Abstract
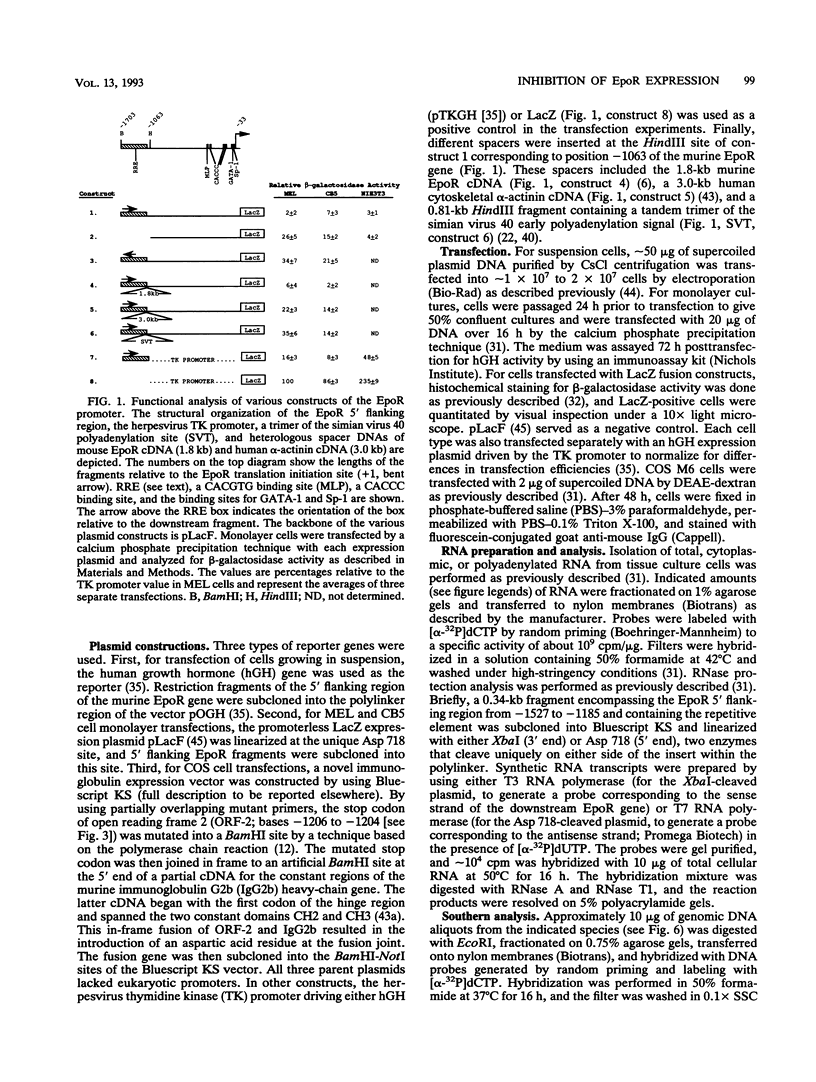
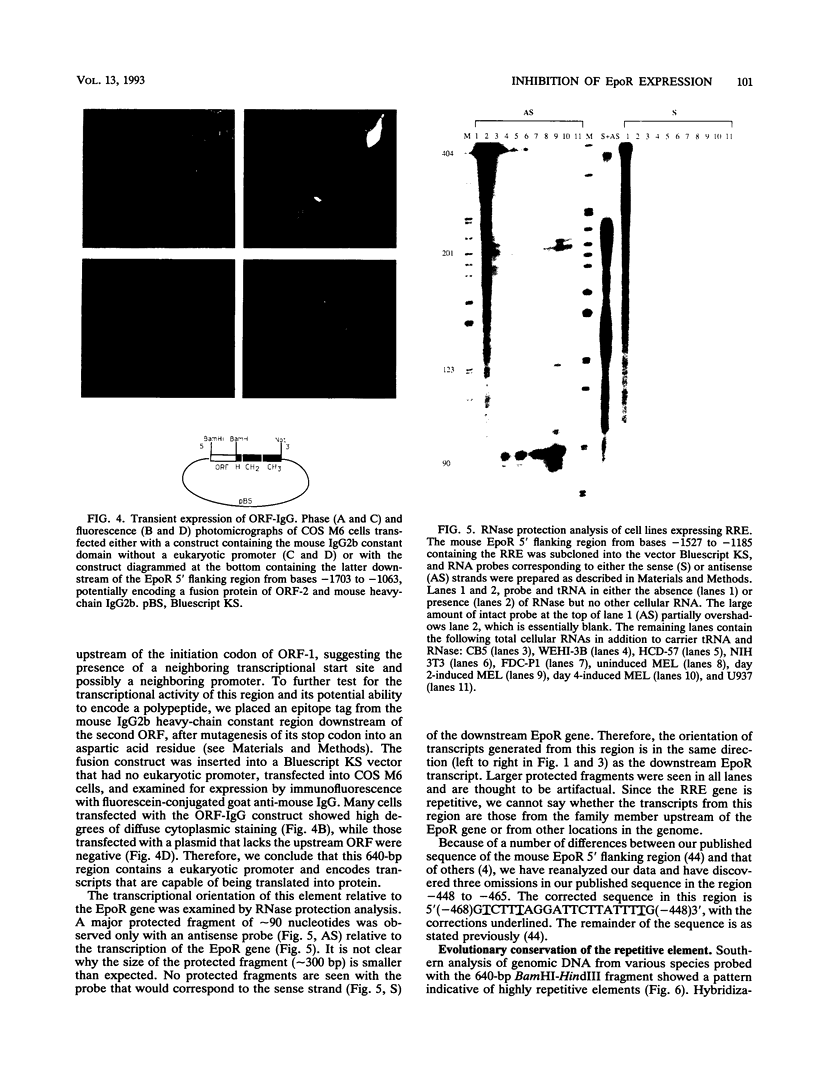
Transcription of the murine erythropoietin receptor (EpoR) gene is inhibited by a novel repetitive element that is located upstream of the EpoR promoter. Reporter gene studies reveal that the inhibitory effect is both distance and orientation dependent. This element is a member of a family of repetitive elements specific to rodents and is present at approximately 10(5) copies per mouse genome. It encodes approximately 500- to 900-bp-long transcripts in both erythroid and nonerythroid cells. RNase protection analysis with a probe from the 5' flanking murine EpoR gene reveals that the direction of transcription is in the sense orientation, relative to the downstream EpoR gene. We suggest that transcriptional inhibition of the EpoR promoter is mediated by read-through transcripts originating in the upstream repetitive element and that this effect may contribute to the basal level of transcription of the murine EpoR gene in erythroid cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anklesaria P., Klassen V., Sakakeeny M. A., FitzGerald T. J., Harrison D., Rybak M. E., Greenberger J. S. Biological characterization of cloned permanent stromal cell lines from anemic Sl/Sld mice and +/+ littermates. Exp Hematol. 1987 Jul;15(6):636–644. [PubMed] [Google Scholar]
- Baniahmad A., Muller M., Steiner C., Renkawitz R. Activity of two different silencer elements of the chicken lysozyme gene can be compensated by enhancer elements. EMBO J. 1987 Aug;6(8):2297–2303. doi: 10.1002/j.1460-2075.1987.tb02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett G., Horiuchi M., Paul M., Pratt R. E., Nakamura N., Dzau V. J. Identification of a negative regulatory element involved in tissue-specific expression of mouse renin genes. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):885–889. doi: 10.1073/pnas.89.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T., Ikawa Y., Todokoro K. GATA-1 transactivates erythropoietin receptor gene, and erythropoietin receptor-mediated signals enhance GATA-1 gene expression. Nucleic Acids Res. 1991 Jul 25;19(14):3843–3848. doi: 10.1093/nar/19.14.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea A. D., Fasman G. D., Lodish H. F. A new hematopoietic growth factor receptor superfamily: structural features and implications for signal transduction. Curr Opin Cell Biol. 1990 Aug;2(4):648–651. doi: 10.1016/0955-0674(90)90106-o. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Lodish H. F., Wong G. G. Expression cloning of the murine erythropoietin receptor. Cell. 1989 Apr 21;57(2):277–285. doi: 10.1016/0092-8674(89)90965-3. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Garland J., Scott D., Scolnick E., Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980 Oct 1;152(4):1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Evans T., Felsenfeld G. The erythroid-specific transcription factor Eryf1: a new finger protein. Cell. 1989 Sep 8;58(5):877–885. doi: 10.1016/0092-8674(89)90940-9. [DOI] [PubMed] [Google Scholar]
- Gregory C. J. Erythropoietin sensitivity as a differentiation marker in the hemopoietic system: studies of three erythropoietic colony responses in culture. J Cell Physiol. 1976 Oct;89(2):289–301. doi: 10.1002/jcp.1040890212. [DOI] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Hyman R., Ralph P., Sarkar S. Cell-specific antigens and immunoglobulin synthesis of murine myeloma cells and their variants. J Natl Cancer Inst. 1972 Jan;48(1):173–184. [PubMed] [Google Scholar]
- Koury M. J., Bondurant M. C. Erythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cells. Science. 1990 Apr 20;248(4953):378–381. doi: 10.1126/science.2326648. [DOI] [PubMed] [Google Scholar]
- Krantz S. B. Erythropoietin. Blood. 1991 Feb 1;77(3):419–434. [PubMed] [Google Scholar]
- Kuramochi S., Ikawa Y., Todokoro K. Characterization of murine erythropoietin receptor genes. J Mol Biol. 1990 Dec 5;216(3):567–575. doi: 10.1016/0022-2836(90)90384-x. [DOI] [PubMed] [Google Scholar]
- Laimins L., Holmgren-König M., Khoury G. Transcriptional "silencer" element in rat repetitive sequences associated with the rat insulin 1 gene locus. Proc Natl Acad Sci U S A. 1986 May;83(10):3151–3155. doi: 10.1073/pnas.83.10.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M., Manley J. L. Transcriptional repression of eukaryotic promoters. Cell. 1989 Nov 3;59(3):405–408. doi: 10.1016/0092-8674(89)90024-x. [DOI] [PubMed] [Google Scholar]
- Lowndes N. F., Bushel P., Mendelsohn L., Wu J., Yen M. Y., Allan M. A short, highly repetitive element in intron -1 of the human c-Ha-ras gene acts as a block to transcriptional readthrough by a viral promoter. Mol Cell Biol. 1990 Sep;10(9):4990–4995. doi: 10.1128/mcb.10.9.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks P. A., Rifkind R. A. Erythroleukemic differentiation. Annu Rev Biochem. 1978;47:419–448. doi: 10.1146/annurev.bi.47.070178.002223. [DOI] [PubMed] [Google Scholar]
- Maxwell I. H., Harrison G. S., Wood W. M., Maxwell F. A DNA cassette containing a trimerized SV40 polyadenylation signal which efficiently blocks spurious plasmid-initiated transcription. Biotechniques. 1989 Mar;7(3):276–280. [PubMed] [Google Scholar]
- Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989 May 4;339(6219):27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Tilly K., Maniatis T. Fine structure genetic analysis of a beta-globin promoter. Science. 1986 May 2;232(4750):613–618. doi: 10.1126/science.3457470. [DOI] [PubMed] [Google Scholar]
- Nepveu A., Marcu K. B. Intragenic pausing and anti-sense transcription within the murine c-myc locus. EMBO J. 1986 Nov;5(11):2859–2865. doi: 10.1002/j.1460-2075.1986.tb04580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H. Globin gene regulation and switching: circa 1990. Cell. 1990 Nov 16;63(4):665–672. doi: 10.1016/0092-8674(90)90133-y. [DOI] [PubMed] [Google Scholar]
- Palacios R., Steinmetz M. Il-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985 Jul;41(3):727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- Patel V. P., Lodish H. F. The fibronectin receptor on mammalian erythroid precursor cells: characterization and developmental regulation. J Cell Biol. 1986 Feb;102(2):449–456. doi: 10.1083/jcb.102.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J. Transcriptional interference and termination between duplicated alpha-globin gene constructs suggests a novel mechanism for gene regulation. Nature. 1986 Aug 7;322(6079):562–565. doi: 10.1038/322562a0. [DOI] [PubMed] [Google Scholar]
- Rogers J. H. The origin and evolution of retroposons. Int Rev Cytol. 1985;93:187–279. doi: 10.1016/s0074-7696(08)61375-3. [DOI] [PubMed] [Google Scholar]
- Sanes J. R., Rubenstein J. L., Nicolas J. F. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986 Dec 1;5(12):3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada K., Krantz S. B., Dai C. H., Koury S. T., Horn S. T., Glick A. D., Civin C. I. Purification of human blood burst-forming units-erythroid and demonstration of the evolution of erythropoietin receptors. J Cell Physiol. 1990 Feb;142(2):219–230. doi: 10.1002/jcp.1041420202. [DOI] [PubMed] [Google Scholar]
- Schmitt R. M., Bruyns E., Snodgrass H. R. Hematopoietic development of embryonic stem cells in vitro: cytokine and receptor gene expression. Genes Dev. 1991 May;5(5):728–740. doi: 10.1101/gad.5.5.728. [DOI] [PubMed] [Google Scholar]
- Selden R. F., Howie K. B., Rowe M. E., Goodman H. M., Moore D. D. Human growth hormone as a reporter gene in regulation studies employing transient gene expression. Mol Cell Biol. 1986 Sep;6(9):3173–3179. doi: 10.1128/mcb.6.9.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya T., Mak T. W. Isolation and induction of erythroleukemic cell lines with properties of erythroid progenitor burst-forming cell (BFU-E) and erythroid precursor cell (CFU-E). Proc Natl Acad Sci U S A. 1983 Jun;80(12):3721–3725. doi: 10.1073/pnas.80.12.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. F., Martin D. I., Zon L. I., D'Andrea A. D., Wong G. G., Orkin S. H. Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature. 1989 Jun 8;339(6224):446–451. doi: 10.1038/339446a0. [DOI] [PubMed] [Google Scholar]
