figure 1. .
(A) Confocal stack showing a fluorescently stained cone and horizontal cell pair used for simultaneous whole-cell recordings. Sulfarhodamine B (magenta; 0.5 mg/mL) was introduced into the cone through the patch electrode near the top of the cell. The horizontal cell was labeled with Lucifer Yellow (yellow, 2 mg/mL). (B) Effects of horizontal cell–membrane potential on cone ICa measured using a ramp voltage protocol (−90 to +60 mV, 0.5 mV/ms). The overlaid traces show measurements of cone ICa when the postsynaptic horizontal cell–membrane potential (Vm) was changed from 0 mV (black) to −40 mV (purple), −70 mV (red), and −90 mV (blue). (C) Changes in horizontal cell Vm had little effect on cone ICa measured in the same cell pair after bath application of lansoprazole (100 μM) for 3 minutes.
Table. .
Clinical Features of Visual Hallucinations in Patients on PPIs
|
Patient |
Age, y |
Sex |
Visual Acuity |
Exudative Disease |
Drug/Duration |
Images |
Reversible |
|
|
Right Eye |
Left Eye |
|||||||
| 1 | 80 | F | 20/40 | 20/400 | Left eye | Omeprazole ×2 days | Blue confetti | Yes |
| 2 | 90 | F | 20/25 | 20/400 | Left eye | Pantoprazole ×2 weeks | Black polka dots | Yes |
| 3 | 98 | F | 20/50 | 20/60 | Right eye | Omeprazole ×5 months | Colored circles | Yes |
| 4 | 90 | F | CF | 20/200 | Right eye | Lansoprazole ×3 years | Black dots, crosses, violet flowers | No |
| 5 | 89 | F | 20/400 | CF | Both | Omeprazole ×4 years | Black dots and colors | No |
CF, counting fingers.
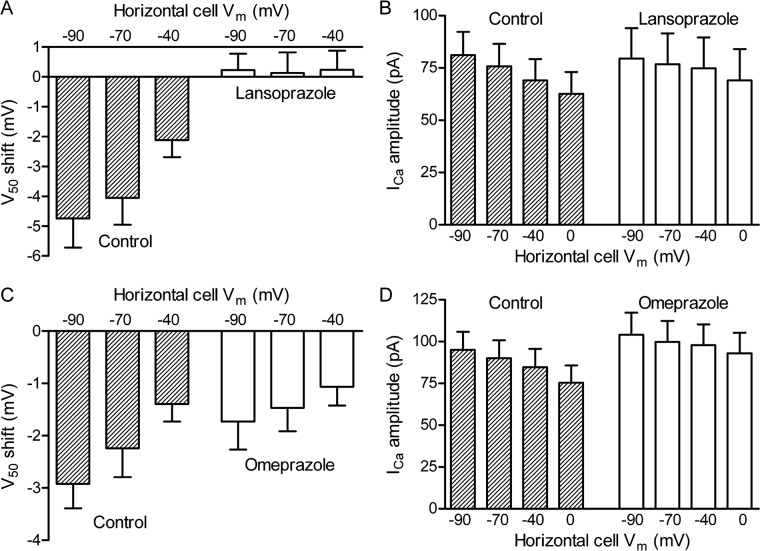
The shift in ICa activation was quantified by determining the voltage at which cone ICa was half maximal (V50). Figure 2A shows the change in cone ICa V50 as a function of horizontal cell–membrane potential. Data were normalized by comparing V50 values measured when the horizontal cell was voltage clamped at −40, −70, or −90 mV to the V50 obtained when the horizontal cell was held at 0 mV. Figure 2B shows the peak amplitude of cone ICa as the horizontal cell hyperpolarizes. In the presence of lansoprazole (Sigma-Aldrich), the cone ICa exhibited no change in V50 in response to hyperpolarization of the horizontal cell and the change in peak current amplitude was diminished. Omeprazole caused a similar but weaker disruption in horizontal cell feedback, as shown by the reduction in the V50 voltage shifts (Fig. 2C) and amplitude changes (Fig. 2D). These effects were restored when omeprazole and lansoprazole were washed out of the bath (not shown), indicating the disruption in horizontal cell-cone feedback was reversible.