- Van Zant G., Goldwasser E. Simultaneous effects of erythropoietin and colony-stimulating factor on bone marrow cells. Science. 1977 Nov 18;198(4318):733–735. doi: 10.1126/science.302986. [DOI] [PubMed] [Google Scholar]
- Warner N. L., Moore M. A., Metcalf D. A transplantable myelomonocytic leukemia in BALB-c mice: cytology, karyotype, and muramidase content. J Natl Cancer Inst. 1969 Oct;43(4):963–982. [PubMed] [Google Scholar]
- Wasylyk B., Wasylyk C., Augereau P., Chambon P. The SV40 72 bp repeat preferentially potentiates transcription starting from proximal natural or substitute promoter elements. Cell. 1983 Feb;32(2):503–514. doi: 10.1016/0092-8674(83)90470-1. [DOI] [PubMed] [Google Scholar]
- Weissman J. D., Singer D. S. A complex regulatory DNA element associated with a major histocompatibility complex class I gene consists of both a silencer and an enhancer. Mol Cell Biol. 1991 Aug;11(8):4217–4227. doi: 10.1128/mcb.11.8.4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Grindlay G. J., Bushel P., Mendelsohn L., Allan M. Negative regulation of the human epsilon-globin gene by transcriptional interference: role of an Alu repetitive element. Mol Cell Biol. 1990 Mar;10(3):1209–1216. doi: 10.1128/mcb.10.3.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssoufian H., McAfee M., Kwiatkowski D. J. Cloning and chromosomal localization of the human cytoskeletal alpha-actinin gene reveals linkage to the beta-spectrin gene. Am J Hum Genet. 1990 Jul;47(1):62–72. [PMC free article] [PubMed] [Google Scholar]
- Youssoufian H., Zon L. I., Orkin S. H., D'Andrea A. D., Lodish H. F. Structure and transcription of the mouse erythropoietin receptor gene. Mol Cell Biol. 1990 Jul;10(7):3675–3682. doi: 10.1128/mcb.10.7.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack D. J., Bennett J., Wang Y., Davenport C., Klaunberg B., Gearhart J., Nathans J. Unusual topography of bovine rhodopsin promoter-lacZ fusion gene expression in transgenic mouse retinas. Neuron. 1991 Feb;6(2):187–199. doi: 10.1016/0896-6273(91)90355-4. [DOI] [PubMed] [Google Scholar]
- Zon L. I., Youssoufian H., Mather C., Lodish H. F., Orkin S. H. Activation of the erythropoietin receptor promoter by transcription factor GATA-1. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10638–10641. doi: 10.1073/pnas.88.23.10638. [DOI] [PMC free article] [PubMed] [Google Scholar]