Figure 2. .
Effects of lansoprazole and omeprazole on feedback from horizontal cells to cone ICa. (A) Voltage shifts in cone ICa were quantified by determining the voltage at which the current was half-maximal (V50). Data are plotted relative to V50 measured when the horizontal cell was voltage clamped at 0 mV. The hatched bars on the left show control data; data from the same cell pairs measured after bath application of lansoprazole (100 μM) are shown by the open bars on the right (N = 10 pairs). Lansoprazole significantly reduced the change in V50 induced by voltage clamping the horizontal cell at −40 (P < 0.0047, paired t-test); −70 (P < 0.0044); and −90 mV (P < 0.0012). (B) Peak amplitude of ICa measured in control and lansoprazole. (C) Voltage shifts measured in control conditions and following bath application of omeprazole (100 μM; N = 10 pairs). Omeprazole significantly reduced the change in V50 induced by voltage clamping the horizontal cell at −70 (P < 0.0066) and −90 mV (P < 0.0044). (D) Peak amplitude of ICa measured in control and omeprazole. Amplitude differences did not attain statistical significance.
Discussion
Our results demonstrate that proton pump inhibitors with the core structure 2-pyridyl-methylsulfinyl benzimidazole can induce or enhance visual hallucinations in wet macular degeneration patients with a breakdown of the outer blood-retinal barrier. Four of the five patients had chronic subretinal neovascular membranes in only one eye, indicating that unilateral disease is sufficient to disrupt visual processing with these drugs. The hallucinations are reversible with timely discontinuation of the proton pump inhibitors, but may be more difficult to reverse after years of drug use.
Our clinical data support the premise that the signals inducing the patterned visual hallucinations in this group of patients are retinal in origin and are dependent on activation of the visual transduction cascade. The patients report their hallucinations are most noticeable in bright light, disappear after 20 to 30 minutes of darkness, and are never present when they awake at night. The patients also report that their images disappeared when the retinal circulation is temporarily decreased and return when the circulation is restored.
The clinical data further suggest that enhancement of the visual hallucinations with proton pump inhibitors may also involve the suppression of normal horizontal cell-cone feedback mechanisms. Patients describe a subjective loss of contrast sensitivity, which is governed by the feedback interactions between horizontal cells and cones and the formation of center-surround receptive fields.11,15,16,25
Simultaneous paired whole-cell recordings from cones and horizontal cells in an animal model showed that these compounds disrupt normal horizontal cell–cone feedback interactions. Previous data from a number of studies has shown that pH neutralizing buffers, such as HEPES, can block horizontal cell feedback,9,10,26 and thereby abolish both color-opponency and the center-surround receptive field arrangement of parasol ganglion cells.13,20 Under our experimental conditions, the effect of lansoprazole (Sigma-Aldrich) and omeprazole (Sigma-Aldrich) on horizontal cell-cone feedback is similar to the effect of HEPES, supporting the idea that the oral proton pump inhibitors may interfere with a proton-related mechanism of surround formation in this patient population.13
Negative feedback from horizontal cells to cones modulates the voltage-dependence of cone calcium currents.27 At the resting membrane potential, the relationship between calcium current amplitude and membrane voltage is quite steep and so small shifts along the voltage axis induced by small changes in proton levels can have large effects on release. The net result of this feedback interaction is to subtract the effects of the average surrounding light levels from more localized changes in illumination falling on the cone, thereby facilitating contrast discrimination.
Photoreceptor calcium currents are uniquely sensitive to the effects of protons on membrane surface charge because of their relatively depolarized resting membrane potential and unusually low threshold for calcium current activation.28 Changes in extracellular pH produce less pronounced effects at most other neuronal synapses because the resting membrane potentials for most neurons are typically below the threshold for calcium current activation.
While pH buffers have consistently been shown to block horizontal cell feedback to photoreceptors, the mechanisms by which they do so remain controversial, in part because changes in pH that have been proposed to influence horizontal-cell-to-cone feedback are likely to be highly localized to the synapse and not readily measurable by conventional techniques (reviewed by Thoreson and Mangel11).
Proton pump inhibitors typically cause an irreversible inhibition of H+ secretion in gastric parietal cells. In our experimental conditions, the disruption of horizontal cell-cone feedback was reversible. One explanation for this apparent discrepancy is that the timing of the drug exposure in our experimental system may have been insufficient for complete inhibition of ATPase activity. The washout of drugs in our system was typically begun 5 to 7 minutes after application, which is less than the time necessary for pyridyl-methylsulfinyl-benzimidazoles to fully inhibit ATPase activity.29 It is notable that the effect of omeprazole (Sigma-Aldrich) was weaker than lansoprazole (Sigma-Aldrich), consistent with its slower rate of inhibition.
However, along with the possibility of species differences, it is worth considering that these drugs may disrupt feedback at sites other than H+ and K+ pumps. For example, these drugs have been shown to inhibit phosphatases,30 Na-K-ATPases,31 and swelling-dependent chloride channels.32 They also alter muscle contractility in various tissues perhaps by acting on L-type calcium channels.33–36 Antibodies to H+-K+-ATPases do not appear to label retinal neurons,37 and the competitive H+, K+ blocker, SCH 28080, did not block horizontal cell–cone feedback in our experimental system. On the other hand, aside from a slight increase in peak amplitude of the calcium current with omeprazole (Sigma-Aldrich) illustrated in Figure 2D, we did not see any consistent changes in the current/voltage relationships of cones during application of these drugs that would suggest actions at other targets. Additional insight into how these drugs alter horizontal cell–cone feedback awaits further study.
The concentrations that were used in the animal model are more than 10-fold higher than plasma concentrations typically attained in patients.38,39 Given that lansoprazole 100 μM (Sigma-Aldrich) completely blocked horizontal cell to cone feedback and such blockade abolishes center-surround receptive fields and color opponency, it is likely that the presence of lansoprazole (Sigma-Aldrich) at higher concentrations in patients would produce much more profound visual disturbances.
We observed interindividual variation in the response to these drugs in AMD patients with exudative disease. Although we do not fully understand why some patients have visual disturbances and others do not, some of this variation could be accounted for by individual differences in the plasma concentrations of these drugs, due to genetic variation in the hepatic cytochrome P450 isoenzymes that metabolize the drugs.38 Patients who are low metabolizers of these drugs have plasma concentrations that are 5-fold higher than the typical rapid metabolizers.
In conclusion, the new idea that visual hallucinations in the Charles Bonnet Syndrome are related to a loss of neuronal feedback inhibition is consistent with the longstanding concept proposed by David Cogan that hallucinations are “release phenomena” that can occur with pathology anywhere in the visual processing pathway.5
Footnotes
Supported by NIH/NCATS UL1 TR000109, NIH Grant EY10542, Research to Prevent Blindness, The Pfeiffer Foundation, The Fonseca Family, and the Scripps Mericos Eye Institute.
Disclosure: A.M. Hanneken, None; N. Babai, None; W.B. Thoreson, None
References
- 1. de Morsier G. Le syndrome de Chalres Bonnet: hallucinations visuelles des vieillards sans deficience. Ann Med Psychol. 1967; 125: 677– 702 [PubMed] [Google Scholar]
- 2. Bonnet C. Essai Analytique sur les Facultes de l'Ame. Copenhagen: A Copenhague, Chez le freres C. & Philibert; A. 1760: 176– 177 [Google Scholar]
- 3. Hedges TR Jr., Bonnet Charles. his life, and his syndrome. Surv Ophthalmol. 2007; 52: 111– 114 [DOI] [PubMed] [Google Scholar]
- 4. Menon GJ, Rahman I, Menon SJ, Dutton GN. Complex visual hallucinations in the visually impaired: the Charles Bonnet Syndrome. Surv Ophthalmol. 2003; 48: 58– 72 [DOI] [PubMed] [Google Scholar]
- 5. Cogan DG. Visual hallucinations as release phenomena. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1973; 188: 139– 150 [DOI] [PubMed] [Google Scholar]
- 6. Rosenbaum F, Harati Y, Rolak L, Freedman M. Visual hallucinations in sane people: Charles Bonnet Syndrome. J Am Geriatr Soc. 1987; 35: 66– 68 [DOI] [PubMed] [Google Scholar]
- 7. Ffytche DH. Visual hallucinations in eye disease. Curr Opin Neurol. 2009; 22: 28– 35 [DOI] [PubMed] [Google Scholar]
- 8. Burke W. The neural basis of Charles Bonnet hallucinations: a hypothesis. J Neurol Neurosurg Psychiatry. 2002; 73: 535– 541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cadetti L, Thoreson WB. Feedback effects of horizontal cell membrane potential on cone calcium currents studied with simultaneous recordings. J Neurophysiol. 2006; 95: 1992– 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hirasawa H, Kaneko A. pH changes in the invaginating synaptic cleft mediate feedback from horizontal cells to cone photoreceptors by modulating Ca2+ channels. J Gen Physiol. 2003; 122: 657– 671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thoreson WB, Mangel SC. Lateral interactions in the outer retina. Prog Retin Eye Res. 2012; 31: 407– 441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mangel SC, Miller RF. Horizontal cells contribute to the receptive field surround of ganglion cells in the rabbit retina. Brain Res. 1987; 414: 182– 186 [DOI] [PubMed] [Google Scholar]
- 13. Davenport CM, Detwiler PB, Dacey DM. Effects of pH buffering on horizontal and ganglion cell light responses in primate retina: evidence for the proton hypothesis of surround formation. J Neurosci. 2008; 28: 456– 464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vigh J, Witkovsky P. Sub-millimolar cobalt selectively inhibits the receptive field surround of retinal neurons. Vis Neurosci. 1999; 16: 159– 168 [DOI] [PubMed] [Google Scholar]
- 15. Kuffler SW. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953; 16: 37– 68 [DOI] [PubMed] [Google Scholar]
- 16. Hartline HK, Wagner HG, Ratliff F. Inhibition in the eye of Limulus. J Gen Physiol. 1956; 39: 651– 673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stell WK, Lightfoot DO. Color-specific interconnections of cones and horizontal cells in the retina of the goldfish. J Comp Neurol. 1975; 159: 473– 502 [DOI] [PubMed] [Google Scholar]
- 18. Burkhardt DA. Synaptic feedback, depolarization, and color opponency in cone photoreceptors. Vis Neurosci. 1993; 10: 981– 989 [DOI] [PubMed] [Google Scholar]
- 19. Vanleeuwen MT, Joselevitch C, Fahrenfort I, Kamermans M. The contribution of the outer retina to color constancy: a general model for color constancy synthesized from primate and fish data. Vis Neurosci. 2007; 24: 277– 290 [DOI] [PubMed] [Google Scholar]
- 20. Crook JD, Manookin MB, Packer OS, Dacey DM. Horizontal cell feedback without cone type-selective inhibition mediates “red-green” color opponency in midget ganglion cells of the primate retina. J Neurosci. 31: 1762– 1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Babai N, Thoreson WB. Horizontal cell feedback regulates calcium currents and intracellular calcium levels in rod photoreceptors of salamander and mouse retina. J Physiol. 2009; 587: 2353– 2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abe H, Yamamoto TY. Diurnal changes in synaptic ribbons of rod cells of the turtle. J Ultrastruct Res. 1984; 86: 246– 251 [DOI] [PubMed] [Google Scholar]
- 23. Balkema GW, Cusick K, Nguyen TH. Diurnal variation in synaptic ribbon length and visual threshold. Vis Neurosci. 2001; 18: 789– 797 [DOI] [PubMed] [Google Scholar]
- 24. Spiwoks-Becker I, Glas M, Lasarzik I, Vollrath L. Mouse photoreceptor synaptic ribbons lose and regain material in response to illumination changes. Eur J Neurosci. 2004; 19: 1559– 1571 [DOI] [PubMed] [Google Scholar]
- 25. Baylor DA, Fuortes MG, O'Bryan PM. Receptive fields of cones in the retina of the turtle. Journal Physiol. 1971; 214: 265– 294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vessey JP, Stratis AK, Daniels BA, et al. Proton-mediated feedback inhibition of presynaptic calcium channels at the cone photoreceptor synapse. J Neurosci. 2005; 25: 4108– 4117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Verweij J, Kamermans M, Spekreijse H. Horizontal cells feedback to cones by shifting the cone calcium-current activation range. Vision Res. 1996; 36: 3943– 3953 [DOI] [PubMed] [Google Scholar]
- 28. Barnes S, Merchant V, Mahmud F. Modulation of transmission gain by protons at the photoreceptor output synapse. Proc Natl Acad Sci U S A. 1993; 90: 10081– 10085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sachs G, Shin JM, Briving C, Wallmark B, Hersey S. The pharmacology of the gastric acid pump: the H+, K+ ATPase. Annu Rev Pharmacol Toxicol. 1995; 35: 277– 305 [DOI] [PubMed] [Google Scholar]
- 30. Delomenede M, Buchet R, Mebarek S. Lansoprazole is an uncompetitive inhibitor of tissue-nonspecific alkaline phosphatase. Acta Biochim Pol. 2009; 56: 301– 305 [PubMed] [Google Scholar]
- 31. Veklich TO, Shkrabak OA, Medvediev VV, Kurs'kyi MD, Kosterin SO. Influence of omeprasole and lansoprasole on Na+, K+ -ATPase and Mg2+ -ATPase activity of the plasmatic membrane of myometrium smooth muscle cells [in Ukrainian]. Ukr Biokhim Zh. 2007; 79: 81– 86 [PubMed] [Google Scholar]
- 32. Schmarda A, Dinkhauser P, Gschwentner M, et al. The gastric H, K-ATPase blocker lansoprazole is an inhibitor of chloride channels. Br J Pharmacol. 2000; 129: 598– 604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aydin C, Sarac B, Koyuncu A, Yildirim S, Sen M, Sarioglu Y. Relaxant effect of omeprazole and lansoprazole in guinea pig gallbladder muscle strips in vitro. J Gastroenterol. 2003; 38: 765– 771 [DOI] [PubMed] [Google Scholar]
- 34. Naseri E, Yenisehirli A. Proton pump inhibitors omeprazole and lansoprazole induce relaxation of isolated human arteries. Eur J Pharmacol. 2006; 531: 226– 231 [DOI] [PubMed] [Google Scholar]
- 35. Yenisehirli A, Onur R. Positive inotropic and negative chronotropic effects of proton pump inhibitors in isolated rat atrium. Eur J Pharmacol. 2005; 519: 259– 266 [DOI] [PubMed] [Google Scholar]
- 36. Yenisehirli A, Onur R. Specific H+/K(+)-ATPase inhibitors decreased contractile responses of isolated rat vas deferens. Pharmacol Res. 2006; 54: 397– 405 [DOI] [PubMed] [Google Scholar]
- 37. Fain GL, Smolka A, Cilluffo MC, et al. Monoclonal antibodies to the H+-K+ ATPase of gastric mucosa selectively stain the non-pigmented cells of the rabbit ciliary body epithelium. Invest Ophthalmol Vis Sci. 1988; 29: 785– 794 [PubMed] [Google Scholar]
- 38. Andersson T, Cederberg C, Edvardsson G, Heggelund A, Lundborg P. Effect of omeprazole treatment on diazepam plasma levels in slow versus normal rapid metabolizers of omeprazole. Clin Pharmacol Ther. 1990; 47: 79– 85 [DOI] [PubMed] [Google Scholar]
- 39. Flouvat B, Delhotal-Landes B, Cournot A, Dellatolas F. Single and multiple dose pharmacokinetics of lansoprazole in elderly subjects. Br J Clin Pharmacol. 1993; 36: 467– 469 [DOI] [PMC free article] [PubMed] [Google Scholar]